Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by zhixi Chen.
Anemarrhena asphodeloides Bunge, a recognized and well-known medicinal plant for more than two thousand years, has demonstrated its effectiveness against cancer. Timosaponin-AIII (TSAIII), as a bioactive steroid saponin isolated from A. asphodeloides, has shown multiple pharmacological activities and has been developed as an anticancer agent.
- steroid saponin
- Timosaponin-AIII
- anticancer
1. Introduction
Cancer, which is characterized by the dysregulated growth and proliferation of cells, has become one of the main causes of death worldwide. It is estimated that the number of new cancer cases increased by 19.3 million in 2020 and may continue to increase to 30.2 million by 2040 [1]. The World Health Organization (WHO) has reported that there will be 27 million new cancer cases and 17.1 million deaths by 2050 [2]. Extensive investigations and understanding of the pathogenesis of cancers have been advanced. Treatment options, such as surgery, chemotherapy, and radiotherapy, have been widely used in clinics. However, current strategies for cancer treatment are unsatisfactory due to their limited safety and effectiveness [1]. Developing efficient and safe chemotherapy agents for cancer treatment is still needed. The anticancer effects of chemotherapy agents could be associated with the disruption of the cell cycle, the induction of apoptosis, the regulation of autophagy, suppression of angiogenesis, the inhibition of migration and invasion, and the improvement of multidrug resistance [3,4][3][4].
Natural products display a role in the prevention and treatment of cancers, and these can be further developed as novel candidates or anticancer agents. Bioactive compounds derived from natural products and primarily traditional medicinal plants are commonly used worldwide [5]. Anemarrhena asphodeloides Bunge (Figure 1), also known as Zhimu in Chinese, has been frequently used in traditional medicine in China, Japan, and Korea to treat various diseases, such as diabetes, hematochezia, arthralgia, coughs, and hemoptysis [6]. A phytochemical investigation into Zhimu has revealed that steroid saponins, phenylpropanoids, flavonoids, alkaloids, anthraquinones, and organic acids are its main