The cerebellum is most renowned for its role in sensorimotor control and coordination, but a growing number of anatomical and physiological studies are demonstrating its deep involvement in cognitive and emotional functions. Recently, the development and refinement of optogenetic techniques boosted research in the cerebellar field and, impressively, revolutionized the methodological approach and endowed the investigations with entirely new capabilities. This translated into a significant improvement in the data acquired for sensorimotor tests, allowing one to correlate single-cell activity with motor behavior to the extent of determining the role of single neuronal types and single connection pathways in controlling precise aspects of movement kinematics. These levels of specificity in correlating neuronal activity to behavior could not be achieved in the past, when electrical and pharmacological stimulations were the only available experimental tools. The application of optogenetics to the investigation of the cerebellar role in higher-order and cognitive functions, which involves a high degree of connectivity with multiple brain areas, has been even more significant. It is possible that, in this field, optogenetics has changed the game, and the number of investigations using optogenetics to study the cerebellar role in non-sensorimotor functions in awake animals is growing. The main issues addressed by these studies are the cerebellar role in epilepsy (through connections to the hippocampus and the temporal lobe), schizophrenia and cognition, working memory for decision making, and social behavior. It is also worth noting that optogenetics opened a new perspective for cerebellar neurostimulation in patients (e.g., for epilepsy treatment and stroke rehabilitation), promising unprecedented specificity in the targeted pathways that could be either activated or inhibited.
- cerebellum
- optogenetics
- sensorimotor system
- non-sensorimotor functions
1. Introduction
Introduction
The first reports on cerebellar structure and the first hypothesis on function pointed out its high degree of connectivity with the rest of the brain and the possible relationship with higher-order functions [1]. However, since the nineteenth century, with the work of Flourens (who provided the first descriptions of the motor syndrome now called ataxia [2]), the cerebellum has been investigated for its role in motor functions, while the role of its extensive connectivity to other brain areas was overlooked. The shift in the cerebellar paradigm is only relatively recent and can be traced back to Schmahmann’s first reports of the Cerebellar Cognitive Affective Syndrome ([3] and the introduction of the Dysmetria of Thought concept in 1998 [4]. Since then, several studies have reported cerebellar abnormalities or lesions at the core of cognitive dysfunctions [5][6][7] as well as higher order function impairments associated with motor syndromes involving the cerebellum [8][9]. While the research on the cerebellar role in sensorimotor integration can rely on a well-defined anatomy and easily measurable motor outputs, investigating the cerebellar role in non-sensorimotor functions proved challenging. To find the role of the cerebellum in these cases, one needs to take into account cerebellar connectivity with other brain regions, such as the hippocampus or the neocortex, that are not usually direct. Moreover, while considering cognitive functions, the involvement of brain cortical associative areas makes the identification of the specific role of the cerebellum hard. Over the last decade, the revolution of technical approaches in neurophysiological investigations has provided new tools and granted unprecedented resolution in determining the effects of neuronal types and single pathways in complex behaviors. In particular, optogenetics proved to be a game-changing tool that neuroscience needed in order to achieve stimulation specificity, in vitro and in vivo, that was not foreseeable only twenty years ago. This technical revolution affected cerebellar research too, allowing huge steps in understanding the cerebellar contribution to both motor and non-motor functions. This review will focus on the use of optogenetics in the cerebellum to analyze behavior, to different extents. Concerning the section about the role of cerebellum in the sensorimotor system, the focus will be on the unprecedented level of detail that optogenetics allow to achieve in terms of specific neuronal types or pathways involved in precise aspects of movement. The non-sensorimotor section will summarize the main findings of the last decade, where the use of optogenetics allowed us to tackle the role of cerebellum in functions and behaviors usually associated with other brain areas. Importantly, this optogenetic revolution in cerebellar investigations might be key in achieving cerebellar stimulation (or inhibition) as a clinical treatment for a broad spectrum of disorders.Herein, we will provide a summary of recent advances in cerebellar optogenetics and its applications to study neuronal circuits in physiological or pathological conditions, highlighting the advantages of photostimulation techniques, as well as emerging questions and future perspectives.
