Soil health is intimately intertwined with ecosystem services. Climate change negatively impacts ecosystem functioning, by altering carbon and nitrogen biogeochemical cycles and shifting nutrient bioavailability, thus hampering food production and exacerbating biodiversity loss. Soil ecosystem services are provided by belowground biota, and as the most abundant metazoans on Earth, nematodes are key elements of soil food webs and reliable bioindicators of soil health.
- abiotic stress
- beneficial nematodes
- ecosystem services
- food webs
- functional ecology
- soil health
- soil microfauna
1. Introduction
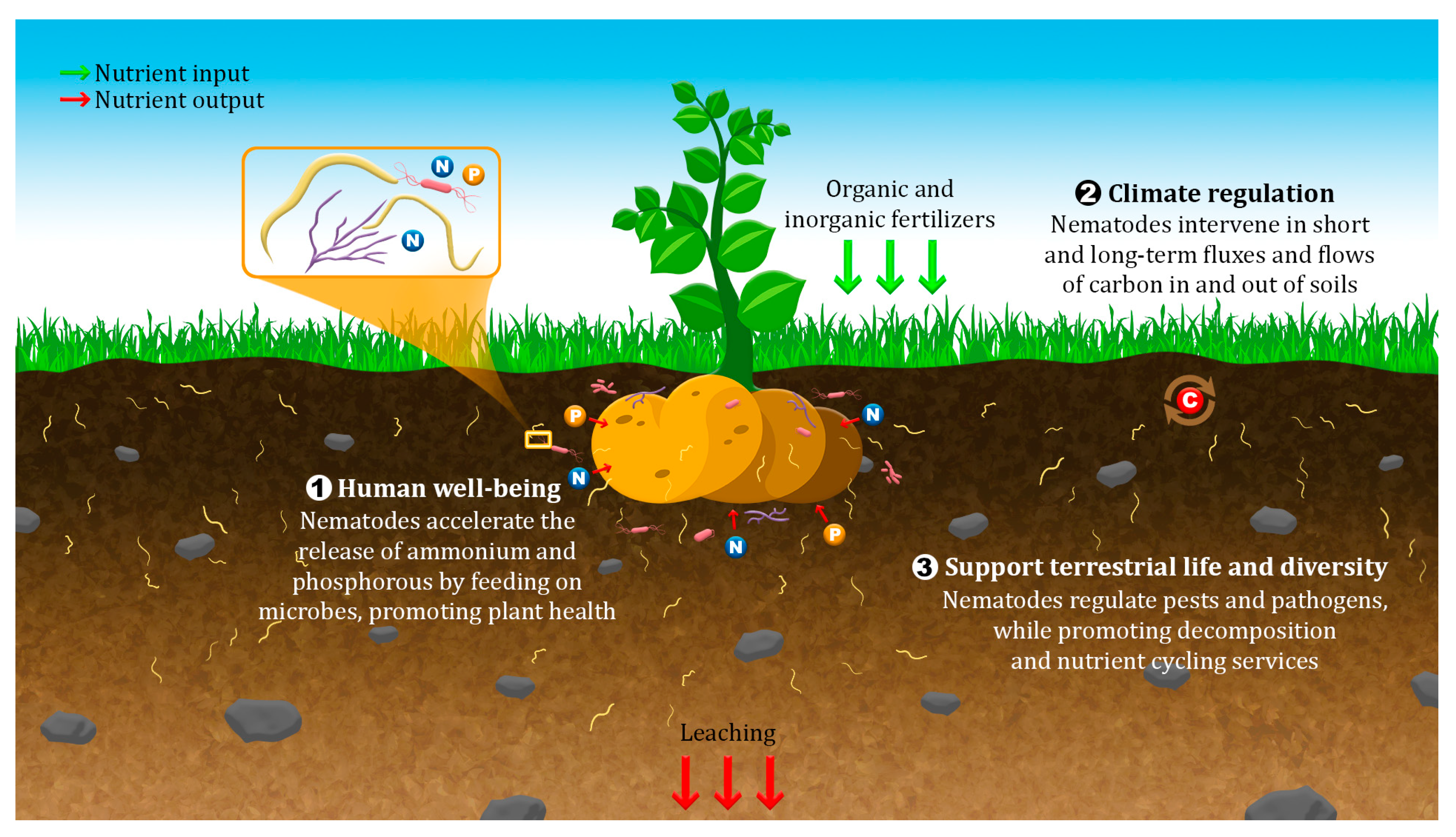
2. Nematode Community Dynamics under Climate Change
2.1. Temperature
|
Factor |
Effects on Soil Nematodes |
Reference |
|
|---|---|---|---|
|
Factor(s) |
Effects on Soil Nematodes |
Reference |
|
Effects on Soil Nematodes |
Reference |
||
|
High temperature |
Reduction in nematode diversity and genera richness |
||
|
Long-term increase in mean annual precipitation | [ |
Decline in total nematode abundance due to predation pressure, and increase in nematode abundance48] | |
2.4. Nutrient Enrichment
|
Factor(s) |
Effects on Soil Nematodes |
Reference |
|---|---|---|
|
Long-term N enrichment |
|
Factors |
Effects on Soil Nematodes |
Reference |
|---|---|---|
|
Reduction in herbivores, with a short-term decrease in bacterivores and fungivores who recover over time, and relative tolerance of omnivores–predators |
[49] |
2.2. Water Stress
|
Intensive land use |
||||||
|
Soil moisture, P addition, and aboveground vegetation |
Increase in bacterivores, fungivores, and herbivores Plant removal dwindled nematode taxon richness and abundance of bacterivores and herbivores; the abundance of fungivores and omnivores–predators increased under the same conditions | |||||
Plant type and water availability had a greater impact on nematode abundance and community composition; drought was detrimental to the total density of nematodes and functional guilds; bacterivores, herbivores, and omnivores were significantly more abundant in soils with legumes |
[72] |
High proportion of plant-parasitic nematodes |
||||
[ | ] |
|||||
|
Soil type |
||||||
Lower biodiversity and C/N ratios in cultivated soils, resulting in a reduction in nematode abundance and diversity; increase in abundance of trophic groups, total abundance, and diversity of nematodes in soils with higher pH and C and N contents |
[57][ |
Long-term N fertilization |
||||
|
Biotic (microbial biomass and competition) and abiotic variables (moisture, salinity, and elevation) |
Greater nematode abundance in fertilized plots, while richness, diversity, and ecological maturity were lower; enriched food web mostly driven by bacterivores and herbivores, with persisting effects overtime | 56] |
Decrease in free-living nematode diversity and evenness |
|||
[ | ||||||
Spatial segregation between two competing bacterivore species, with contrasting responses to abiotic factors: one best adapted to high salinity, lower temperatures, and low moisture environments, while the other thrives at higher temperatures, higher soil moisture, and lower salinity |
Land use conversion |
High N deposition Negative impacts on soil taxa across a large spatial scale, but agronomic practices limit the climatic constraints on belowground biodiversity |
Decrease in most nematode trophic groups and community diversity under understory addition of N compared to canopy addition of N |
|||
|
Climatic, soil, and historical factors | ||||||
Current factors, particularly climate, are more influential than historical factors in shaping nematode diversity patterns on a broader scale |
Water scarcity |
Short-term N and P enrichment under soil acidification Reduction in total nematode abundance, and decrease in relative abundance of bacterivores, fungivores, and predators; relative abundance of herbivores unaffected |
Nematode variables, including community structure, were largely unaffected by short-term nutrient enrichment under soil acidification |
|||
[75] | ||||||
|
Liming treatments |
Lower bacterivore and fungivore abundance, marginal increase in omnivores and decrease in fungivores, higher maturity and structure index, and lower prey:predator ratio |
2.3. Land Use
|
Factor(s) | |||||||
|---|---|---|---|---|---|---|---|
Monoculture | |||||||
|
Increase in fungivores, and decrease in bacterivores, herbivores, and omnivores–predators |
Interacting nematode and microbial communities minimally impacted by liming, with an increase in omnivores and predators, who keep bacterivores under control; stronger interaction in the presence of an abundant microbial community |
||||||
[ | ] |
||||||
[ |
Monoculture, tillage, and manure |
Increase in bacterivores and fungivores, and decrease in omnivores–predators |
Long-term organic amendments and mineral fertilization |
Positive effect on the abundance of most functional guilds by organic amendments, which enhanced the energy transfer among nematode communities, while increasing the relative allocation of energy flux to bacterivores and fungivores and decreasing the relative allocation to herbivores [60][ |
|||
|
Increasing aridity across a large spatial scale |
Decline in total and relative nematode abundance of each functional guild under increasing aridity; taxonomic richness of total nematode community and functional guilds decreased under moisture scarcity; at the dry end of the aridity gradient, richness of bacterivores was higher, while herbivores declined steadily; richness of fungivores and omnivores–predators remained relatively stable up to a certain point, before dropping steeply | 59] |
