Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Fatih Yanar and Version 2 by Rita Xu.
Organic and inorganic nanoparticles (NPs) have attracted significant attention due to their unique physico-chemical properties, which have paved the way for their application in numerous fields including diagnostics and therapy. Hybrid nanomaterials consisting of organic nanocompartments (e.g., liposomes, micelles, poly (lactic-co-glycolic acid) NPs, dendrimers, or chitosan NPs) encapsulating inorganic NPs (quantum dots, or NPs made of gold, silver, silica, or magnetic materials) have been researched for usage in vivo as drug-delivery or theranostic agents.
- organic nanoparticles
- inorganic nanoparticles
- hybrid nanoparticles
1. Introduction
Nanoparticles (NPs) are submicroscopic particles with dimensions typically ranging between 1 and 100 nanometers (nm) in diameter. The unique physico-chemical properties of NPs, including their small size, large surface-to-volume ratio, and unique optical behaviour, make them a suitable candidate system for usage in the field of nanomedicine, especially in drug delivery and bioimaging applications. Examples of commonly employed NPs in nanomedicine include organic particulate systems, such as liposomes, micelles, dendrimers, poly (lactic-co-glycolic acid) (PLGA), and chitosan NPs, as well as inorganic NPs such as quantum dots (QDs) and NPs made of gold (AuNPs), silver (AgNPs), silica (SNPs), or magnetic materials (MNPs).
NP-based drug-delivery systems (DDSs) are designed for delivering a drug (or a combination of drugs) to a specific region within the body, in order to primarily cause damage to target cells whilst reducing side-effects due to systemic drug distribution in off-target regions. For example, it has been demonstrated that drug-encapsulating liposomes can enhance targeting and treatment efficiency compared with the free form of the drug [1]. Notably, DDSs have been successful in treating cancer, as well as a wide range of other diseases and conditions. For example, they have shown potential for improving treatment of infectious diseases [2], respiratory diseases [3], hypertension [4], diabetes [5], and for targeting the brain vasculature to enable drug transport across the blood–brain barrier [6].
Building upon the achievements of liposomal products, it has become increasingly apparent that NPs hold potential for overcoming widely recognized challenges such as those associated with the delivery of poorly water-soluble drugs, the transport of drugs across tight epithelial barriers, the intracellular delivery of large molecules, and the co-delivery of two or more drugs [7]. The range of applications has also extended beyond drug delivery to include detection of proteins, tissue engineering, tumour diagnosis, purification of molecules/cells, and biomedical imaging [8]. In recent years, the use of different types of NPs in nanomedicine has increased significantly, largely due to their ability to lower toxicity and improve bioavailability of therapeutic payloads, as well as for their applicability as contrast agents in biomedical imaging.
Although organic and inorganic NPs individually are cornerstones of nanomedicine, these nanoparticulate systems can also be used in combination to achieve multifunctional features. These can be obtained via encapsulation or surface modification of the NPs, resulting in hybrid (organic–inorganic) nanoplatforms with enhanced diagnostic and/or therapeutic performance. Significant efforts have been dedicated to the development of hybrid nanoplatforms with a core–shell architecture where inorganic NPs are encapsulated within the aqueous core of organic nanocompartments, or where inorganic NPs are stabilised by an outer layer of organic compounds. These hybrid nanoplatforms have been evaluated for different applications [9][10][11][9,10,11], since the combination of organic/inorganic NPs is thought to elicit synergistic effects and to also improve the effectiveness and safety of inorganic NPs.
Inorganic NPs have characteristics of large surface area and high reactivity, which can lead to agglomeration or degradation [12]. Moreover, they generally exhibit poor solubility in biological fluids, which can potentially trigger an immune response. The protection of inorganic NPs using an organic ‘shield’ can provide a physical barrier that prevents direct contact of inorganic NPs with biological structures, thereby reducing unwanted toxic effects. The presence of an organic shell or coating also improves the solubility, stability, and biocompatibility of inorganic NPs, while promoting or enabling targeted delivery and cellular uptake. Additionally, surface functionalization of inorganic NPs can allow application in targeted delivery and controlled release. Overall, the presence of an organic compartment (i.e., shell or coating) can allow and extend the use of inorganic NPs in drug delivery and biomedical imaging. As a result, hybrid organic/inorganic nanoplatforms have attracted significant attention in recent years and have been extensively studied in the literature.
