Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jamir Pitton Rissardo and Version 2 by Rita Xu.
Cenobamate (CNB), ([(R)-1-(2-chlorophenyl)-2-(2H-tetrazol-2-yl)ethyl], is a novel tetrazole alkyl carbamate derivative. In November 2019, the Food and Drug Administration approved Xcopri
®
, marketed by SK Life Science Inc., (Paramus, NJ, USA) for adult focal seizures. The European Medicines Agency approved Ontozry
®
by Arvelle Therapeutics Netherlands B.V.(Amsterdam, The Neatherlands) in March 2021. Cenobamate is a medication that could potentially change the perspectives regarding the management and prognosis of refractory epilepsy.
- cenobamate
- YKP3089
- Xcopri
- Ontozry
- epilepsy
1. Introduction
Epilepsy affects more than seventy million individuals worldwide, corresponding to an age-standardized prevalence of 621.5 per 100,000 people [1]. Approximately 3 million adults and almost 500,000 children in the United States have epilepsy [2]. Increased life expectancies and more people surviving events that can lead to epilepsy are expected to raise the number of people with epilepsy [3]. The estimated annual costs in the United States of acute seizure care are around USD 12.5 billion [4]. In this context, the burden of drug-resistant epilepsy (DRE) is believed to be significantly higher due to the number of antiseizure medications (ASMs) used concomitantly and the possible high incidence of adverse events [5][6][5,6].
Seizure freedom is a primary goal in the treatment of epilepsy. Only half of the individuals with epilepsy will become seizure-free with their first ASM [7]. Also, more than one in every three patients with epilepsy will have uncontrolled seizures despite adequate management and anticonvulsant therapy [8]. In this context, failure to achieve sustained seizure freedom with the rational use of two anti-seizure drugs administered alone or in combination defines drug-resistant epilepsy [9].
Uncontrolled epilepsy, compared to epilepsy in general, is associated with ten-to-fifteen-fold more frequent mortality secondary to traumatic injury, drowning, suicide, and sudden unexpected death from epilepsy (SUDEP) [10]. Also, some types of childhood epilepsies are related to neuronal damage leading to epileptic encephalopathy, resulting in lifelong disabilities [11]. In addition, low employment rates and lower high school graduation rates can hinder individuals with epilepsy from reaching their maximum potential [12]. Therefore, poor control of seizures can lead to a higher risk of experiencing physical and psychological disorders, causing worse healthcare outcomes, increased healthcare needs, and decreased quality of life [13].
Many new ASMs have been discovered during the last three decades, with more than twenty new ASMs approved [14]. In this context, these new anticonvulsants have improved the spectrum of side effects, increased routes of administration, and reduced the severity of epilepsy, leading to better compliance and treatment adherence [15][16][15,16]. But, there were no significant changes in the proportion of individuals affected by DRE. Interestingly, the prevalence of DRE in the 1980s was sixty-three percent, and in 2014 this number was sixty-four percent [17].
2. Historical Aspects of Cenobamate
Epilepsy is a neurological disorder characterized by recurrent and unprovoked seizures [18][20]. It is believed that excessive excitability in neural tissues can contribute to the abnormal electrical activities leading to epilepsy [19][21]. ASMs acting on voltage-gated sodium channels have been utilized for the pharmacologic management of epilepsy because these channels are essential for generating and conducting action potentials [20][22]. Also, several point mutations in voltage-gated sodium channels, which exhibit increased persistent sodium currents, have been identified in patients with epilepsy [21][23]. Some ASMs, such as phenytoin and lamotrigine, are known to affect persistent sodium currents [22][24]. In 1951, during rat studies to develop a new anxiolytic drug, meprobamate was observed to have antiseizure activity [23][25]. Ten years later, Frank Berger at Wallace Laboratories noted the remarkable efficacy of felbamate in controlling abnormal electrical activity in animal models of epilepsy (Figure 1) [24][26]. In 2008, Johnson & Johnson submitted a new application for carisbamate, which was approved by the U.S. Food and Drug Administration [25][27]. But, two years later, carisbamate was removed from the market due to insignificant superiority over a placebo in a randomized controlled trial [26][28].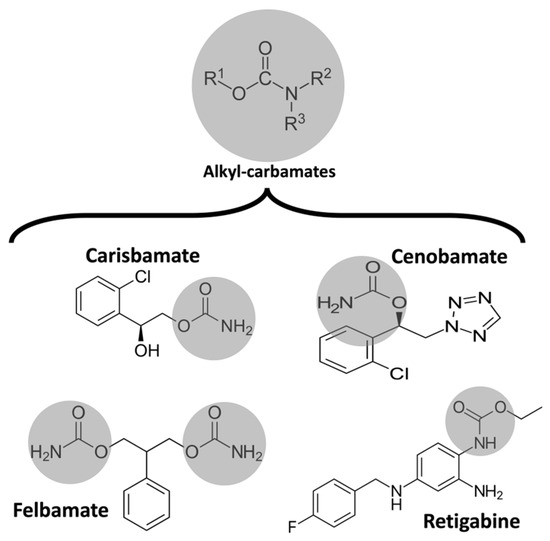
Figure 1. Chemical structure of some alkyl-carbamates with antiseizure activity. Carisbamate, cenobamate, felbamate, and retigabine (ezogabine). Note that felbamate is a dicarbamate. The other drugs are monocarbamates.