|||||
|
Vegetation succession |
Positive effect on nematode abundance, diversity, complexity of community structure, and diversity-weighted abundance |
][ |
Liming, P, and zinc inputs |
P input significantly increased nematode diversity and genera; bacterivores and herbivores were the most abundant trophic groups, and predators the least common; nematode biodiversity was unaffected by liming, and nematode diversity and maturity were reduced in the absence of liming [61] |
|||
[ |
Livestock grazing |
Decline in bacterivores, herbivores, and omnivores–predators abundance, lower total nematode abundance, and no detrimental effect on fungivore abundance |
|||||
|
Land degradation |
Lower nematode trophic diversity |
||||||
2.5. Combined Stressors
[ | |||||
] | |||||
[ | |||||
] | |||||
|
Drought and fertilization |
Drought favored bacterivores and fungivores, and likely had detrimental effects on higher trophic levels; fertilization caused a prominent increase in bacterivores and an equally significant drop in fungivores |
||||
|
Elevated CO2 and N, warming, and drought |
Increase in nematode density at elevated N and ambient CO2, and ambient N and elevated CO2 |
||||
|
Warming and precipitation |
Decrease in nematode abundance, especially of bacterivores and herbivores (with minor effects on fungivores), under artificial warming, but the nematode community diversity and functions remained stable; decrease in nematode abundance, especially of bacterivores and omnivores–predators, under reduced precipitation, with fungivores and herbivores relatively insensitive to water stress; increase in nematode abundance and community diversity with water availability |
||||
|
Intensive land use |
|||||
|
Ecological and edaphic factors |
Generalists equally abundant in all ecosystems, with specialists dominating agricultural landscapes and less abundant in low disturbed soils; highest richness and diversity in grasslands and dairy farms, with low abundances in shrubland–woodland habitats |
Reduced nematode abundance and diversity with increasing altitude, with bacterivores consistently the dominant group; nematode diversity was mostly influenced by temperature and moisture; decrease in nematode abundance with increasing soil acidity; nematode diversity and richness were directly proportional to nutrient (N and P) levels |
|||
[ |
Plant resource-use strategies |
||||
|
N deposition under reduced water availability |
Plants with acquisitive strategies promoted nematode abundance, but fewer opportunistic nematodes in the rhizosphere of acquisitive plants compared to conservative plants |
Reduced nematode abundance and diversity under N addition and reduced water input; synergistic effects of N addition and reduced water input on soil nematode communities at higher trophic levels; sole addition of N was more detrimental to the nematode community |
|||
[ |
Agricultural practices |
||||
|
Returning agricultural residues | (i) Conventional practices decrease abundance, trophic structure, and taxonomic richness of nematode communities; (ii) agroecological practices enhance the functional and taxonomic diversity of nematodes: total nematode abundance and absolute abundance of fungivores, herbivores, and omnivores–predators; reduction in herbivore abundance in crop rotation; increase in omnivores–predators in cover crops; increase in bacterivores and fungivores in organic fertilization; reduction in nematode abundance and food web structure in monoculture and pesticide application, while copiotrophic nematodes are favored |
Nematode diversity was lower in treatments with conventional chemical NPK fertilizers; positive correlation between omnivore–predator abundance and ecosystem multifunctionality and soil fertility |
|||
[ |
Spatial distance and environmental filtering |
Plant type altered β-diversity of nematodes; spatial turnover was the primary process driving total β-diversity of the nematode community |
|||
|
Vegetation restoration with varying degrees of degradation |
Strong effects on soil nematodes observed under low degradation |
||||
|
Crop-tree thinning |
Increase in abundance of soil nematodes, along with the relative abundance of herbivores in all systems; increase in proportion of stress-tolerant enrichment and general opportunists |
Harvest frequency and legume density |
Legume addition and density were drivers of total nematode abundance, especially bacterivores, while improving metabolic activities of total nematodes, bacterivores, and omnivores–predators; positive effects of legume addition subsided after increased harvesting frequency |
||
|
Agricultural practices |
Plant diversity enhanced the activity of beneficial nematodes; positive effects of nematodes on plant growth and function associated with higher values in soil pH and cation contents |
3. Nematode Contributions to Soil Health
4. How Nematodes Promote Soil Resilience