constituents [6]. In addition, steroid saponin constituents, such as Timosaponin-AIII (TSAIII, Figure 1), Timosaponin-BII (TSBII), sarsasaponin, xanthones, and anemarsaponin, are one of the natural compound classes. Steroid saponins structurally consist of a lipophilic steroidal derivative and a hydrophilic glycoside moiety. Due to different sugar moiety substituents and sapogenins, saponins have diverse compounds [7].
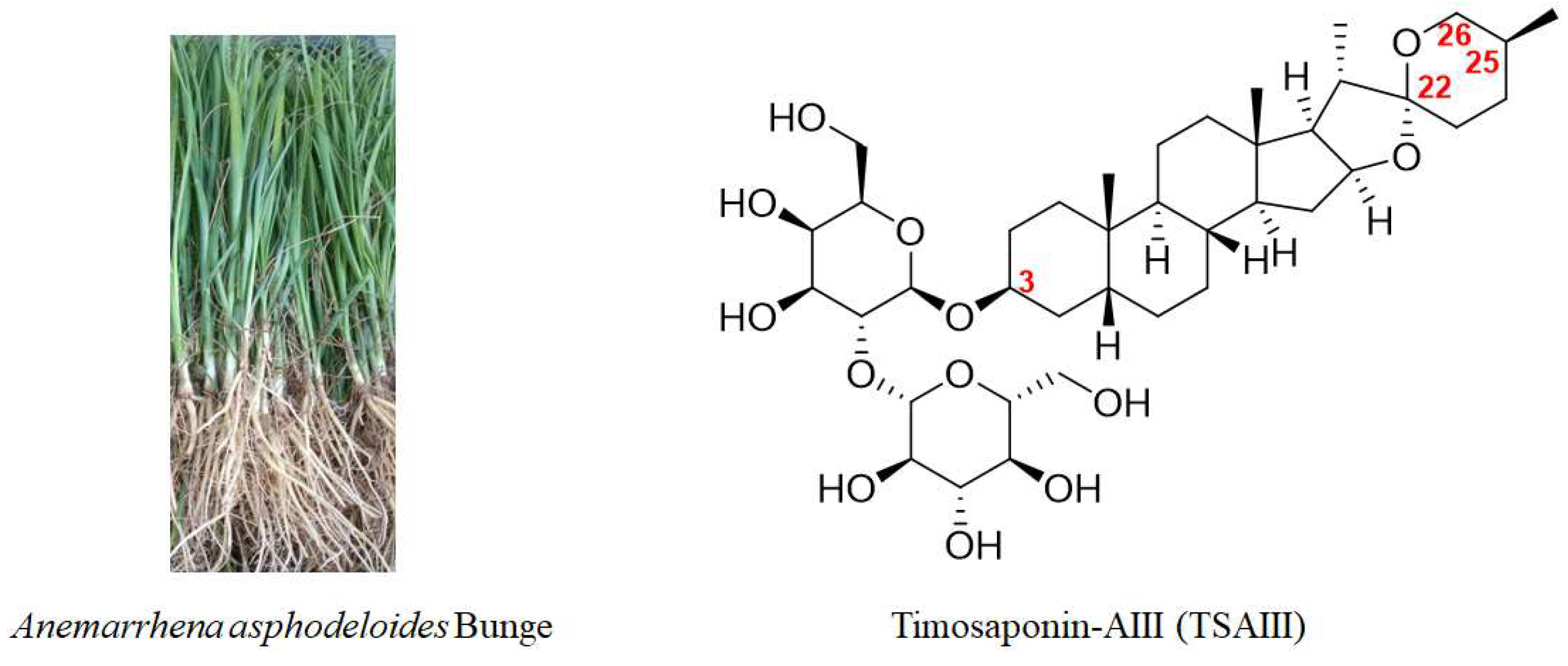
Figure 1.
A picture of
Anemarrhena asphodeloides
Bunge and the chemical structure of TSAIII.
Pharmacological assays for screening bioactive ingredients in Zhimu show that TSAIII is one of the natural steroidal saponins with multiple biological activities, such as antipyretics, anti-inflammation, antioxidation, antiplatelet aggregation, and antidepression [8]. Importantly, TSAIII has become one of the markers for the quality control of various A. asphodeloides Bunge-containing formulae, including Guizhi/Shaoyang/Zhimu formula, Zhimu/Bihe formula, TongGuan Wan, and Rhizoma anemarrhenae/Phellodendron formula [6,9,10,11,12][6][9][10][11][12]. Natural saponins have been demonstrated to have anticancer effects [13], and TSAIII has shown efficacy in different diseases, including Alzheimer’s disease (AD), diabetes, colitis, and cancer [8,14,15,16][8][14][15][16]. TSAIII has been proposed as a potent anticancer agent [8,9][8][9]. Recently, research on anticancer drugs has entered the fast lane. Informative data on the pharmacological activities of TSAIII are explosive. In this article, the anticancer effects of TSAIII are comprehensively updated.
2. Chemical Structure, Biotransformation, and Structure–Activity Relationship of TSAIII
TSAIII (Figure 1) has an IUPAC name: (2S,3R,4S,5S,6R)-2-[(2R,3R,4S,5R,6R)- 4,5-dihydroxy-6-(hydroxymethyl)-2-[(1R,2S,4S,5′S,6R,7S,8R,9S,12S,13S,16S,18R)-5′,7,9,13- tetramethylspiro [5-oxapentacyclo [10.8.0.02,9.04,8.013,18]icosane-6,2′-oxane]-16-yl] oxyoxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol), which is a molecular formula of C39H64O13, and it has a molecular weight of 740.92 g/mol. TSAIII is a white to off-white solid with various pharmacological activities and is soluble in methanol, butanol, 80% ethanol, and aqueous pentanol; however, it is insoluble in water.