The first reports on cerebellar structure and the first hypothesis on function pointed out its high degree of connectivity with the rest of the brain and the possible relationship with higher-order functions [1]. However, since the nineteenth century, with the work of Flourens (who provided the first descriptions of the motor syndrome now called ataxia [2]), the cerebellum has been investigated for its role in motor functions, while the role of its extensive connectivity to other brain areas was overlooked. The shift in the cerebellar paradigm is only relatively recent and can be traced back to Schmahmann’s first reports of the Cerebellar Cognitive Affective Syndrome ([3] and the introduction of the Dysmetria of Thought concept in 1998 [4]. Since then, several studies have reported cerebellar abnormalities or lesions at the core of cognitive dysfunctions [5-7] as well as higher order function impairments associated with motor syndromes involving the cerebellum [8,9]. While the research on the cerebellar role in sensorimotor integration can rely on a well-defined anatomy and easily measurable motor outputs, investigating the cerebellar role in non-sensorimotor functions proved challenging. To find the role of the cerebellum in these cases, one needs to take into account cerebellar connectivity with other brain regions, such as the hippocampus or the neocortex, that are not usually direct. Moreover, while considering cognitive functions, the involvement of brain cortical associative areas makes the identification of the specific role of the cerebellum hard. Over the last decade, the revolution of technical approaches in neurophysiological investigations has provided new tools and granted unprecedented resolution in determining the effects of neuronal types and single pathways in complex behaviors. In particular, optogenetics proved to be a game-changing tool that neuroscience needed in order to achieve stimulation specificity, in vitro and in vivo, that was not foreseeable only twenty years ago. This technical revolution affected cerebellar research too, allowing huge steps in understanding the cerebellar contribution to both motor and non-motor functions. This review will focus on the use of optogenetics in the cerebellum to analyze behavior, to different extents. Concerning the section about the role of cerebellum in the sensorimotor system, the focus will be on the unprecedented level of detail that optogenetics allow to achieve in terms of specific neuronal types or pathways involved in precise aspects of movement. The non-sensorimotor section will summarize the main findings of the last decade, where the use of optogenetics allowed us to tackle the role of cerebellum in functions and behaviors usually associated with other brain areas. Importantly, this optogenetic revolution in cerebellar investigations might be key in achieving cerebellar stimulation (or inhibition) as a clinical treatment for a broad spectrum of disorders.Herein, we will provide a summary of recent advances in cerebellar optogenetics and its applications to study neuronal circuits in physiological or pathological conditions, highlighting the advantages of photostimulation techniques, as well as emerging questions and future perspectives.
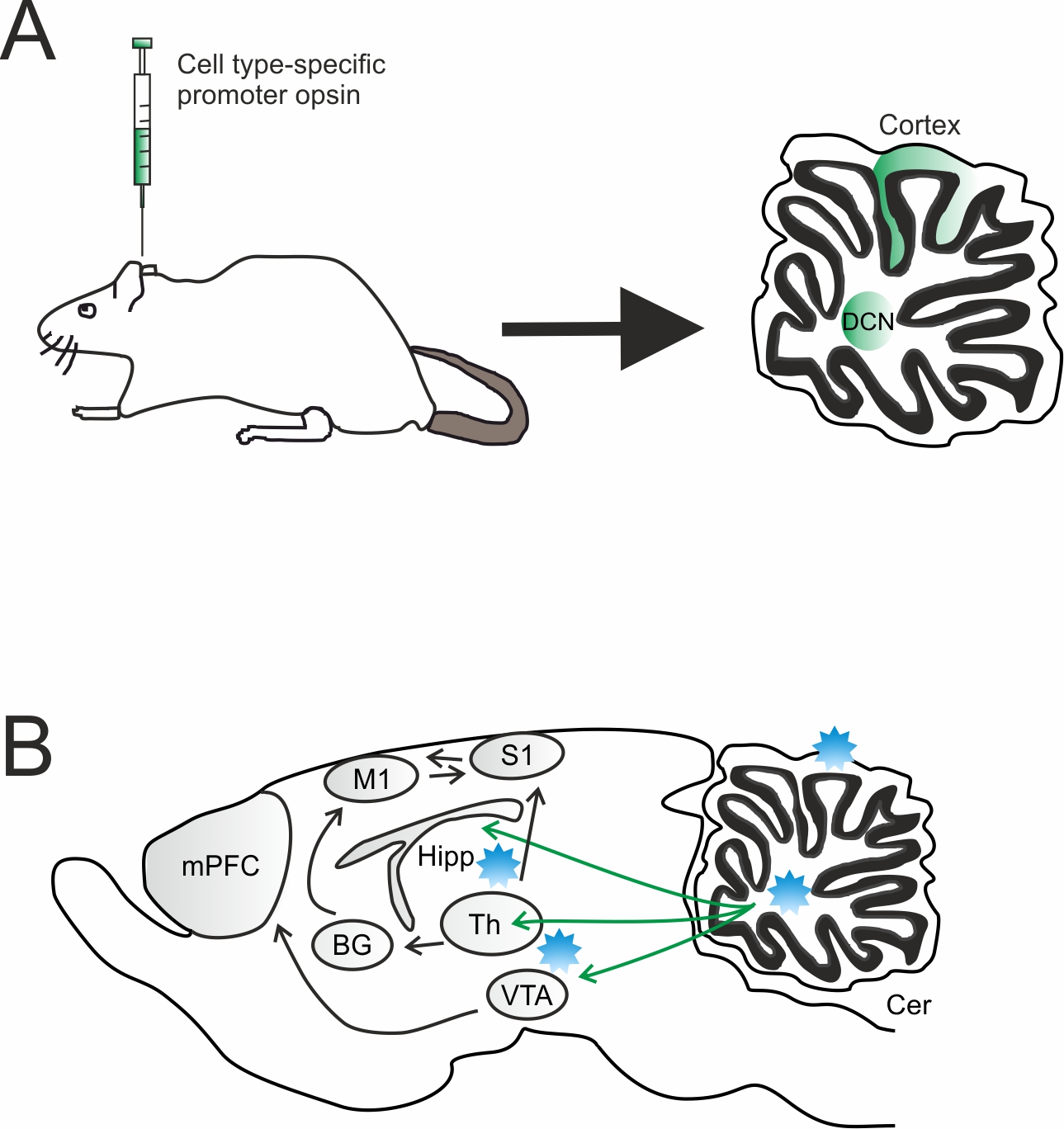
Graphical Abstract: Overview of virus injection and optogenetic stimulation sites. A) Sagittal section showing the infected cerebellar regions. A DNA vector encoding an opsin can be efficiently packaged into adeno-associated virus (AAV) and injected into the targeted cerebellar region [cortex or deep nuclei (DCN)]. In order to achieve cell type specific opsin expression, a cell type-specific promoter or Cre-Lox recombination approach can be used. B) Light is delivered via an optical fiber placed either over the cell bodies to target all projection neurons or in a downstream region to target a specific projection. Cer, cerebellum; mPFC, medial prefrontal cortex; Th, thalamus; BG, basal ganglia; VTA, ventral tegmental area; Hipp, hippocampus; M1, premotor cortex; S1, somatosensory cortex; DCN, deep cerebellar nuclei.