2. Biomedical Applications of Hybrid Nanoparticles
Hybrid NPs have been utilised as powerful tools in biomedical applications, especially for targeted drug delivery, bioimaging, and theranostics. Concerning applications in drug delivery, hybrid NPs—like other nanomedicines—can deliver drugs by active or passive targeting. Active targeting typically involves some modification on the particle surface, e.g., using charged lipids, antibodies, or attachment of ligands that enable the NP to bind to the receptors of the target cells and to cross biological membranes more effectively (Figure 1c). Active targeting therefore reduces undesired side-effects of drugs, while providing high therapeutic efficacy by allowing high dosing at the diseased site [13][54].
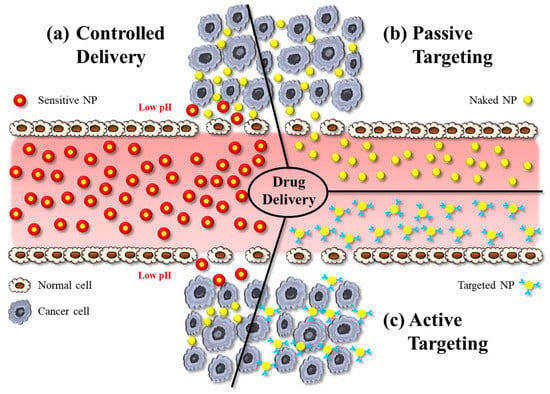
Figure 1. The illustration shows various approaches in nanoparticle-based drug delivery: (a) controlled delivery, (b) active targeting, and (c) passive targeting. NP: nanoparticle.
Passive targeting is the accumulation of NPs at pathological sites due to the EPR effect, whereby the increased vascular permeability enables enhanced extravasation of NPs and drugs (Figure 1b). Notably, the size of intercellular gaps in the vascular endothelium of these pathological regions increases by about 1 μm after exposure to inflammatory mediators. This helps NPs, for example, at tumour sites much more effectively than in physiological tissues [14][55]. The delivery of the payload to cells occurs via the interaction between the NP and the cell membrane, which can occur through different mechanisms. NPs can indirectly enter the cytoplasm by endocytosis, resulting in the delivery of the payload. An alternative process is fusion, whereby NP layers merge with the cell membrane, resulting in direct delivery of the payload. Another mechanism of interaction between NPs and cell membranes is lipid exchange, which involves the exchange of bilayer materials between the NP layers and the cell membrane. It is important to note that these interactions can trigger the immune system. Therefore, it is crucial to develop the surface chemistry of NPs in such a way to make them unrecognizable by the RES [15][17].
Controlled drug delivery, on the other hand, refers to the regulation of the release of a payload from a nanocarrier system (Figure 1c). These systems are designed to initiate drug release in response to an exogenous or endogenous trigger such as pH, temperature, or light. Sustained drug-delivery systems on the other hand provide prolonged release of a drug over a certain period of time. In DDSs designed for sustained release, the drug is typically encapsulated within the NP or a matrix system, and drug release occurs via diffusion [16][56].
Another important biomedical application of NPs is in biomedical imaging, i.e., as contrast agents. This approach has the potential to provide more accurate information about a given disease condition compared with traditional clinical imaging. Owing to the aforementioned targeting capabilities and the small size of NPs, nanoparticulate contrast agents can be directed to the targeted area, thus enabling accurate detection and imaging of a biological target tissue. This approach can be applied in fluorescence imaging, magnetic resonance imaging (MRI), computerized tomography (CT), ultrasound (US), and multimodal imaging [17][57].
Fluorescence imaging often involves the use of fluorophores or fluorescent dye molecules attached to NPs. These compounds can absorb light at specific wavelengths and emit light at longer wavelengths, when excited by an appropriate light source. Fluorophores can be targeted to specific cell types or cell structures for various purposes including protein analysis, gene detection, diagnostics, and real-time monitoring [18][58]. Fluorescence imaging can provide vital information, especially when NIR light is utilised, due to improved tissue penetration of light and reduced autofluorescence, allowing for enhanced imaging sensitivity. Fluorescence imaging can be implemented by conjugating fluorescent dyes with NPs or by encapsulating fluorescent agents within NPs.
