Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Gabriela Patrichi and Version 2 by Wendy Huang.
Matrix metalloproteinases (MMPs) are a family of 25 proteolytic enzymes (zinc-dependent endopeptidases) present in the extracellular matrix whose known role is to degrade its structural components. A primary role of these proteases has been that of cleaving extracellular matrix proteins under certain physiological and pathological conditions. Physiologically, they are involved in different processes, mediating different activities of cell proliferation and differentiation and tissue repair, as well as in the mechanisms of apoptosis and angiogenesis, or cell migration.
- matrix metalloproteinases
- cardiovascular disease
- atherosclerosis
- hypertension
- myocardial infarction
1. Introduction
MMP activity is tightly regulated by proteolytic activation and is inhibited by tissue inhibitors of metalloproteinases (TIMPs), and an imbalance between these two components results in the development of many diseases, including cardiovascular diseases, neurodegenerative diseases and cancer, but also in inflammatory processes such as arthritis or fibrotic changes. Both intracellular and extracellular MMPs are responsible for the development of cardiovascular disease—including post cardiac transplant rejection [1][2][10,13].
In addition to TIMPs, the expression and the activity of these enzymes can also be influenced by other metabolic pathways, chemical agents and cell signaling molecules (different hormones, growth factors, and cytokines such as insulin or insulin-like growth factor-1, leptin, adiponectin, and corticosteroids). Moreover, gene expression was also found to be involved in the upregulation of MMPs [3][14].
Cardiovascular disease is a major concern and remains the leading cause of morbidity and mortality worldwide. In addition to the numerous known risk factors that favor the development of various heart diseases, MMPs also contribute to the development of these pathologies. Thus, alterations in the expression of these proteases lead to increased risk of cardiovascular morbidity and mortality.
MMP has been shown to present broad roles in the pathogenesis of multiple cardiovascular diseases, not limited to transplant pathology. Among these, an important contribution in the process of atherosclerosis or in myocardial ischemia and reperfusion lesions was mentioned. Multiple studies also show the involvement of MMPs in diabetes, myocardial infarction, aneurysm, hypertension, and cardiomyopathies [4][5][6][39,40,41].
2. Atherosclerosis
MMPs have been associated with atherosclerosis, a degenerative disease characterized by lipid accumulation on the intimal surface of the arteries, with the formation over time of atheromatous plaques by various promoting factors. These proteases have the ability to degrade atheromatous plaques, thereby destroying the structure of the vascular wall [7][42]. Moreover, Lee et al. demonstrated (in a study on atherosclerosis) the involvement of MMPs in inflammatory processes, and showed that the decrease in the degree of inflammatory infiltrate is due to inhibition of the elastase system through activation of MMPs, triggering the production of local growth factors and creating a vicious circle of initiating a new inflammatory process with a role in atherosclerotic plaque formation [8][43]. MMP2, MMP3, MMP9, and MMP14 are thought to promote vascular damage by their proaterogenic and proinflammatory effects. They act at the level of both smooth muscle cells and endothelial cells [9][44]. Increased levels of MMP2 and MMP9 degrade the extracellular matrix and lead to rupture of vulnerable arterial plaques, increasing plaque instability [3][14]. Thus, these protease actions together contribute to complications secondary to atherosclerotic lesions. There are also numerous trigger factors for plaque destruction, including influenza virus (which is associated with overexpression of MMP13), and carotid stenosis (associated with increased levels of MMP1, MMP7, and MMP10) [10][11]. Numerous studies in rats have revealed that MMP2 overexpression is frequently found in various cardiac pathomechanisms resulting in troponin I proteolysis, cardiac mitochondrial dysfunction, left ventricular remodeling, and systolic heart damage [11][12][45,46]. MMP2 is known to be involved in the degradation of cardiac sarcomeric proteins such as troponin I, desmin, and alpha actin, leading to contractile dysfunction, as well as in vascular smooth muscle cells, where it produces angiotensin II-mediated damage with secondary appearance of inflammation and triggering of the immune response [13][47].3. Ischemia and Reperfusion Injuries
MMP2 is activated in the myocardium following cytotoxic anthracycline treatment in ischemia and reperfusion injury, and can also be triggered by certain proinflammatory cytokines or by diabetic cardiomyopathy, through degradation of cardiac proteins or impairment of myocardial contractility [14][15][48,49]. Disruption of the major compatibility system with loss of myosin filaments and sarcomere disarrangement underlies the pathomechanism of dilated cardiomyopathy, with MMP9 also contributing [16][50]. In myocardial ischemia and reperfusion injury, MMP2 acts at the intracellular level by degrading proteins responsible for myocardial contractility, causing a decrease in the affinity of contractile myofilaments for calcium, with the secondary appearance of contractile dysfunction [17][51].4. Hypertension