Overview of virus injection and optogenetic stimulation sites. A) Sagittal section showing the infected cerebellar regions. A DNA vector encoding an opsin can be efficiently packaged into adeno-associated virus (AAV) and injected into the targeted cerebellar region [cortex or deep nuclei (DCN)]. In order to achieve cell type specific opsin expression, a cell type-specific promoter or Cre-Lox recombination approach can be used. B) Light is delivered via an optical fiber placed either over the cell bodies to target all projection neurons or in a downstream region to target a specific projection. Cer, cerebellum; mPFC, medial prefrontal cortex; Th, thalamus; BG, basal ganglia; VTA, ventral tegmental area; Hipp, hippocampus; M1, premotor cortex; S1, somatosensory cortex; DCN, deep cerebellar nuclei.
2. Brief Overview of the Cerebellar Anatomy and Microcircuits Organization
Brief Overview of the Cerebellar Anatomy and Microcircuits Organization
The cerebellum is part of the hindbrain, located above the brainstem, and is characterized by two lateral hemispheres and a medial region called the vermis. The whole cerebellum is organized in the deep cerebellar nuclei (DCN), enclosed in the white matter, and in the three layers characterizing the cerebellar cortex. Throughout the whole cortex, the network organization is repeated, with very few differences among regions. In essence, the first set of inputs is conveyed by the mossy fibers, making excitatory synaptic contacts with granule cells and Golgi cells in the granular layer (the inner cortical layer of the cerebellum). Granule cells are excitatory neurons that make synaptic contacts (through the ascending axon or the parallel fibers) with the Golgi cells, molecular layer interneurons, and Purkinje cell (PC) dendrites (in the molecular layer, the external layer of the cerebellar cortex; in between, PCs soma originate the Purkinje cell layer). Interestingly, granule cells are the only excitatory neurons in the cerebellar cortex (except for unipolar brush cells in specific regions), giving rise to an inhibitory organization in feedback and feedforward loops that endow the cortical processing with peculiar features (for a detailed review of this topic see [10][11][12]). Another set of inputs comes from the climbing fibers, originating in the inferior olive. Climbing fibers directly contact PCs, which provide the sole output of the cerebellar cortex. PCs axons make inhibitory synaptic contacts with DCN neurons. The DCN are composed of three sets of nuclei (from medial to lateral): the fastigial, the interpositus (that includes the globose and emboliform nuclei in primates), and the dentate nuclei. Both mossy and climbing fibers send collaterals to the DCN before entering the cerebellar cortex. Therefore, the DCN can compare and integrate the input signals conveyed to the cerebellum and the result of cortical processing of those same inputs. Several studies report that this anatomical organization of cerebellar microcircuits can be divided into modules, operating in loops with the regions of origin of their inputs, being the inferior olive or the rest of the brain [13]. The cerebellar role in both sensorimotor and non-sensorimotor functions likely relies on the brain regions involved in these loops. This is also evident from the recent advances in cerebellar development studies, though this topic is beyond the scope of this review (see for details on this subject [14][15]).