As highly adaptable animals, with diverse roles in ecosystem functioning, the physiological and life history traits of nematodes make them less susceptible to environmental changes compared to larger fauna higher-up in the food web. Indeed, these characteristics could prove useful for the resistance and resilience of soils to natural and anthropogenic changes. To better comprehend the role of bacterivores in maintaining functional stability of ecosystems under disturbance, Chen et al. [97][96] studied their contributions to promoting soil resistance and resilience under copper and heat stress. The relative shifts in two dominant bacterivore genera, Acrobeloides and Protorhabditis, responded differently to disturbance. Protorhabditis exhibited greater resistance and resilience to copper stress compared to Acrobeloides, while both genera displayed higher resilience only by the end of the experiment under heat stress. Indeed, bacterivores showed a positive effect on soil resilience under thermal stress starting at 28 days. The increase in relative abundance of bacterivores did not significantly affect soil resistance in terms of microbiota but it improved soil resilience to copper stress. The differences in responses of soil function to disturbance highlight the role of bacteria-feeding nematodes in promoting ecosystem stability under stress. To determine the effects of soil properties, rainfall, and temperature on soil nematodes, da Silva et al. [98][97] analyzed the changes in nematode community structure under contrasting types of land use in a seasonally dry tropical forest in Brazil. Nematofauna composition in the secondary forest differed in abundance and richness compared to agricultural systems, being expectedly higher in the former and lower in the latter, with bacterivores and omnivore–predators more susceptible to the type of land use. The variation in taxonomic composition among the studied sites was strongly related to soil properties, monthly mean rainfall, and temperature, which accounted for 65.42% of the total variation. These results further indicate that anthropogenic activities, expressed by the conversion of native vegetation to cropping systems, which modify soil characteristics, as well as climate variables, negatively affect the structure and composition of nematode communities. Nevertheless, changes in nematode community composition and structure can be reversed by allowing the fields to undergo secondary forest regeneration after abandonment. Seeking to identify the major ecological predictors of soil invertebrate diversity, Bastida et al. [99][98] surveyed 83 locations in six continents, from polar to arid climates, to study three soil invertebrates: nematodes, arachnids, and rotifers. Different ecosystem types such as forest, grasslands, and shrublands were included in the survey and nematodes were the most abundant, accounting for 43% of all taxa surveyed. Aridity was detrimental to the diversity of nematodes, whereas forest, plant richness, and annual net primary productivity were positively correlated. These findings exposed potential vulnerabilities of soil invertebrates to climate change in locations where hotter temperatures may occur in the future. Moreover, deforestation processes and increase in aridity may reduce nematode diversity, providing evidence of the importance of vegetation and climate for the diversity of soil invertebrates. Considering an increasing likelihood of extreme climatic events, Majdi et al. [100][99] exposed five species of free-living bacterivorous nematodes to a wide range of temperatures under controlled conditions, and their population growth rates and body-size distributions were measured. Body size at maturity was inversely proportional to temperature, mature females were laying a smaller number of eggs at higher temperatures, and a prevalence of early juvenile stages resulted in reduced body-mass structure with increasing temperature. Additionally, closely related species like Plectus acuminatus and P. cf. velox had very different thermal tolerance ranges, with the population growth of most tested species declining between 25 and 30 °C, and A. nanus exhibiting the broadest thermal tolerance range. This study demonstrated how thermic stress can induce changes in the growth and size–structure of bacterivores. To investigate the community-weighted mean body mass of soil nematodes, Andriuzzi et al. [101][100] studied the role of water availability in the body size of these invertebrates across a gradient of precipitation in North American grasslands, ranging from arid to semiarid and mesic conditions. An increase in nematode community-weighted mean mass from arid to mesic conditions was observed, but no effects were reported at the arid site. When grouping community-weighted mean mass by feeding habits, only plant-parasitic nematodes showed a positive response to water input in semiarid and mesic conditions. This suggests that aridity acts as a buffer against large-bodied nematodes, limiting community body size shifts in response to extreme events, either drought or rainfall. Aiming to study the latitudinal variation in soil nematode communities under climate warming-related range-expanding and native plants, Wilschut et al. [102][101] showed that the composition of soil nematode communities changes across a latitudinal gradient, but not their richness or abundance, with plant species identity (both range-expanding and native plant species) being the strongest predictor of this shift. These findings further indicate that this variation is less dependent on soil characteristics, such as pH and soil moisture. In addition, plant species that expand their range due to climate warming may experience advantages by being free from nematode herbivory in their new habitat. A plant removal experiment was set up by Wang et al. [103][102] to better understand how dominant vegetation changes impact nematode assemblages. Edaphic properties, especially soil C and N content, were the primary drivers of nematode community structure and community-weighted mean