MRI is one of the most commonly applied methods for disease diagnosis and monitoring in the clinic for various medical conditions. The fundamental principle of MRI is based on the movement of protons within a strong magnetic field. This provides high-resolution images of internal body structures in multiple planes. Commonly employed contrast agents in MRI include gadolinium-based NPs and superparamagnetic materials, due to their remarkable magnetic properties [19][59]. These contrast agents help enhance the visibility of targeted areas, improving the accuracy of the diagnostic process.
US imaging is also one of the most commonly employed methods for medical imaging, due to its simplicity of usage, safety, and real-time imaging capabilities. In this approach, sound waves (with a frequency >20 kHz) are generated by an extracorporeal transducer positioned in contact with the body. As these waves penetrate tissues, they encounter biological structures with different acoustical properties. These differences in properties between structures (i.e., mainly in density and compressibility) result in reflections of the ultrasound wave, which are captured by a probe and are then converted into images [20][60]. Different types of contrast agents are used in US imaging in order to enhance the acoustical properties mismatch between the vasculature and surrounding tissues; these include gas-filled (e.g., microbubbles with a core of a heavy gas, like perfluorocarbon), solid-based (e.g., silica NPs), and liquid-based (e.g., perfluorooctyl bromide) particles. Some of these NP systems present a core–shell design configuration. It is important to note that the aforementioned imaging approaches primarily focus on providing single imaging modalities; however, advancements have allowed the development of multifunctional NPs serving as imaging agents with multimodal imaging capabilities. These NPs can incorporate single (e.g., silicon naphthalocyanine) [21][61] or multiple imaging agents, enabling the simultaneous use of distinct imaging modalities [22][62]. This approach allows complementary information to be captured from different imaging techniques, thereby also improving accuracy and reliability of diagnosis.
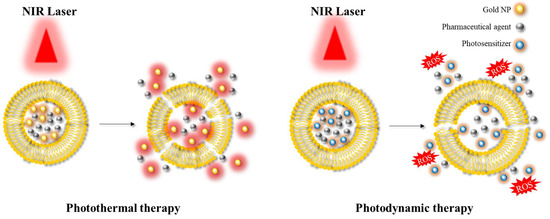
Another significant area of application is photoablation therapy, which can be classified into two main modalities: photodynamic therapy (PDT) and photothermal therapy (PTT) [23][24][40,63] (Figure 2). PDT involves the use of (initially) non-toxic compounds called photosensitizers, which are exposed to light at a specific wavelength (e.g., in the NIR) resulting in the formation of toxic compounds. These toxic products can react with hydroxyl ions or water, and in turn form reactive oxygen species (ROS) that can cause cell death. This approach is mainly employed in the context of anticancer therapy, as the production of ROS can induce suppression of tumour growth. In PTT, the targeted area is irradiated using a light source at a specific wavelength similar to PDT. This light energy is converted into heat energy by specific materials, such as AuNPs or AgNPs, resulting in hyperthermia and cell death. PDT and PTT are examples of common therapeutic approaches utilising hybrid platforms.

Figure 2. Illustration depicting the application of photothermal therapy and photodynamic therapy. NIR: near-infrared; ROS: reactive oxygen species.
2.1. Liposomes-Based Hybrid Platforms
Liposomes are one of the most commonly used nanocarriers for integration into hybrid platforms in nanomedicine. These platforms combine the unique advantages of liposomes and inorganic NPs, offering multi-functional features.