The cerebellum is part of the hindbrain, located above the brainstem, and is characterized by two lateral hemispheres and a medial region called the vermis. The whole cerebellum is organized in the deep cerebellar nuclei (DCN), enclosed in the white matter, and in the three layers characterizing the cerebellar cortex. Throughout the whole cortex, the network organization is repeated, with very few differences among regions. In essence, the first set of inputs is conveyed by the mossy fibers, making excitatory synaptic contacts with granule cells and Golgi cells in the granular layer (the inner cortical layer of the cerebellum). Granule cells are excitatory neurons that make synaptic contacts (through the ascending axon or the parallel fibers) with the Golgi cells, molecular layer interneurons, and Purkinje cell (PC) dendrites (in the molecular layer, the external layer of the cerebellar cortex; in between, PCs soma originate the Purkinje cell layer). Interestingly, granule cells are the only excitatory neurons in the cerebellar cortex (except for unipolar brush cells in specific regions), giving rise to an inhibitory organization in feedback and feedforward loops that endow the cortical processing with peculiar features (for a detailed review of this topic see [10-12]). Another set of inputs comes from the climbing fibers, originating in the inferior olive. Climbing fibers directly contact PCs, which provide the sole output of the cerebellar cortex. PCs axons make inhibitory synaptic contacts with DCN neurons. The DCN are composed of three sets of nuclei (from medial to lateral): the fastigial, the interpositus (that includes the globose and emboliform nuclei in primates), and the dentate nuclei. Both mossy and climbing fibers send collaterals to the DCN before entering the cerebellar cortex. Therefore, the DCN can compare and integrate the input signals conveyed to the cerebellum and the result of cortical processing of those same inputs. Several studies report that this anatomical organization of cerebellar microcircuits can be divided into modules, operating in loops with the regions of origin of their inputs, being the inferior olive or the rest of the brain [13]. The cerebellar role in both sensorimotor and non-sensorimotor functions likely relies on the brain regions involved in these loops. This is also evident from the recent advances in cerebellar development studies, though this topic is beyond the scope of this review (see for details on this subject [14,15]).
3. Pros and Cons of Optogenetics
Pros and Cons of Optogenetics
The obvious advantage of optogenetics is the possibility to modify neuronal activity in a cell-specific or region-specific manner. This is usually achieved using promoters for proteins expressed in specific neuronal subtypes or by localizing the viral injection to confined areas. The second main advantage is the possibility to activate or inhibit the target neurons. Previously, neuronal inhibition was achieved by local lesions, pharmacological tools, or decreasing the temperature in the brain region of interest. All of these approaches have evident disadvantages. Electrical lesions are usually not precisely localized and are not reversible. Optogenetic inhibition of neuronal activity can be achieved with millisecond precision and brief duration, if needed. The pharmacological tool lacks temporal precision, and the reversibility might not be complete. The drop in local temperature is usually achieved by perfusing cold solutions, lacking both temporal and spatial precisions. The high spatial and temporal resolutions of optogenetic modulation of neuronal activity, together with the specificity of cell types and pathways, led to a definitive advance in the study of neuronal activity and connectivity on the basis of complex behaviors and pathological conditions. This was even more evident in the last few years, where technological improvements made miniaturized devices available, allowing us to modify the activity of selected neuronal types and specific connections during a wide range of behaviors and behavioral tasks, providing causal relationships between the optogenetically targeted neurons and the observed behavior.
Though the pros of optogenetics are impressive, some technical issues need to be taken into account. First of all, the need to inject viral constructs when genetically modified animals are not available. Different viral batches may come with different titer and, in every case, the dilution and the volume of the injections must be calibrated each time. This is true even when using the same batch but changing injection location, since different brain areas show different infection levels with the same construct. Moreover, the specificity of the cell type is allowed only where the target cells have unique markers, not common to other cells in the surrounding areas. This condition is not always achievable. Finally, the amount of opsins expressed on neuronal membranes might differ from trial to trial, making the net effect of light-driven neuronal activation/inhibition difficult to reproduce consistently. Nevertheless, the pros of optogenetics use in neuroscience mostly overcomes the cons, and its unique features need to be taken into account when designing experiments and interpreting results. Before proceeding, another critical issue is worth pointing out. Optogenetics is indeed a revolutionary method to excite or inhibit neurons, since it involves the opening of ion channels on their membranes, generating ion fluxes to modify membrane voltage. This condition is very similar to the physiological processes involved during neuronal activity, but it is essential to keep in mind that optogenetic stimulation is very different from the physiological condition. It is not possible to have control over the amount of currents induced in a single neuron or to affect only those neurons that are physiologically activated together by a common pathway. Every neuron expressing the opsins will react when illuminated, therefore activating or inhibiting entire regions. This condition is quantitatively different from physiological activation in terms of current amplitude in single neurons and the number of neurons affected. Though this specification was necessary, a stimulation method acting directly on neurons and mimicking the exact physiological activation is not available at the moment.