biomass, with no observable short-term effects resulting from vegetation removal. However, long-term effects on nematode assemblages are expectable due to nutrient flow mediated by shifts in vegetation composition. To characterize and explore the relationship between soil biota and plant diversity and productivity, Bennett et al. [104][103] carried out a long-term experiment. Plant species richness had a positive effect on fungi, including increased arbuscular mycorrhizal fungi, while reducing plant-parasitic nematodes. Overall, soil biota resistance to disturbance increased with plant diversity, highlighting the importance of plant species richness for belowground communities. To evaluate the impacts of various measures of trophic diversity, climate, and soil environmental factors across three spatial scales, Wu et al. [105][104] conducted a field survey on the stability of ecosystems on the Mongolian Plateau. Soil biota diversity, including α-and β-diversity, positively contributed to ecosystem stability, with soil nematode diversity and trophic groups associated with higher ecosystem stability. The relatively low abundance of herbivores may have contributed to enhanced plant performance by increasing root exudation, which stimulated microbial activity and nutrient availability. The positive association of soil biota diversity with ecosystem stability was similar to that of plant diversity in some cases. Similarly, an increase in the abundance of higher trophic levels such as omnivores–predators and microbial-feeding nematodes may have resulted in improved nutrient transfer to plants, leading to enhanced plant productivity and maintaining ecosystem stability through top–down effects. Severe anthropogenic impacts often lead to simplified soil food webs, with limited top–down control by omnivores–predators, ultimately compromising ecosystem functioning and impairing their natural ability to mitigate the effects of climate change. It is therefore crucial to restore the complexity of soil food webs to enhance soil resilience to climate change.References
- Brevik, E.C.; Cerdà, A.; Mataix-Solera, J.; Pereg, L.; Quinton, J.N.; Six, J.; Van Oost, K. The Interdisciplinary Nature of SOIL. SOIL 2015, 1, 117–129.
- Doran, J.W.; Zeiss, M.R. Soil Health and Sustainability: Managing the Biotic Component of Soil Quality. Appl. Soil. Ecol. 2000, 15, 3–11.
- Lal, R. Soil Health and Climate Change: An Overview. In Soil Health and Climate Change; Singh, B.P., Cowie, A.L., Chan, K.Y., Eds.; Springer: Berlin, Heidelberg, 2011; Volume 29, pp. 3–24. ISBN 978-3-642-20256-8.
- Holling, C.S. Resilience and Stability of Ecological Systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23.
- Alcamo, J. Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: A Framework for Assessment; Island Press: Washington, DC, USA, 2005; pp. 49–70.
- Bardgett, R.D.; van der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511.
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological Linkages Between Aboveground and Belowground Biota. Science 2004, 304, 1629–1633.
- US Department of Agriculture Soil Health. Available online: https://www.nrcs.usda.gov/conservation-basics/natural-resource-concerns/soils/soil-health (accessed on 17 February 2023).
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The Concept and Future Prospects of Soil Health. Nat. Rev. Earth Environ. 2020, 1, 544–553.
- Girija Veni, V.; Srinivasarao, C.; Sammi Reddy, K.; Sharma, K.L.; Rai, A. Soil Health and Climate Change. In Climate Change and Soil Interactions; Prasad, M.N.V., Pietrzykowski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 751–767. ISBN 978-0-12-818032-7.
- Tripathi, A.; Pandey, V.; Ranjan, M.R. Climate Change and Its Impact on Soil Properties. In Climate Change and the Microbiome; Choudhary, D.K., Mishra, A., Varma, A., Eds.; Springer: Cham, Switzerland, 2021; Volume 63, pp. 139–153. ISBN 978-3-030-76863-8.
- Rillig, M.C.; van der Heijden, M.G.A.; Berdugo, M.; Liu, Y.-R.; Riedo, J.; Sanz-Lazaro, C.; Moreno-Jiménez, E.; Romero, F.; Tedersoo, L.; Delgado-Baquerizo, M. Increasing the Number of Stressors Reduces Soil Ecosystem Services Worldwide. Nat. Clim. Chang. 2023, 13, 478–483.
- Conant, R.T.; Ryan, M.G.; Ågren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.M.; et al. Temperature and Soil Organic Matter Decomposition Rates - Synthesis of Current Knowledge and a Way Forward. Glob. Chang. Biol. 2011, 17, 3392–3404.
- Hopkins, F.M.; Torn, M.S.; Trumbore, S.E. Warming Accelerates Decomposition of Decades-Old Carbon in Forest Soils. Proc. Natl. Acad. Sci. USA 2012, 109, E1753–E1761.
- Lorenzen, S. The Phylogenetic Systematics of Freeliving Nematodes; Platt, H.M., Ed.; Ray Society: London, UK, 1994.
- Kergunteuil, A.; Campos-Herrera, R.; Sánchez-Moreno, S.; Vittoz, P.; Rasmann, S. The Abundance, Diversity, and Metabolic Footprint of Soil Nematodes Is Highest in High Elevation Alpine Grasslands. Front. Ecol. Evol. 2016, 4.
- Holterman, M.; Schratzberger, M.; Helder, J. Nematodes as Evolutionary Commuters between Marine, Freshwater and Terrestrial Habitats. Biol. J. Linn. Soc. 2019, 128, 756–767.
- Sapir, A. Why Are Nematodes so Successful Extremophiles? Commun. Integr. Biol. 2021, 14, 24–26.
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S.S. Feeding Habits in Soil Nematode Families and Genera-an Outline for Soil Ecologists. J. Nematol. 1993, 25, 315–331.
- Bongers, T. The Maturity Index: An Ecological Measure of Environmental Disturbance Based on Nematode Species Composition. Oecologia 1990, 83, 14–19.