Xing et al. demonstrated the implementation of PTT by employing a liposomal system encapsulating doxorubicin (Dox), i.e., a chemotherapeutic compound and AuNPs as inorganic NPs [23][40]. When the hybrid platform was irradiated by NIR light, the liposome layers became permeable to both Dox and AuNPs, allowing the release of both payloads. It was reported that this platform resulted in effective tumour suppression with a cell growth inhibition rate of up to 78.28%. In a similar study, Koga et al. utilised AuNPs to achieve controlled drug release via PTE. Their results showed that the hybrid system was capable of releasing >80% of the drug in less than 1 min upon NIR irradiation [25][64]. Another approach, investigated by Lv et al., employed thermosensitive liposomes encapsulating Au nanorods, MNPs, and Dox for targeted delivery assisted by PTE [26][74]. This platform showed superparamagnetic properties and enabled controlled release, with approximately 95% of the drug released after 3 h of irradiation using a 980 nm laser beam. This approach was found to be highly effective in treating bladder tumour cells. Among many studies investigating liposome–AuNP hybrid platforms as DDSs, Li et al. demonstrated that incorporating hollow AuNPs in liposomes resulted in greater efficacy both in terms of the hyperthermia achieved within the targeted tissue and the drug (Dox) release profile, compared with liposomes loaded with solid AuNPs [27][72]. It is worth mentioning that, in addition to employing chemotherapeutic agents (such as Dox) as encapsulated payloads in liposomes for targeted and controlled drug-delivery applications, various other types of molecules have also been encapsulated in nanocompartments, including miRNA inhibitors [28][66] and fish oil protein [29][65]. Grafals-Ruiz et al. proposed a liposomal system for the treatment of glioblastoma (GBM), one of the most common types of brain tumours [28][66]. The system comprised liposomes encapsulating AuNPs functionalized with oligonucleotide miRNA inhibitors. In addition, the liposomes were conjugated to either apolipoprotein E (ApoE) or rabies virus glycoprotein. The hybrid nanoplatform was investigated in GBM syngeneic mice by intravenous administration. Results showed that the expression of miRNA-92b was effectively inhibited. Furthermore, compared with controls, liposomes conjugated with ApoE accumulated to a greater extent at the tumour tissue, suggesting improved targeted delivery.
Liposomal platforms have also been employed for enhanced bioimaging applications. A hybrid nanoplatform consisting of liposomes encapsulating AuNPs, perfluorocarbon, and Dox has been utilised for image-guided PTT [30][68], with positive outcomes in terms of both bioimaging and drug delivery. Another study conducted by Prasad et al. synthesized liposomes encapsulating AuNPs along with emissive graphene QDs for application in in vivo bioimaging and NIR-mediated cancer therapy [31][73]. The liposomes also encapsulated Dox as a chemotherapeutic drug, and their surface was functionalized with folic acid (FA) as a targeting ligand. The developed theranostic system demonstrated capability for in vivo bioimaging of tumour tissue using NIR light (wavelength of 750 nm). Moreover, it offered PDT and chemotherapeutic performance. Notably, NIR light exposure resulted in the generation of ROS, resulting in tumour reduction.
In a similar theranostic approach by Li et al., perfluorocarbon was encapsulated in liposomes by film dispersion, with the aim of developing US contrast agents that are more effective at penetrating into a target tissue than conventional, micrometer-sized contrast agents [30][68]. This hybrid system also encapsulated Dox along with hollow AuNPs, to achieve PTT upon exposure to NIR light (wavelength of 808 nm). In vivo fluorescence imaging demonstrated the accumulation of NPs at the targeted area in a 4T1 tumour model. NIR illumination also resulted in localized hyperthermia leading to significant Dox release. The nanoplatform was also found effective at performing US image-guided PTT and chemotherapy. Charest et al. developed a liposomal formulation of AuNPs with carboplatin and evaluated its radiosensitizing potential [32][67]. The study found that simultaneous administration of low doses of carboplatin and AuNPs through encapsulation in liposomal nanocarriers resulted in effective radiosensitization.
The investigation of liposomal encapsulation of QDs, MNPs, and SNPs was also conducted for enhanced therapeutic efficacy. Chen et al. examined liposomes encapsulating both CdSe QDs modified with oleic acid and superparamagnetic iron oxide NPs (SPIONs), for the treatment of hepatocellular carcinoma [33][76]. This strategy led to the synthesis of magnetic fluorescent liposomes with multifunctional properties. The platform could label and image cancer cells with high biocompatibility, suggesting that it has the potential for improved targeted drug delivery. A study by Sun et al. demonstrated the capabilities of liposomes encapsulating MSNs, for triple-modal image-guided cancer therapy as a theranostic drug-delivery platform [34][80]. In this research, gadolinium-doped MSNs were encapsulated in liposomes along with Dox. Liposomes were coated with FA to prevent the leakage of Dox and for achieving targeted delivery. The nanoplatform was also conjugated with indocyanine green (ICG) to enable triple-modal imaging through NIR irradiation. Results demonstrated that ICG enabled PTT and PDT, while allowing NIR fluorescence imaging and photoacoustic imaging (PAI). The addition of gadolinium also enabled MRI capabilities. In vitro and in vivo studies showed improved antitumour effects with good imaging contrast, suggesting that this theranostic platform is a candidate for image-guided phototherapy. Overall, the system was successful at providing triple-modal imaging in a single platform, capable of NIR fluorescence imaging, PAI, and MRI.