Indeed, optogenetics has moved cerebellar research ahead by allowing us to disentangle intertwined pathways at a spatiotemporal precision unmatched by other techniques. In particular, optogenetic tools allowed for (1) the genetic specificity and anatomical strategies controlling the electrical activity of selected cerebellar neurons, (2) the targeting of projections between cerebellum and other brain regions by delivering light to opsin-expressing axons, (3) the link to data obtained from in vitro/in vivo electrophysiological recordings and targeted cerebellar neurons during behavioral tasks and (4) closed-loop interventions in which optical cerebellar stimulation is guided by real-time readouts of ongoing activity [16].
In the following sections, the use of optogenetic tools to dissect the cerebellar role in behavior is divided for investigations of the sensorimotor and non-sensorimotor functions.
4. Sensorimotor Functions
Sensorimotor Functions
The renowned function of the cerebellum is the integration of sensorimotor information. Despite the first reports on this topic dating back almost two centuries ago, the underlying physiological mechanisms remain incompletely defined still at the neuronal and microcircuit levels. The recent development of optogenetics boosted neurophysiological research, providing a suitable tool to investigate the impact of specific neurons or pathways on behavior. Indeed, optogenetics is now primarily employed to stimulate specific components of the cerebellar circuit in order to investigate the cerebellar contribution to perception and motor control. The cerebellum is involved in loops with the cerebral cortex, including motor and sensory areas. Inputs coming to the cerebellum send collaterals to the DCN before entering the cerebellar cortex. The DCN are in the position to integrate the “raw” signal sent to the cerebellum with the results of the cortical processing of the same input. In turn, the DCN convey the integrated signal back to the brain regions of origin. These sensorimotor cortico-cerebellar loops play a crucial role in the fine control of voluntary movements [17][18].
The renowned function of the cerebellum is the integration of sensorimotor information. Despite the first reports on this topic dating back almost two centuries ago, the underlying physiological mechanisms remain incompletely defined still at the neuronal and microcircuit levels. The recent development of optogenetics boosted neurophysiological research, providing a suitable tool to investigate the impact of specific neurons or pathways on behavior. Indeed, optogenetics is now primarily employed to stimulate specific components of the cerebellar circuit in order to investigate the cerebellar contribution to perception and motor control. The cerebellum is involved in loops with the cerebral cortex, including motor and sensory areas. Inputs coming to the cerebellum send collaterals to the DCN before entering the cerebellar cortex. The DCN are in the position to integrate the “raw” signal sent to the cerebellum with the results of the cortical processing of the same input. In turn, the DCN convey the integrated signal back to the brain regions of origin. These sensorimotor cortico-cerebellar loops play a crucial role in the fine control of voluntary movements [17,18].
The main findings made possible by optogenetics in the investigation of the cerebellar role in sensorimotor control and learning include cerebellar mechanisms involved in sensorimotor integration and voluntary movement [17], associative learning [19][20] (Figure 1), eye movements in monkeys [21], movement kinematics [22][23][24], and movement disorders [25].
The main findings made possible by optogenetics in the investigation of the cerebellar role in sensorimotor control and learning include cerebellar mechanisms involved in sensorimotor integration and voluntary movement [17], associative learning [19,20] (Figure 1), eye movements in monkeys [21], movement kinematics [22-24], and movement disorders [25].
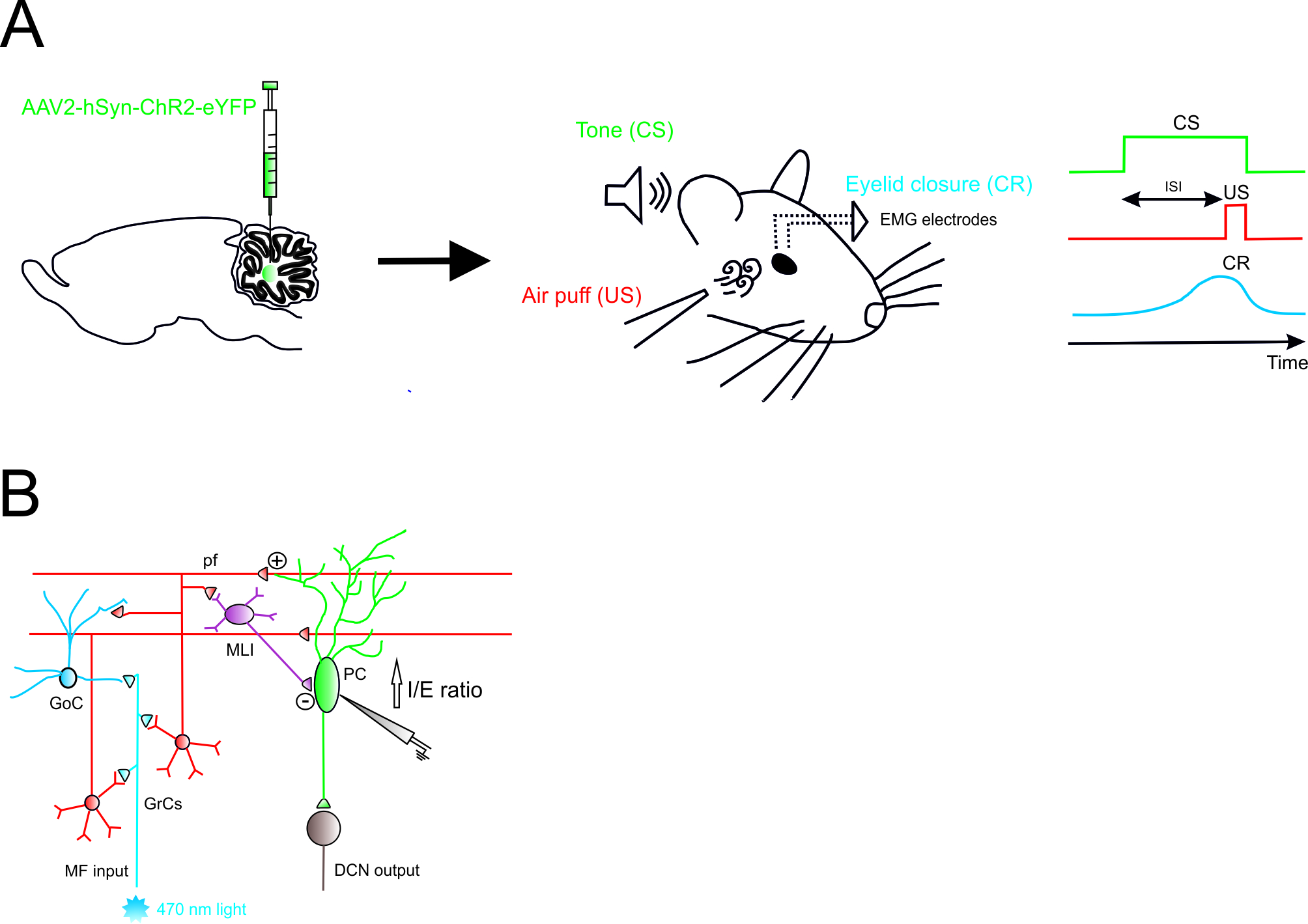
Figure 1.
Role of Excitatory Cerebellar Nucleocortical Circuit in controlling associative motor learning