- Yeates, G.W.; Bongers, T. Nematode Diversity in Agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 113–135.
- Yeates, G.W. Nematodes as Soil Indicators: Functional and Biodiversity Aspects. Biol. Fertil. Soils 2003, 37, 199–210.
- Bongers, T.; Ferris, H. Nematode Community Structure as a Bioindicator in Environmental Monitoring. Trends Ecol. Evol. 1999, 14, 224–228.
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A Framework for Soil Food Web Diagnostics: Extension of the Nematode Faunal Analysis Concept. Appl. Soil. Ecol. 2001, 18, 13–29.
- Ferris, H. Contribution of Nematodes to the Structure and Function of the Soil Food Web. J. Nematol. 2010, 42, 63–67.
- Ferris, H.; Venette, R.C.; Scow, K.M. Soil Management to Enhance Bacterivore and Fungivore Nematode Populations and Their Nitrogen Mineralisation Function. Appl. Soil. Ecol. 2004, 25, 19–35.
- Paul, E.A. Soil Microbiology, Ecology and Biochemistry, 4th ed.; Paul, E.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780125468077.
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil Nematode Abundance and Functional Group Composition at a Global Scale. Nature 2019, 572, 194–198.
- Bach, E.M.; Ramirez, K.S.; Fraser, T.D.; Wall, D.H. Soil Biodiversity Integrates Solutions for a Sustainable Future. Sustainability 2020, 12, 2662.
- Neher, D.A. Role of Nematodes in Soil Health and Their Use as Indicators. J. Nematol. 2001, 33, 161–168.
- Martin, T.; Wade, J.; Singh, P.; Sprunger, C.D. The Integration of Nematode Communities into the Soil Biological Health Framework by Factor Analysis. Ecol. Indic. 2022, 136, 108676.
- Sánchez-Moreno, S.; Ferris, H. Nematode Ecology and Soil Health. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; Sikora, R.A., Coyne, D., Hallmann, J., Timper, P., Eds.; CAB International: Wallingford, UK, 2018; pp. 62–86.
- Heděnec, P.; Jiménez, J.J.; Moradi, J.; Domene, X.; Hackenberger, D.; Barot, S.; Frossard, A.; Oktaba, L.; Filser, J.; Kindlmann, P.; et al. Global Distribution of Soil Fauna Functional Groups and Their Estimated Litter Consumption across Biomes. Sci. Rep. 2022, 12, 17362.
- Wilschut, R.A.; Geisen, S. Nematodes as Drivers of Plant Performance in Natural Systems. Trends Plant. Sci. 2021, 26, 237–247.
- Topalović, O.; Hussain, M.; Heuer, H. Plants and Associated Soil Microbiota Cooperatively Suppress Plant-Parasitic Nematodes. Front. Microbiol. 2020, 11, 313.
- Tu, C.; Koenning, S.R.; Hu, S. Root-Parasitic Nematodes Enhance Soil Microbial Activities and Nitrogen Mineralization. Microb. Ecol. 2003, 46, 134–144.
- Topalović, O.; Geisen, S. Nematodes as Suppressors and Facilitators of Plant Performance. New. Phytol. 2023, 238, 2305–2312.
- Nielsen, U.N.; Ayres, E.; Wall, D.H.; Li, G.; Bardgett, R.D.; Wu, T.; Garey, J.R. Global-Scale Patterns of Assemblage Structure of Soil Nematodes in Relation to Climate and Ecosystem Properties. Glob. Ecol. Biogeogr. 2014, 23, 968–978.
- Yan, D.; Yan, D.; Song, X.; Yu, Z.; Peng, D.; Ting, X.; Weng, B. Community Structure of Soil Nematodes under Different Drought Conditions. Geoderma 2018, 325, 110–116.
- Stevnbak, K.; Maraldo, K.; Georgieva, S.; Bjørnlund, L.; Beier, C.; Schmidt, I.K.; Christensen, S. Suppression of Soil Decomposers and Promotion of Long-Lived, Root Herbivorous Nematodes by Climate Change. Eur. J. Soil. Biol. 2012, 52, 1–7.
- Ferris, H.; Griffiths, B.S.; Porazinska, D.L.; Powers, T.O.; Wang, K.-H.; Tenuta, M. Reflections on Plant and Soil Nematode Ecology: Past, Present and Future. J. Nematol. 2012, 44, 115–126.
- A’Bear, A.D.; Jones, T.H.; Boddy, L. Potential Impacts of Climate Change on Interactions among Saprotrophic Cord-Forming Fungal Mycelia and Grazing Soil Invertebrates. Fungal Ecol. 2014, 10, 34–43.
- Ayres, E.; Wall, D.; Simmons, B.; Field, C.; Milchunas, D.; Morgan, J.; Roy, J. Belowground Nematode Herbivores Are Resistant to Elevated Atmospheric CO2 Concentrations in Grassland Ecosystems. Soil. Biol. Biochem. 2008, 40, 978–985.
- Blankinship, J.C.; Niklaus, P.A.; Hungate, B.A. A Meta-Analysis of Responses of Soil Biota to Global Change. Oecologia 2011, 165, 553–565.
- Cesarz, S.; Reich, P.B.; Scheu, S.; Ruess, L.; Schaefer, M.; Eisenhauer, N. Nematode Functional Guilds, Not Trophic Groups, Reflect Shifts in Soil Food Webs and Processes in Response to Interacting Global Change Factors. Pedobiologia 2015, 58, 23–32.
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil Biodiversity and Human Health. Nature 2015, 528, 69–76.
- De Deyn, G.B.; Raaijmakers, C.E.; van Ruijven, J.; Berendse, F.; van der Putten, W.H. Plant Species Identity and Diversity Effects on Different Trophic Levels of Nematodes in the Soil Food Web. Oikos 2004, 106, 576–586.