It has been reported that the content of TSAIII in the rhizomes of A. asphodeloides is approximately 0.19–0.28% [17]. However, another study reported that the content of TSAIII is too low to be detected in a Zhimu/Baihe formula [18]. Interestingly, TSBII has a much higher level and can be transformed into TSAIII. β-D-glycosidase, as a key enzyme, has been used to produce TSAIII by hydrolyzing TSBII (Figure 2) from the crude extract liquid of the rhizomes of A. asphodeloides. Potentially, approximately 7 g of TSAIII can be isolated from 1 kg of the rhizomes of A. asphodeloides by five-step preparation. The optimal conditions for this include pH4.0, 55 °C, 2 h, and 600 U/g of β-D-glycosidase [17]. TSBII can be hydrolyzed and isomerized by the fungus Colletotrichum gloeosporioides to generate TSAIII, and TSAIII can also be produced by Aspergillus niger [19]. However, TSAIII could be further transformed into sarsasapogenin by removing the sugar chain at the C-3 position (Figure 2); this could induce a low yield of TSAIII [9,20][9][20]. Thus, an efficient industrial process for TSAIII production is essential.
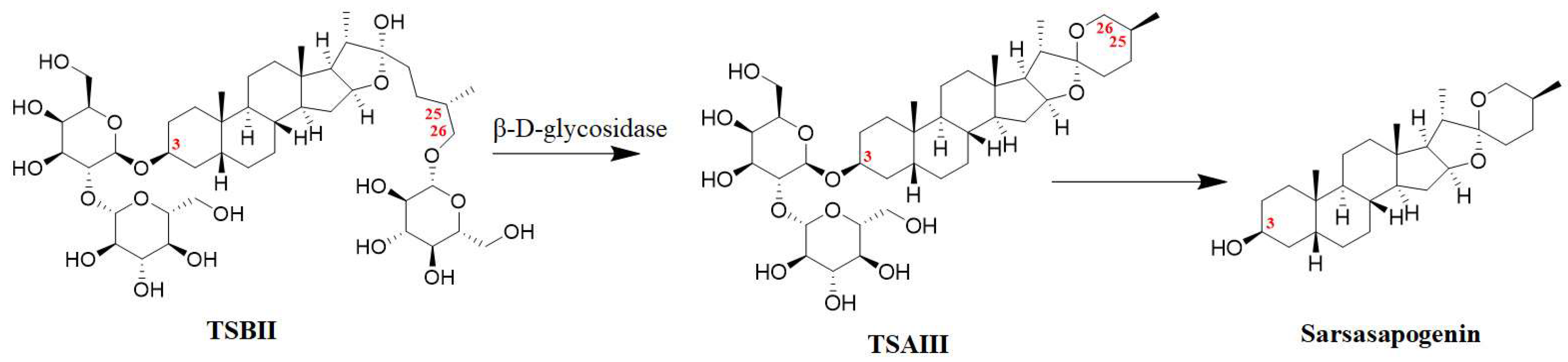
Figure 2. The biotransformation of TSAIII: the transformation of TSBII into TSAIII could be regulated by β-D-glycosidase, and TSAIII could be further transformed into sarsasapogenin by removing the sugar chain at the C-3 position.