3. Pharmacology and Mechanism of Action
CNB’s mechanism of action has yet to be completely understood. Interestingly, CNB was discovered purely by phenotype-based screening, and its presumed dual mechanism of action was only described years after the first studies [43][46]. CNB can reduce repetitive neuronal firing by inhibiting voltage-gated sodium currents. It may enhance the fast and slow inactivation of sodium channels and potently inhibit the non-inactivating persistent component of the sodium channel current, which has already been observed with other ASMs [44][47]. Noteworthily, CNB had little effect on the peak component of transient sodium currents induced by brief depolarizing step pulses. But, CNB strongly inhibited the noninactivating persistent component of sodium currents [45][48]. Therefore, CNB may modify excitability in principal neurons without compromising inhibitory interneurons [46][49]. Also, CNB was revealed to be a positive allosteric modulator of the γ-aminobutyric acid (GABA) ion channel. This effect was similar for all tested GABAA receptors containing six different alpha subunits (α1β2γ2 or α2-6β3γ2). Nakamura et al., studied the effects of CNB in rat hippocampal CA3 neurons. They observed that CNB had little effect on the peak component of transient sodium current induced by brief depolarizing step pulses. Still, CNB potently inhibited the non-inactivating persistent component of sodium currents. Also, it inhibited the sodium currents evoked by slow voltage-ramp stimuli [45][48]. Noteworthily, the effect of CNB in sodium current in hippocampal rat neurons was concentration-dependent [40][43]. Sharma et al., assessed the effects of CNB on GABAergic neurotransmission, specifically its effects on GABAA receptors mediating inhibitory postsynaptic currents and tonic conductance in rodent hippocampal neurons. The authors found that CNB is a positive allosteric modulator of high-affinity GABAA receptors, activated by GABA at a site independent of the benzodiazepine binding site, and efficiently enhances tonic conductance inhibition in hippocampal neurons [47][50]. CNB may resemble barbiturate action because of increased tonic and phasic inhibition through GABAA receptor activation [48][51]. It is worth mentioning that these mechanisms were already observed in animal studies with neurosteroids [49][52]. Also, the effect on both phases of GABAA receptor activation could partially explain the efficacy of CNB in managing status epilepticus [50][53]. The CNB terminal half-life of 50 to 60 h allows this drug to be taken once a day. Noteworthily, this terminal half-life increases with increasing doses of CNB from 30 h (CNB 10 mg) to 76 h (CNB 750 mg). The area under the plasma concentration versus time curve (AUC) increases more than proportionally after the administration of single doses of CNB ranging from 5 to 750 mg. However, after multiple doses of CNB at the steady state, AUC increases linearly with increasing doses within the 50–500 mg/day dose range. Plasma CNB concentrations are steady after approximately two weeks of once-daily dosing. The tablets should be swallowed whole and not crushed or chewed. CNB pharmacokinetics have been reported to be consistent regarding gender, race, and age. Patients with mild-to-moderate (Clcr 30 to 90 mL/min) and severe (Clcr 30 mL/min) renal impairment and those with mild-to-moderate hepatic impairment should be treated with caution and reduced dose. There are no data regarding CNB’s pharmacokinetics in individuals with end-stage renal disease (Clcr < 15 mL/min) undergoing hemodialysis and those with severe hepatic impairment. Vernillet et al., studied the mass balance and the metabolic profiling of CNB in humans. Eight CNB metabolites (M1, M2a, M2b, M3, M5, M6, M7, and M11) were identified across plasma, urine, and feces. CNB was the main plasma radioactive component, and M1 was the only metabolite detected in plasma (>98% and <2% total radioactivity AUC, respectively). All detected metabolites were found in urine; unchanged CNB accounted for approximately six percent. CNB metabolites appeared to be formed slowly [37][40]. Greene et al., assessed the effect of CNB on the single-dose pharmacokinetics of multiple cytochrome P450 probes in healthy subjects. They observed that CNB induces CYP2B6 activity, exhibits a dose-dependent induction of CYP3A4/5 activity, inhibits CYP2C19 activity, and has a negligible effect on CYP2C9 activity [51][57]. RWesearchers calculated the chemical and pharmacological properties of CNB using the SwissADME tool (Figure 2). These properties can help identify compounds suitable for oral use [52][58]. All the parameters analyzed were within the normal range, except for an insaturation slightly higher than those desired for oral molecules, which can reduce oral bioavailability.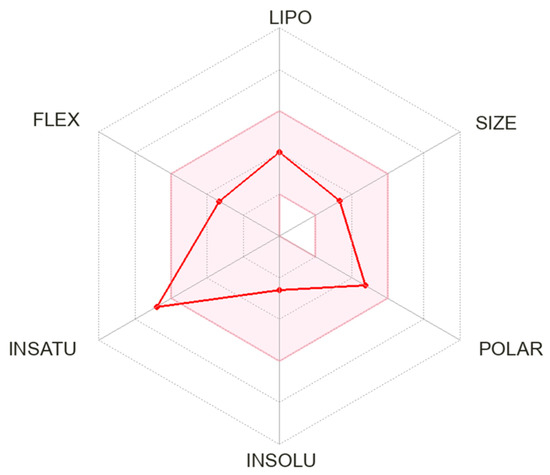
Figure 2. Physicochemical properties of cenobamate. The pink area represents the optimal range for each property. Abbreviation: LIPO: lipophilicity; FLEX: flexibility; INSATU: saturation; INSOLU: solubility.