- Wang, J.; Li, M.; Zhang, X.; Liu, X.; Li, L.; Shi, X.; Hu, H.; Pan, G. Changes in Soil Nematode Abundance and Composition under Elevated and Canopy Warming in a Rice Paddy Field. Plant. Soil. 2019, 445, 425–437.
- Liu, Y.; Wang, W.; Liu, P.; Zhou, H.; Chen, Z.; Suonan, J. Plant-Soil Mediated Effects of Long-Term Warming on Soil Nematodes of Alpine Meadows on the Qinghai–Tibetan Plateau. Biology 2022, 11, 1596.
- Franco, A.L.C.; Gherardi, L.A.; de Tomasel, C.M.; Andriuzzi, W.S.; Ankrom, K.E.; Shaw, E.A.; Bach, E.M.; Sala, O.E.; Wall, D.H. Drought Suppresses Soil Predators and Promotes Root Herbivores in Mesic, but Not in Xeric Grasslands. Proc. Natl. Acad. Sci. USA 2019, 116, 12883–12888.
- Franco, A.L.C.; Gherardi, L.A.; de Tomasel, C.M.; Andriuzzi, W.S.; Ankrom, K.E.; Bach, E.M.; Guan, P.; Sala, O.E.; Wall, D.H. Root Herbivory Controls the Effects of Water Availability on the Partitioning between Above- and Below-ground Grass Biomass. Funct. Ecol. 2020, 34, 2403–2410.
- Franco, A.L.C.; Guan, P.; Cui, S.; Tomasel, C.M.; Gherardi, L.A.; Sala, O.E.; Wall, D.H. Precipitation Effects on Nematode Diversity and Carbon Footprint across Grasslands. Glob. Chang. Biol. 2022, 28, 2124–2132.
- Yang, Q.; Veen, G.F.; Wagenaar, R.; Manrubia, M.; ten Hooven, F.C.; van der Putten, W.H. Temporal Dynamics of Range Expander and Congeneric Native Plant Responses during and after Extreme Drought Events. Ecol. Monogr. 2022, 92, e1529.
- Homet, P.; Ourcival, J.-M.; Gutiérrez, E.; Domínguez-Begines, J.; Matías, L.; Godoy, O.; Gómez-Aparicio, L. Short- and Long-Term Responses of Nematode Communities to Predicted Rainfall Reduction in Mediterranean Forests. Soil. Biol. Biochem. 2023, 179, 108974.
- Siebert, J.; Ciobanu, M.; Schädler, M.; Eisenhauer, N. Climate Change and Land Use Induce Functional Shifts in Soil Nematode Communities. Oecologia 2020, 192, 281–294.
- Renčo, M.; Gömöryová, E.; Čerevková, A. The Effect of Soil Type and Ecosystems on the Soil Nematode and Microbial Communities. Helminthologia 2020, 57, 129–144.
- Li, X.; Zhu, H.; Geisen, S.; Bellard, C.; Hu, F.; Li, H.; Chen, X.; Liu, M. Agriculture Erases Climate Constraints on Soil Nematode Communities across Large Spatial Scales. Glob. Chang. Biol. 2020, 26, 919–930.
- Krashevska, V.; Kudrin, A.A.; Widyastuti, R.; Scheu, S. Changes in Nematode Communities and Functional Diversity with the Conversion of Rainforest into Rubber and Oil Palm Plantations. Front. Ecol. Evol. 2019, 7.
- Yogaswara, D.A.; Kasmara, H.; Hermawan, W. Using Nematode Community to Evaluate Banana Soil Food Web in Mekargalih, Cianjur, West Java. Pertanika J. Trop. Agric. Sci. 2021, 44, 465–483.
- Gao, D.; Wang, F.; Li, J.; Yu, S.; Li, Z.; Zhao, J. Soil Nematode Communities as Indicators of Soil Health in Different Land Use Types in Tropical Area. Nematology 2020, 22, 595–610.
- Wang, B.; Wu, L.; Chen, D.; Wu, Y.; Hu, S.; Li, L.; Bai, Y. Grazing Simplifies Soil Micro-food Webs and Decouples Their Relationships with Ecosystem Functions in Grasslands. Glob. Chang. Biol. 2020, 26, 960–970.
- Han, X.; Li, Y.; Du, X.; Li, Y.; Wang, Z.; Jiang, S.; Li, Q. Effect of Grassland Degradation on Soil Quality and Soil Biotic Community in a Semi-Arid Temperate Steppe. Ecol. Process. 2020, 9, 63.
- Vazquez, C.; Goede, R.G.M.; Korthals, G.W.; Rutgers, M.; Schouten, A.J.; Creamer, R. The Effects of Increasing Land Use Intensity on Soil Nematodes: A Turn towards Specialism. Funct. Ecol. 2019, 33, 2003–2016.
- Zhang, C.; Wang, J.; Ren, Z.; Hu, Z.; Tian, S.; Fan, W.; Chen, X.; Griffiths, B.S.; Hu, F.; Liu, M. Root Traits Mediate Functional Guilds of Soil Nematodes in an Ex-Arable Field. Soil. Biol. Biochem. 2020, 151, 108038.
- Puissant, J.; Villenave, C.; Chauvin, C.; Plassard, C.; Blanchart, E.; Trap, J. Quantification of the Global Impact of Agricultural Practices on Soil Nematodes: A Meta-Analysis. Soil. Biol. Biochem. 2021, 161, 108383.
- Xiong, D.; Wei, C.; Wang, X.; Lü, X.; Fang, S.; Li, Y.; Wang, X.; Liang, W.; Han, X.; Bezemer, T.M.; et al. Spatial Patterns and Ecological Drivers of Soil Nematode Β-diversity in Natural Grasslands Vary among Vegetation Types and Trophic Position. J. Anim. Ecol. 2021, 90, 1367–1378.