The sugar chain at the C-3 position in TSAIII is indispensable to its pharmacological effects. The IC50 values of TSAIII and sarsasapogenin in lowing Aβ42 production in N2A-APPswe cells are 2.3 μM and 53 μM, respectively. In addition, disaccharide TSAIII has shown higher activity in reducing the production of Aβ42 compared with monosaccharide TSAI (IC50 = 6.1 μM). However, trisaccharide TSAV (IC50 = 4.2 μM) exhibits a lower activity than TSAIII [21]. Interestingly, an intact F ring is essential for TSAIII and sarsasapogenin to reduce Aβ42 production [21]. One study reported that an α-D-1,4-glucopyranosyl group at the C-4-OH of the glucosyl group in TSAIII could decrease the cytotoxicity of HL60 cells [22]. A structure–activity relationship study showed that the substituents at the C-3 and C-26 positions played a key role in selective anticancer activity [23,24][23][24]. Specifically, linking an amine (N-methyl piperazinyl, piperidyl, piperzinyl, pyrrolidinyl, N,N-dimethylamino, and N,N-diethylamino) to the C-3 or C-26 position and presenting a methoxyl group at the C-3 position could increase the selective cytotoxic activity of sarsasapogenin. In addition, the opening of the F ring in sarsasapogenin might enhance the antiproliferative activity [23].
3. Pharmacokinetic Profiles of TSAIII
Pharmacokinetic studies have exhibited a crucial role in novel drug development. The detection of TSAIII in animal blood plasma after the oral administration of TSAIII or TSAIII-containing formulae has been performed. After the oral administration of free TSAIII (6.8 mg/kg) in healthy male SD rats, the Cmax, Tmax, AUC0-t, and T1/2 of TSAIII were 18.2 ± 3.1 ng/mL, 2.3 ± 0.57 h, 150.5 ± 29.2 ng·h/mL, and 4.9 ± 2.0 h, respectively [25]. Another study reported that the values of T1/2, Cmax, and AUC were 2.74 ± 1.68 h, 105.7 ± 14.9 ng/mL, and 921.8 ± 289.0 ng·h/mL after the intragastrical administration of TSAIII (25 mg/kg) in male SD rats [26]. Comparably, the values of these were 15.1 ± 2.3 h, 77.28 ± 21.47 ng/mL, and 916.61 ± 208.43 ng·h/mL after the intragastrical administration of sarsasapogenin (25 mg/kg) [27]. Interestingly, these parameters for TSAIII could be 22.2 ± 6.5 ng/mL, 3.15 ± 0.62 h, 206.0 ± 45.1 ng·h/mL, and 9.9 ± 2.8 h, respectively, after the oral administration of Zhimu/Baihe formula (containing 6.4 mg/kg TSAIII). The improved pharmacokinetic profiles might be associated with the increased biotransformation of TSAIII and synergistic effects [25].
After the oral administration of the saponin extract from Rhizoma anemarrhenae, eight saponins were detected in the rat plasma. The pharmacokinetic profiles showed that TSAIII had the highest values of Tmax (7.85 h) and t1/2 (9.77 h). These suggested that TSAIII had a long body residence time and slow excretion [28]. Another study reported that after an oral administration of the 7 g/kg Zhimu/Bai formula, the level of TSAIII in the portal vein plasma and the systemic plasma in male Wistar rats was 1656.7 ± 121.1 ng/mL/h and 650.5 ± 45.2 ng/mL/h, respectively, indicating 60.7% of the liver extraction rate and the dramatic liver first-pass effect. The values of Cmax, Tmax, and t1/2 were 5085.3 ± 1581.5 ng/mL, 6 h, and 12.1 h, respectively. These suggested the comparable possibility of liver accumulation in TSAIII [18]. It has also been reported that Cmax, Tmax, and T1/2 of TSAIII were 94.4 ± 3.8 ng/mL, 336.0 ± 53.7 min, and 211.8 ± 98.8 min after the oral administration of the Zhimu/Bai formula (10 g/kg, containing 19.2 mg/kg of TSAIII) in male SD rats. In the other group, these values were 84.3 ± 8.6 ng/mL, 312 ± 65.7 min, and 104.2 ± 10.3 min after the oral administration of the Zhimu extract (10 g/kg, containing 18.7 mg/kg of TSAIII) [29].