.
(
A
)
Left
, schematic viral injection of AAV2-hSyn-ChR2-eYFP targeting the interposed nucleus.
Right
, in the eyeblink conditioning (EBC) paradigm, a conditioned stimulus (CS, tone) is presented before the onset of an unconditioned stimulus (US, air puff). The inter-stimulus interval (ISI) is the time interval between CS and US onset. The two stimuli normally co-terminate. During the training, mice learn to generate a delayed conditioned response (CR) to the CS before the onset of the expected US. (
B
) Mossy fiber (MF) afferents can influence deep cerebellar nuclei (DCN) output via projections onto granule cells (GrCs). GrCs can directly affect Purkinje cell (PC) activity via parallel fiber connections or indirectly via feedforward GrC-MLI-PC processing. The EBC paradigm increases the inhibitory/excitatory (I/E) ratio in PCs, indicating a preferential enhancement of the feedforward inhibitory GrC-MLI-PC pathway.
5. Non-Sensorimotor Functions
While the cerebellar role in sensorimotor functions and motor coordination is well established, the nature of its impact on cognition and emotion remains more difficult to address. The connections involved are usually indirect and the convergence of more inputs to associative areas makes it incredibly difficult to detect the specific role of the cerebellum among the contributions of other brain areas. To further complicate the picture, the behavioral counterpart to the cerebellar involvement in non-sensorimotor functions is difficult to retrieve. The understanding of the cerebellar involvement in non-sensorimotor functions was first prompted by the development of functional magnetic resonance imaging (fMRI), during the past 25 years. Recent anatomical, structural and functional evidence has revealed that cerebellar activation is associated with addiction, social cognition and emotional processing [26][27][28][29]. Furthermore, cerebellar lesions are implicated in cognitive disorders and abnormal social behavior such as in autism spectrum disorders (ASD), cognitive affective syndrome, schizophrenia and epilepsy [29][30][31][32][33][34][35][36]. Notably, ASD patients report both motor dysfunctions together with non-motor symptoms, suggesting that cerebellar impairment might indeed contribute to both [31][36][37], likely depending on the connected brain areas [38]. In non-human primates, tract-tracing investigations have demonstrated that topographically distinct regions (called output channels) of the DCN project to different cortical areas: with dorsal parts sending efferent fibers to the motor cortex and ventral parts to the prefrontal and parietal cortexes, which are generally involved in cognitive and higher-order executive functions [39][40]. Recently, neuroimaging studies have reported a similar connectivity topography in human DCN [41][42]. The prefrontal cortex and its extensive connections with other cortical, subcortical and brain stem areas have extensively been investigated. These studies provided evidence for two different pathways through which DCN communicate with the prefrontal cortex. The main pathway involves glutamatergic neurons located in the DCN, which connect to primary thalamic nuclei such as ventrolateral, ventromedial and, additionally, centrolateral nuclei [39][43][44]. The connectivity among the DCN and thalamic nuclei is yet to be fully characterized. It is unclear whether the cerebellum projects to the mediodorsal thalamic nuclei, which are known to provide the main thalamic inputs to the prefrontal cortex [45][46]. Recently, reciprocal connectivity between the prefrontal cortex and ventral thalamic nuclei (specifically ventromedial) has been shown [45][47][48]. The second pathway involves DCN projections to the ventral tegmental area (VTA) which, in turn, send dopaminergic fibers to the prefrontal cortex [49]. A growing body of evidence has reported that VTA dopaminergic projections in the prefrontal cortex, in addition to influencing stress-related function and working memory [50][51], mediate many of the higher-order cognitive functions, including reward, motivation, attention and behavioral flexibility [52][53][54][55]. Notably, alterations in dopaminergic neurotransmission in the prefrontal cortex has been shown in a number of patients diagnosed with schizophrenia and autism [56][57][58]. Recently, it has been shown that the electrical stimulation of DCN was able to indirectly evoke the release of dopamine in the prefrontal cortex (dentate-reticulotegmental-peduncolopontine-VTA-prefrontal cortex pathway) [59][60], suggesting a cerebellar contribution to reward driven behaviors.