- Wang, B.; Zhu, Y.; Chen, X.; Chen, D.; Wu, Y.; Wu, L.; Liu, S.; Yue, L.; Wang, Y.; Bai, Y. Even Short-term Revegetation Complicates Soil Food Webs and Strengthens Their Links with Ecosystem Functions. J. Appl. Ecol. 2022, 59, 1721–1733.
- Yin, H.; Su, Y.; Liu, S.; Li, X.; Li, X.; Fan, C.; Guan, P.; Xie, Z.; Wang, S.; Scheu, S.; et al. Consistent Response of Nematode Communities to Management of Coniferous Plantations. For. Ecosyst. 2022, 9, 100045.
- Zhao, J.; Zhang, W.; Liu, X.; Yang, R.; Xiao, D.; He, X.; Wang, K. Grass Harvesting Eliminates the Beneficial Effects of Legume Addition on Soil Nematode Communities in a Tall Grass Pasture. Agric. Ecosyst. Environ. 2023, 349, 108468.
- Trap, J.; Ranoarisoa, M.P.; Raharijaona, S.; Rabeharisoa, L.; Plassard, C.; Mayad, E.H.; Bernard, L.; Becquer, T.; Blanchart, E. Agricultural Practices Modulate the Beneficial Activity of Bacterial-Feeding Nematodes for Plant Growth and Nutrition: Evidence from an Original Intact Soil Core Technique. Sustainability 2021, 13, 7181.
- Chen, D.; Xing, W.; Lan, Z.; Saleem, M.; Wu, Y.; Hu, S.; Bai, Y. Direct and Indirect Effects of Nitrogen Enrichment on Soil Organisms and Carbon and Nitrogen Mineralization in a Semi-arid Grassland. Funct. Ecol. 2019, 33, 175–187.
- Shaw, E.A.; Boot, C.M.; Moore, J.C.; Wall, D.H.; Baron, J.S. Long-Term Nitrogen Addition Shifts the Soil Nematode Community to Bacterivore-Dominated and Reduces Its Ecological Maturity in a Subalpine Forest. Soil. Biol. Biochem. 2019, 130, 177–184.
- Liu, T.; Mao, P.; Shi, L.; Eisenhauer, N.; Liu, S.; Wang, X.; He, X.; Wang, Z.; Zhang, W.; Liu, Z.; et al. Forest Canopy Maintains the Soil Community Composition under Elevated Nitrogen Deposition. Soil. Biol. Biochem. 2020, 143, 107733.
- Xiao, H.; Wang, B.; Lu, S.; Chen, D.; Wu, Y.; Zhu, Y.; Hu, S.; Bai, Y. Soil Acidification Reduces the Effects of Short-term Nutrient Enrichment on Plant and Soil Biota and Their Interactions in Grasslands. Glob. Chang. Biol. 2020, 26, 4626–4637.
- Wan, B.; Hu, Z.; Liu, T.; Yang, Q.; Li, D.; Zhang, C.; Chen, X.; Hu, F.; Kardol, P.; Griffiths, B.S.; et al. Organic Amendments Increase the Flow Uniformity of Energy across Nematode Food Webs. Soil. Biol. Biochem. 2022, 170, 108695.
- Varga, I.; Benković-Lačić, T.; Lončarić, Z.; Popović, B.; Brmež, M. Liming, Phosphorus and Zinc Influence on Soil Nematode Community Structure at Hot Pepper. Hortic. Sci. 2019, 46, 65–71.
- Olatunji, O.A.; Gong, S.; Tariq, A.; Pan, K.; Sun, X.; Chen, W.; Zhang, L.; Dakhil, M.A.; Huang, D.; Tan, X. The Effect of Phosphorus Addition, Soil Moisture, and Plant Type on Soil Nematode Abundance and Community Composition. J. Soils Sediments 2019, 19, 1139–1150.
- Caruso, T.; Hogg, I.D.; Nielsen, U.N.; Bottos, E.M.; Lee, C.K.; Hopkins, D.W.; Cary, S.C.; Barrett, J.E.; Green, T.G.A.; Storey, B.C.; et al. Nematodes in a Polar Desert Reveal the Relative Role of Biotic Interactions in the Coexistence of Soil Animals. Commun. Biol. 2019, 2, 63.
- Li, X.; Chen, X.; Zhu, H.; Ren, Z.; Jiao, J.; Hu, F.; Liu, M. Effects of Historical Legacies on Soil Nematode Communities Are Mediated by Contemporary Environmental Conditions. Ecol. Evol. 2020, 10, 6732–6740.
- Neilson, R.; Caul, S.; Fraser, F.C.; King, D.; Mitchell, S.M.; Roberts, D.M.; Giles, M.E. Microbial Community Size Is a Potential Predictor of Nematode Functional Group in Limed Grasslands. Appl. Soil. Ecol. 2020, 156, 103702.
- Xiong, D.; Wei, C.; Wubs, E.R.J.; Veen, G.F.; Liang, W.; Wang, X.; Li, Q.; Putten, W.H.; Han, X. Nonlinear Responses of Soil Nematode Community Composition to Increasing Aridity. Glob. Ecol. Biogeogr. 2020, 29, 117–126.
- Siebert, J.; Sünnemann, M.; Auge, H.; Berger, S.; Cesarz, S.; Ciobanu, M.; Guerrero-Ramírez, N.R.; Eisenhauer, N. The Effects of Drought and Nutrient Addition on Soil Organisms Vary across Taxonomic Groups, but Are Constant across Seasons. Sci. Rep. 2019, 9, 639.
- Thakur, M.P.; Del Real, I.M.; Cesarz, S.; Steinauer, K.; Reich, P.B.; Hobbie, S.; Ciobanu, M.; Rich, R.; Worm, K.; Eisenhauer, N. Soil Microbial, Nematode, and Enzymatic Responses to Elevated CO2, N Fertilization, Warming, and Reduced Precipitation. Soil. Biol. Biochem. 2019, 135, 184–193.