Hypertension is a common condition in the population, characterized by increased systolic and diastolic pressure above 140/90 mmHg. The presence of this disease underlies the development of other known cardiovascular diseases. An alteration of the large vascular walls, consequent with microcirculatory damage and the appearance of inflammation, is determined by the action of MMP2 and MMP9 on endothelial cells with stimulation of the release of pro-inflammatory factors [6][41]. Elevated levels of MMP and TIMP1 activity have been observed in patients with primary hypertension associated with vascular wall thickening and stiffening [18][52]. MMP9 acts at the level of the extracellular matrix in arterial hypertension, through collagen degradation consequent to vascular wall alteration and the development of hypertension. It is also involved in the process of arterial wall remodeling in myocardial vessels as a result of increased blood pressure and altered wall elasticity. Implicitly, this compensatory remodeling affects both the structure of the myocardium and the vessels, creating a vicious circle that in time will lead to other cardiovascular pathologies [19][53]. Various studies have reported increased levels of MMP9 and TIMP1 in hypertensive patients without secondary cardiac damage, and increased levels of MMP2 in patients with cardiac damage. MMP1 and TIMP1 activity correlates with the extent of organ damage in hypertensive patients [20][19]. Other studies have revealed that increased levels of MMP7, MMP9, and TIMP1 are predictive factors in the development of left ventricular hypertrophy [21][54]. If these proteases play an important role in the pathogenesis of cardiovascular disease, the use of MMP inhibitors could be therapeutically beneficial in preventing the development of complications.5. Myocardial Infarction
Increased levels of MMP9 have also been reported in induced myocardial infarction in mice [22][55]. MMPS play an important role both in atherosclerotic plaque stability (when maintained within normal limits) and in vessel rupture and obstruction with secondary ischemia and necrosis (when there is increased expression of the activity of these proteases). Therefore, even in myocardial infarction, it is necessary to have a balance between MMPs and TIMPs to limit the occurrence of this pathology. However, when this occurs, an inflammatory infiltrate accumulates at the site of myocardial necrosis, predominantly with neutrophils but also with inflammatory mediators that determine the activation of MMP9 which in turn contributes to the pathomechanism of post-infarction ventricular remodeling and secondary to terminal cardiac damage [23][56]. As MMP9 is secreted by neutrophils, it follows that high levels of this protease is present in myocardial infarction and participates in the process of post-infarct remodeling, impeding tissue repair and angiogenesis [24][57]. MMPs make an important contribution to the ventricular remodeling process after myocardial infarction or viral infection by contributing to the cleavage of collagen and elastin structure, ultimately leading to hypertrophy and compensatory dilation [25][26][6,58]. These changes result in end-stage heart failure and, ultimately, death [27][4].6. Aortic Aneurysm
Aortic aneurysm is a dilatation of the vascular wall due to alteration of structural components of the wall (collagen and elastin). It is a condition not to be due to its significant complications, with the risk of wall emboli and even rupture. Thus, MMP2, MMP9, and MMP12 have been shown to contribute to the degradation of the extracellular matrix by decreasing elastin levels and increasing collagen levels. Consequently, an inflammatory process occurs, with accumulation of macrophages, neutrophils, LT and pro-inflammatory cytokines leading to fragmentation of elastic fibers, fibroblastic proliferation or fibrosis, and, in more severe stages, wall destruction [28][59].7. Cardiac Rejection Pathology
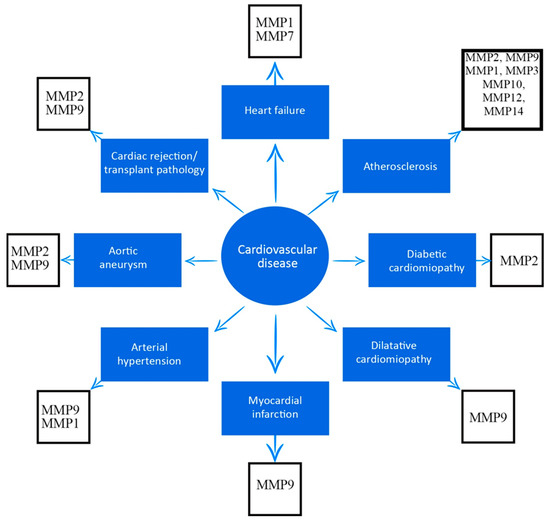
In cardiac rejection pathology, the influx and persistence of raised levels of CD3 and CD68 inflammatory infiltrate aggravates the acute cellular rejection stage of the graft and, implicitly, the increased degree of rejection is associated with high levels of pro-inflammatory cytokines (IL-6, TNF alpha, TGF beta) and an elevated level of MMP9; however, not of TIMP1, which has an anti-inflammatory role. Thus, MMP9 is responsible for the influx of LT and monocytes into the transplanted heart and is considered a marker for the inflammatory response in cardiac rejection. MMP9 activity has been found to increase in direct proportion to the degree of rejection, with peak levels of MMP9 expression occurring in advanced stages of rejection, and it may in the future be considered as an important parameter to be included in standard evaluation and diagnostic criteria [29][30][31][5,60,61]. Another important parameter to follow in the rejection phenomenon is the process of fibrosis. Elevated expression of MMP2 and MMP9, as well as TIMP1 and TIMP2, correlates with increased expression of collagen types II and III, which in turn progressively increase with the degree of graft rejection. TIMP1 and TIMP2 activate fibroblast proliferation in the extracellular matrix and regulate collagen synthesis and production in the transplanted heart graft. MMP9 is considered a surrogate marker for the degree of inflammation in the transplanted heart, and is used to distinguish between a rejected and an unrejected heart [29][32][33][5,62,63]. As emphasized in this research, several MMPs have been associated with cardiovascular disease, and are outlined in Figure 1.
Figure 1.
Association between matrix metalloproteinases and cardiovascular diseases.