While the cerebellar role in sensorimotor functions and motor coordination is well established, the nature of its impact on cognition and emotion remains more difficult to address. The connections involved are usually indirect and the convergence of more inputs to associative areas makes it incredibly difficult to detect the specific role of the cerebellum among the contributions of other brain areas. To further complicate the picture, the behavioral counterpart to the cerebellar involvement in non-sensorimotor functions is difficult to retrieve. The understanding of the cerebellar involvement in non-sensorimotor functions was first prompted by the development of functional magnetic resonance imaging (fMRI), during the past 25 years. Recent anatomical, structural and functional evidence has revealed that cerebellar activation is associated with addiction, social cognition and emotional processing [26-29]. Furthermore, cerebellar lesions are implicated in cognitive disorders and abnormal social behavior such as in autism spectrum disorders (ASD), cognitive affective syndrome, schizophrenia and epilepsy [29-36]. Notably, ASD patients report both motor dysfunctions together with non-motor symptoms, suggesting that cerebellar impairment might indeed contribute to both [31,36,37], likely depending on the connected brain areas [38]. In non-human primates, tract-tracing investigations have demonstrated that topographically distinct regions (called output channels) of the DCN project to different cortical areas: with dorsal parts sending efferent fibers to the motor cortex and ventral parts to the prefrontal and parietal cortexes, which are generally involved in cognitive and higher-order executive functions [39,40]. Recently, neuroimaging studies have reported a similar connectivity topography in human DCN [41,42]. The prefrontal cortex and its extensive connections with other cortical, subcortical and brain stem areas have extensively been investigated. These studies provided evidence for two different pathways through which DCN communicate with the prefrontal cortex. The main pathway involves glutamatergic neurons located in the DCN, which connect to primary thalamic nuclei such as ventrolateral, ventromedial and, additionally, centrolateral nuclei [39,43,44]. The connectivity among the DCN and thalamic nuclei is yet to be fully characterized. It is unclear whether the cerebellum projects to the mediodorsal thalamic nuclei, which are known to provide the main thalamic inputs to the prefrontal cortex [45,46]. Recently, reciprocal connectivity between the prefrontal cortex and ventral thalamic nuclei (specifically ventromedial) has been shown [45,47,48]. The second pathway involves DCN projections to the ventral tegmental area (VTA) which, in turn, send dopaminergic fibers to the prefrontal cortex [49]. A growing body of evidence has reported that VTA dopaminergic projections in the prefrontal cortex, in addition to influencing stress-related function and working memory [50,51], mediate many of the higher-order cognitive functions, including reward, motivation, attention and behavioral flexibility [52-55]. Notably, alterations in dopaminergic neurotransmission in the prefrontal cortex has been shown in a number of patients diagnosed with schizophrenia and autism [56-58]. Recently, it has been shown that the electrical stimulation of DCN was able to indirectly evoke the release of dopamine in the prefrontal cortex (dentate-reticulotegmental-peduncolopontine-VTA-prefrontal cortex pathway) [59,60], suggesting a cerebellar contribution to reward driven behaviors.