The metabolites of TSAIII, including deglycosylated, glycosylated, and hydroxylated products, were identified, and these could be detected in the heart, urine, and feces. Only the parent form was detected in the plasma, liver, and kidney [30]. Another study reported that 19 metabolites were detected and identified from the rat plasma, bile, urine, and feces after a single oral dose of 400 mg/kg of TSAIII. Consistently, TSAIII underwent deglycosylation, oxidation, dehydrogenation, F-ring cleavage, and isotype reactions [31]. Salt processing, as a traditional processing method for Chinese herbs, has been reported to increase the content of TSAIII due to its increased transformation from TSBII. The AUC and Cmax of TSAIII after the oral administration of extracts of the unprocessed A. rhizoma were 16.5 μg·h/L and 4.5 μg/L, respectively. However, these values decreased to 7.2 μg·h/L and 2.18 μg/L, respectively, after the oral administration of the processed samples. The explanation for these discrepancies in the pharmacokinetic parameters of TSAIII is complex. For example, salt processing could increase the metabolism and excretion of TSAIII [32].
It has been reported that hydrophobicity and low bioavailability limit the antitumor efficacy and application of TSAIII. Indeed, new delivery systems for TSAIII have been developed. The anti-CD44 antibody, as a tumor-target ligand, has been used to improve liposome accumulation in tumors by specifically interacting with CD44 receptors. It has been reported that anti-CD44 antibody-modified TSAIII-loaded liposomes (CD44-TSAIII-LP) could increase the circulation time, the bioavailability, the tumor-target accumulation, and the antitumor activity of TSAIII, demonstrating a potential agent for the therapeutic management of CD44-positive cancers [33]. A grapheme oxide-based nanocomposite hydrogel (GPP) has been developed to improve the bioavailability of TSAIII, enhance the efficacy of photothermal therapy, and elevate its protective activity against tumor development. Specifically, the encapsulation rate of GPP-TSAIII is 66.36%, and this delivery system was found to have a slower release and higher uptake of TSAIII in mouse melanoma B16F10 cells in vitro [34].
References
- Çetinkaya, M.; Baran, Y. Therapeutic Potential of Luteolin on Cancer. Vaccines 2023, 11, 554.
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407.
- Sleire, L.; Førde, H.E.; Netland, I.A.; Leiss, L.; Skeie, B.S.; Enger, P. Drug repurposing in cancer. Pharmacol. Res. 2017, 124, 74–91.
- Megyesfalvi, Z.; Gay, C.M.; Popper, H.; Pirker, R.; Ostoros, G.; Heeke, S.; Lang, C.; Hoetzenecker, K.; Schwendenwein, A.; Boettiger, K.; et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA A Cancer J. Clin. 2023.
- Liang, X.; Luo, D.; Luesch, H. Advances in exploring the therapeutic potential of marine natural products. Pharmacol. Res. 2019, 147, 104373.
- Wang, Y.; Dan, Y.; Yang, D.; Hu, Y.; Zhang, L.; Zhang, C.; Zhu, H.; Cui, Z.; Li, M.; Liu, Y. The genus Anemarrhena Bunge: A review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2014, 153, 42–60.
- Ur Rahman, S.; Ismail, M.; Khurram, M.; Ullah, I.; Rabbi, F.; Iriti, M. Bioactive Steroids and Saponins of the Genus Trillium. Molecules 2017, 22, 2156.
- Han, F.Y.; Song, X.Y.; Chen, J.J.; Yao, G.D.; Song, S.J. Timosaponin AIII: A novel potential anti-tumor compound from Anemarrhena asphodeloides. Steroids 2018, 140, 125–130.
- Lin, Y.; Zhao, W.R.; Shi, W.T.; Zhang, J.; Zhang, K.Y.; Ding, Q.; Chen, X.L.; Tang, J.Y.; Zhou, Z.Y. Pharmacological Activity, Pharmacokinetics, and Toxicity of Timosaponin AIII, a Natural Product Isolated From Anemarrhena asphodeloides Bunge: A Review. Front. Pharmacol. 2020, 11, 764.