- Zhang, G.; Sui, X.; Li, Y.; Jia, M.; Wang, Z.; Han, G.; Wang, L. The Response of Soil Nematode Fauna to Climate Drying and Warming in Stipa Breviflora Desert Steppe in Inner Mongolia, China. J. Soils Sediments 2020, 20, 2166–2180.
- Nisa, R.U.; Tantray, A.Y.; Kouser, N.; Allie, K.A.; Wani, S.M.; Alamri, S.A.; Alyemeni, M.N.; Wijaya, L.; Shah, A.A. Influence of Ecological and Edaphic Factors on Biodiversity of Soil Nematodes. Saudi J. Biol. Sci. 2021, 28, 3049–3059.
- Wang, H.; Liu, G.; Huang, B.; Wang, X.; Xing, Y.; Wang, Q. Long-Term Nitrogen Addition and Precipitation Reduction Decrease Soil Nematode Community Diversity in a Temperate Forest. Appl. Soil. Ecol. 2021, 162, 103895.
- Li, J.; Zhao, J.; Liao, X.; Yi, Q.; Zhang, W.; Lin, H.; Liu, K.; Peng, P.; Wang, K. Long-Term Returning Agricultural Residues Increases Soil Microbe-Nematode Network Complexity and Ecosystem Multifunctionality. Geoderma 2023, 430, 116340.
- Ferris, H. Form and Function: Metabolic Footprints of Nematodes in the Soil Food Web. Eur. J. Soil. Biol. 2010, 46, 97–104.
- Zhang, X.; Ferris, H.; Mitchell, J.; Liang, W. Ecosystem Services of the Soil Food Web after Long-Term Application of Agricultural Management Practices. Soil. Biol. Biochem. 2017, 111, 36–43.
- Wood, J.R.; Holdaway, R.J.; Orwin, K.H.; Morse, C.; Bonner, K.I.; Davis, C.; Bolstridge, N.; Dickie, I.A. No Single Driver of Biodiversity: Divergent Responses of Multiple Taxa across Land Use Types. Ecosphere 2017, 8, e01997.
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452.
- van den Hoogen, J.; Geisen, S.; Wall, D.H.; Wardle, D.A.; Traunspurger, W.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Ferris, H.; Bardgett, R.D.; et al. A Global Database of Soil Nematode Abundance and Functional Group Composition. Sci. Data 2020, 7, 103.
- Karuri, H. Nematode Community Structure and Functional Guilds Differ in Tea Fields and Tropical Forest. Geoderma 2021, 392, 115006.
- Jiang, Y.; Wang, Z.; Liu, Y.; Han, Y.; Wang, Y.; Wang, Q.; Liu, T. Nematodes and Their Bacterial Prey Improve Phosphorus Acquisition by Wheat. New Phytol. 2023, 237, 974–986.
- Zheng, J.; Dini-Andreote, F.; Luan, L.; Geisen, S.; Xue, J.; Li, H.; Sun, B.; Jiang, Y. Nematode Predation and Competitive Interactions Affect Microbe-Mediated Phosphorus Dynamics. mBio 2022, 13, 3.
- Chen, X.; Xue, W.; Xue, J.; Griffiths, B.S.; Liu, M. Contribution of Bacterivorous Nematodes to Soil Resistance and Resilience under Copper or Heat Stress. Soil. Ecol. Lett. 2020, 2, 220–229.
- da Silva, J.V.C.d.L.; Hirschfeld, M.N.C.; Cares, J.E.; Esteves, A.M. Land Use, Soil Properties and Climate Variables Influence the Nematode Communities in the Caatinga Dry Forest. Appl. Soil. Ecol. 2020, 150, 103474.
- Bastida, F.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Gallardo, A.; García-Velázquez, L.; García, C.; Hart, S.C.; Pérez, C.A.; Santos, F.; et al. Climatic Vulnerabilities and Ecological Preferences of Soil Invertebrates across Biomes. Mol. Ecol. 2020, 29, 752–761.
- Majdi, N.; Traunspurger, W.; Fueser, H.; Gansfort, B.; Laffaille, P.; Maire, A. Effects of a Broad Range of Experimental Temperatures on the Population Growth and Body-Size of Five Species of Free-Living Nematodes. J. Biol. 2019, 80, 21–36.
- Andriuzzi, W.S.; Franco, A.L.C.; Ankrom, K.E.; Cui, S.; de Tomasel, C.M.; Guan, P.; Gherardi, L.A.; Sala, O.E.; Wall, D.H. Body Size Structure of Soil Fauna along Geographic and Temporal Gradients of Precipitation in Grasslands. Soil. Biol. Biochem. 2020, 140, 107638.
- Wilschut, R.A.; Geisen, S.; Martens, H.; Kostenko, O.; Hollander, M.; Hooven, F.C.; Weser, C.; Snoek, L.B.; Bloem, J.; Caković, D.; et al. Latitudinal Variation in Soil Nematode Communities under Climate Warming-related Range-expanding and Native Plants. Glob. Chang. Biol. 2019, 25, 2714–2726.
- Wang, X.; Xiao, S.; Yang, X.; Liu, Z.; Zhou, X.; Du, G.; Zhang, L.; Guo, A.; Chen, S.; Nielsen, U.N. Dominant Plant Species Influence Nematode Richness by Moderating Understory Diversity and Microbial Assemblages. Soil. Biol. Biochem. 2019, 137, 107566.
- Bennett, J.A.; Koch, A.M.; Forsythe, J.; Johnson, N.C.; Tilman, D.; Klironomos, J. Resistance of Soil Biota and Plant Growth to Disturbance Increases with Plant Diversity. Ecol. Lett. 2020, 23, 119–128.
- Wu, L.; Chen, H.; Chen, D.; Wang, S.; Wu, Y.; Wang, B.; Liu, S.; Yue, L.; Yu, J.; Bai, Y. Soil Biota Diversity and Plant Diversity Both Contributed to Ecosystem Stability in Grasslands. Ecol. Lett. 2023, 26, 858–868.