2.2. Micelle-Based Hybrid Platforms
Micelles are widely used as organic NPs in biomedical applications, acting as nanocompartments through the creation of shells or coatings, as well as for encapsulating inorganic NPs.
Volsi et al. studied the design of a theranostic micellar nanoplatform for targeted cancer therapy [35][86]. The polymeric micellar structure consisted of α,β-poly(N-hydroxyethyl)-DL-aspartamide functionalized with lipoic acid (LA), PEG as a hydrophilic moiety, and FA as a targeting moiety which was able to self-assemble in aqueous solution. The platform also encapsulated Dox and Au core–shell QD NPs. Experiments showed that the nanocompartment was stable and efficient at targeting/delivering Dox to MCF7 cells, as well as capable of exploiting heat generation by PTE of QD-Au NPs. It was suggested that this theranostic hybrid system has potential for cancer treatment considering its enhanced drug-delivery behaviour and imaging capabilities, which can assist in diagnostics and therapy monitoring purposes.
Micellar nanocompartments were also examined by Li et al. for pH-sensitive delivery and MRI imaging [36][90]. Researchers synthesized poly(ethylene glycol)-b-poly(β-benzyl L-aspartate) and aminolyzed it with N,N-diisopropylamino ethylamine and N,N-dibutalamino ethylamine at different molar ratios, for the development of an amphiphilic block copolymer capable of encapsulating SPIONs and Dox. The system was designed to encapsulate its payloads, i.e., drugs or contrast agents, under neutral pH conditions, providing stability and preventing premature release of payloads during circulation or storage. The nanoplatform was also designed to provide triggered release in weak acidic environments, typically found in certain pathological conditions. Experiments demonstrated effective uptake by HepG2 cells and successful release of Dox at low pH conditions, demonstrating the nanoplatform’s potential for therapeutic purposes. In addition, fluorescence and MRI studies revealed that the weak positive charge of the hybrid system contributed to longer blood circulation in vivo. The system thus exhibited successful pH-sensitive tumour targeting with efficient and non-invasive MRI visibility, allowing improved non-invasive image-guided therapy. Furthermore, it had a minimal side-effects profile, while displaying impressive anticancer outcomes. Further studies focusing on the applicability of micellar nanocompartments for enhanced tracking and biomolecular detection have been also carried out by encapsulating QDs and SPIONs [37][87].
2.3. PLGA-Based Hybrid Platforms
PLGA-based hybrid systems have recently emerged as promising platforms, especially for drug delivery and bioimaging applications.
Luo et al. utilised PLGA NPs for the encapsulation of anti-PD-1 peptide and hollow Au nanoshells for improved immunotherapy achieved by PD-1 blocking combined with PTT [38][91]. This hybrid system demonstrated long-term activation of the immune system over 40 days, which could also be accelerated using NIR laser illumination. It was also revealed that multiple irradiations using NIR laser illumination enhanced the antitumour effect, resulting in the inhibition of primary tumours as well as distant tumours. The therapy was also capable of enhancing immune cell activation.
In another study conducted by Galliani et al., PLGA-based hybrid platforms were designed to implement PDT for enhanced cancer therapy [24][63]. Chlorophyllin–copper complex and CdSe/ZnS core–shell QDs were encapsulated successfully in the PLGA nanocompartments. Irradiation at 365 nm by UV resulted in the generation of ROS due to fluorescence resonance energy transfer between QDs and chlorophyllin. It was indicated by the authors that this platform has potential for PDT as it could generate ROS upon irradiation; however, further analysis is required to assess the underlying mechanisms and optimize the formulation.
As reported by Jin et al., a PLGA-based nanoplatform was designed for multimodal imaging and US-triggered drug delivery. SPIONs and Dox were successfully encapsulated in PLGA-based nanocompartments conjugated with PEG and FA [39][94]. In vitro experiments demonstrated the potential for US and MRI contrast imaging, as well as increased targeting ability due to FA conjugation. Focused US was utilised as a remote-control technique to trigger Dox release and induce cell membrane permeabilization. These findings highlighted the promising application potential of this system as a tool for US- and MRI-guided drug delivery in anticancer therapy.