- Tang, Y.H.; Sun, Z.L.; Fan, M.S.; Li, Z.X.; Huang, C.G. Anti-diabetic effects of TongGuanWan, a Chinese traditional herbal formula, in C57BL/KsJ-db/db mice. Planta Med. 2012, 78, 18–23.
- Tang, Z.; Li, G.; Yang, J.; Duan, J.; Qian, D.; Guo, J.; Zhu, Z.; Song, Z. Anemarrhena asphodeloides Non-Steroidal Saponin Components Alter the Pharmacokinetic Profile of Its Steroidal Saponins in Rat. Molecules 2015, 20, 11777–11792.
- Yang, B.; Liu, Z.; Wang, Q.; Chai, Y.; Xia, P. Pharmacokinetic comparison of seven major bioactive components in normal and depression model rats after oral administration of Baihe Zhimu decoction by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2018, 148, 119–127.
- Xu, X.H.; Li, T.; Fong, C.M.; Chen, X.; Chen, X.J.; Wang, Y.T.; Huang, M.Q.; Lu, J.J. Saponins from Chinese Medicines as Anticancer Agents. Molecules 2016, 21, 1326.
- Tundis, R.; Bonesi, M.; Menichini, F.; Loizzo, M.R. Recent Knowledge on Medicinal Plants as Source of Cholinesterase Inhibitors for the Treatment of Dementia. Mini Rev. Med. Chem. 2016, 16, 605–618.
- Wang, N.; Xu, P.; Wang, X.; Yao, W.; Wang, B.; Wu, Y.; Shou, D. Timosaponin AIII attenuates inflammatory injury in AGEs-induced osteoblast and alloxan-induced diabetic osteoporosis zebrafish by modulating the RAGE/MAPK signaling pathways. Phytomedicine 2020, 75, 153247.
- Lim, S.M.; Jeong, J.J.; Kang, G.D.; Kim, K.A.; Choi, H.S.; Kim, D.H. Timosaponin AIII and its metabolite sarsasapogenin ameliorate colitis in mice by inhibiting NF-κB and MAPK activation and restoring Th17/Treg cell balance. Int. Immunopharmacol. 2015, 25, 493–503.
- Lu, L.; Liu, Y.; Ding, Y.; Hou, J.; Zhang, Y.; Xue, H.; Zhang, T. Preparation of highly purified timosaponin AIII from rhizoma anemarrhenae through an enzymatic method combined with preparative liquid chromatography. Nat. Prod. Res. 2016, 30, 2364–2367.
- Xie, Y.; Zhou, X.; Pei, H.; Chen, M.C.; Sun, Z.L.; Xue, Y.R.; Tian, X.T.; Huang, C.G. Metabolism, pharmacokinetics, and hepatic disposition of xanthones and saponins on Zhimu treatments for exploratively interpreting the discrepancy between the herbal safety and timosaponin A3-induced hepatotoxicity. Acta Pharmacol. Sin. 2018, 39, 1923–1934.
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent Advances in Biotransformation of Saponins. Molecules 2019, 24, 2365.
- King, F.W.; Fong, S.; Griffin, C.; Shoemaker, M.; Staub, R.; Zhang, Y.L.; Cohen, I.; Shtivelman, E. Timosaponin AIII is preferentially cytotoxic to tumor cells through inhibition of mTOR and induction of ER stress. PLoS ONE 2009, 4, e7283.
- Sy, L.K.; Lok, C.N.; Wang, J.Y.; Liu, Y.; Cheng, L.; Wan, P.K.; Leung, C.T.; Cao, B.; Kwong, W.L.; Chang, R.C.; et al. Identification of “sarsasapogenin-aglyconed” timosaponins as novel Aβ-lowering modulators of amyloid precursor protein processing. Chem. Sci. 2016, 7, 3206–3214.
- Wang, Y.Z.; Feng, B.; Huang, H.Z.; Kang, L.P.; Cong, Y.; Zhou, W.B.; Zou, P.; Cong, Y.W.; Song, X.B.; Ma, B.P. Glucosylation of steroidal saponins by cyclodextrin glucanotransferase. Planta Med. 2010, 76, 1724–1731.
- Yin, Y.; Zhao, X.C.; Wang, S.J.; Gao, P.Y.; Li, L.Z.; Ikejima, T.; Song, S.J. Synthesis and biological evaluation of novel sarsasapogenin derivatives as potential anti-tumor agents. Steroids 2015, 93, 25–31.
- Wang, W.; Wang, D.; Wang, Z.; Yao, G.; Li, X.; Gao, P.; Li, L.; Zhang, Y.; Wang, S.; Song, S. Synthesis of new sarsasapogenin derivatives with cytotoxicity and apoptosis-inducing activities in human breast cancer MCF-7 cells. Eur. J. Med. Chem. 2017, 127, 62–71.
- Liu, Z.; Dong, X.; Ding, X.; Chen, X.; Lv, L.; Li, Y.; Chai, Y. Comparative pharmacokinetics of timosaponin B-II and timosaponin A-III after oral administration of Zhimu-Baihe herb-pair, Zhimu extract, free timosaponin B-II and free timosaponin A-III to rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 926, 28–35.
- Liu, Y.; Pu, Y.; Zhang, T.; Ding, Y.; Wang, B.; Cai, Z. Rapid and Sensitive Determination of Timosaponin AIII in Rat Plasma by LC-MS/MS and Its Pharmacokinetic Application. Int. J. Mol. Sci. 2013, 14, 3656–3670.
- Yang, B.; Liu, Z.; Hu, J.; Lai, X.; Xia, P. Quantitative determination of sarsasapogenin in rat plasma using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1022, 213–219.
- Wang, H.Q.; Lan, F.; Zhang, Y.H.; Xia, J.E.; Gong, X.M.; Liu, M. Identification and pharmacokinetics of saponins in Rhizoma anemarrhenae after oral administration to rats by HPLC-Q-TOF/MS and HPLC-MS/MS. Acta Pharm. 2021, 71, 567–585.
- Li, G.; Tang, Z.; Yang, J.; Duan, J.; Qian, D.; Guo, J.; Zhu, Z.; Liu, H. Simultaneous determination of five components in rat plasma by UPLC-MS/MS and its application to a comparative pharmacokinetic study in Baihe Zhimu Tang and Zhimu extract. Molecules 2015, 20, 6700–6714.
- Jia, Y.; Wu, B.; Fan, M.; Wang, J.; Huang, J.; Huang, C. High-performance liquid chromatography-electrospray ionization tandem mass spectrometry for metabolism study of timosaponin AIII. J. Chromatogr. Sci. 2014, 52, 418–422.
- Sun, Y.; Liu, L.; Peng, Y.; Liu, B.; Lin, D.; Li, L.; Song, S. Metabolites characterization of timosaponin AIII in vivo and in vitro by using liquid chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 997, 236–243.
- Wang, X.; Yu, Y.; Pei, L.; Gao, H. Comparison of the pharmacokinetics of timosaponin AIII, timosaponin BIII, and mangiferin extracted from crude and salt-processed Anemarrhenae Rhizoma by UPLC-MS/MS. RSC Adv. 2023, 13, 11919–11928.
- Lu, L.; Ding, Y.; Zhang, Y.; Ho, R.J.; Zhao, Y.; Zhang, T.; Guo, C. Antibody-modified liposomes for tumor-targeting delivery of timosaponin AIII. Int. J. Nanomed. 2018, 13, 1927–1944.
- Huang, X.; He, Y.; Zhang, M.; Lu, Z.; Zhang, T.; Wang, B. GPP-TSAIII nanocomposite hydrogel-based photothermal ablation facilitates melanoma therapy. Expert Opin. Drug Deliv. 2023, 1–19.
More