Kumar et al. aimed to develop implantable nanoplatforms composed of PLGA-based nanocompartments encapsulating docetaxel and Cy7.5 (fluorophore) conjugated with SNPs [40][95]. In vivo studies demonstrated efficient sustained drug release near tissues. The docetaxel-loaded spacers exhibited suppression of tumour growth compared with the control over 16 days, demonstrating improved therapeutic efficacy. It is important to note that this system was also suitable for contrast imaging due to its fluorescent moiety (Cy7.5), with potential for use in disease monitoring.
2.4. Dendrimer-Based Hybrid Platforms
In a study by Ghosh et al., a dendrimer-based hybrid platform was utilised for targeted gene delivery for the treatment of triple-negative breast cancer (TNBC) [41][97]. The authors successfully synthesized carbon QDs conjugated with PAMAM dendrimers of different generations. RGDS peptides were further conjugated to the nanoplatform to be able to target the αvβ3 integrin, which is known to be overexpressed in TNBC. Among different conjugates, QD–PAMAM conjugate 3 showed superior capabilities for gene complexation and protection against enzymatic digestion. Furthermore, it exhibited efficient detection of Cu (II) ions, with a fluorescence quenching efficiency of 93%. It is important to note that TNBC often has higher levels of Cu (II) ions, which offers potential for the detection of the metastatic phase of TNBC.
In another approach, a pH-sensitive and self-fluorescent nanoplatform was developed using mesoporous SNPs and PAMAM dendrimers [42][99]. It was found that the inclusion of PAMAM dendrimers provided improved encapsulation efficiency and additional eligible reaction sites for modifications. In addition, the structure of PAMAM dendrimers affected the drug-release performance and prevented burst release. The fluorescence behaviour of this hybrid system offers the capability for potential biological tracking and bio-detection. Importantly, PAMAM dendrimers served as both pH-sensitive capping agents and self-fluorescent agents, possessing multiple functions in a single platform. This research demonstrated the versatility of dendrimer-based systems for developing multifunctional and biocompatible drug-delivery platforms for biomedical imaging, diagnosis, and simultaneous therapy.
Dendrimer-based nanocompartments have also been utilised for the application in multimodal image-guided cancer therapy [43][100]. A theranostic nanoplatform was developed, comprising generation 5 poly(amidoamine) dendrimer-stabilized AuNPs embedded with ultrasmall IONPs, capable of MR/CT/PAI-guided PTT and radiotherapy (RT). This multifunctional nanoplatform induced significant cell death under laser irradiation. In vivo experiments demonstrated accumulation within the tumour tissue along with MRI, CT, and PAI imaging enhancement capabilities. These findings suggest that this nanoplatform has the potential for image-guided cancer therapy, leading to improved diagnosis and treatment while minimizing side-effects.
2.5. Chitosan-Based Hybrid Platforms
Liu et al. developed a theranostic platform by incorporating cysteine functionalized AuNPs into chitosan/tripolyphosphate NPs (modified with polyacrylic acid), aiming for improved cellular uptake, high loading capacity, controlled release, and efficient bioimaging [44][101]. This hybrid platform was also loaded with Dox as the chemotherapeutic agent. In vitro experiments showed sustained drug release for up to 48 h under acidic conditions; however, drug release was accelerated at higher pH values. The hybrid platform also showed greater cellular uptake compared to free Dox. In vivo studies revealed that the drug accumulation could be tracked, which was confirmed by CT scans. Notably, significant inhibition of tumour growth compared with free Dox was observed in vivo. These findings demonstrated the potential of this hybrid platform as a theranostic tool for tumour treatment.
A study by Gholami et al. explored chitosan-based hybrid platforms loaded with SPIONs and Dox for treating glioblastoma [45][103]. Drug-release tests demonstrated a rapid release of Dox at pH 5.5, which resembles the pH of the tumour microenvironment, indicating pH-dependent drug-release capability. In addition, the cellular internalization of this hybrid platform was confirmed through fluorescence microscopy. Overall, the study demonstrated the potential of this formulation for both diagnosis and treatment of glioblastoma.
Chen et al. investigated a nanoplatform comprising aptamer-modified graphene QDs and magnetic chitosan for the treatment of hepatocellular carcinoma [46][105]. It was designed to utilise an aptamer for active targeting and graphene QDs for PTT. In vitro experiments demonstrated the internalization of this hybrid platform in cancer cells and subsequent NIR-triggered drug release. Additionally, the platform showed low cytotoxicity profiles and enhanced accumulation at the tumour site in vivo, which was further validated by imaging. Overall, this system has potential for combined photothermal chemotherapy in cancer treatment.