The recent findings in the main non-sensorimotor functions addressed using optogenetics are summarized in Table 1, covering the main topics of reward and social behavior [61] (Figure 2), working memory and decision making [62], schizophrenia and cognition [63][64][65], temporal lobe epilepsy and absence seizure [66][67][68], and control of blood pressure.
The recent findings in the main non-sensorimotor functions addressed using optogenetics are summarized in Table 1, covering the main topics of reward and social behavior [61] (Figure 2), working memory and decision making [62], schizophrenia and cognition [63-65], temporal lobe epilepsy and absence seizure [66-68], and control of blood pressure.
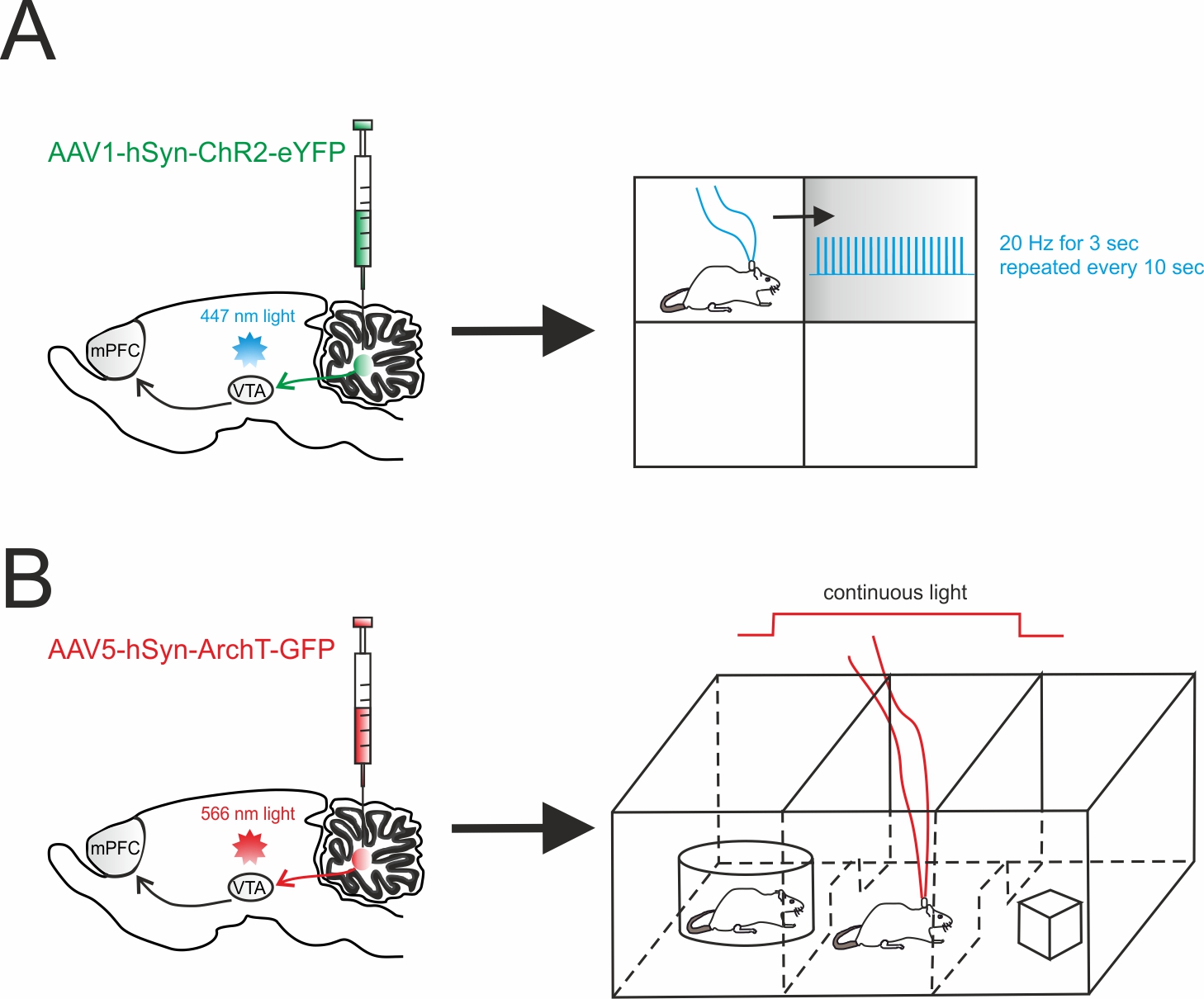
Figure 2.
Cerebellar influence on reward and sociability
.
(
A
)
Left
, schematic viral injection of AAV1-hSyn-ChR2-eYFP targeting the deep cerebellar nuclei (DCN). Optical fibers were implanted into the ventral tegmental area (VTA), bilaterally.
Right
, mice were allowed to explore a square chamber, divided in quadrants, one of which was associated to a reward. Whenever the mouse entered in the reward quadrant, cerebellar axons innervating the VTA were optically stimulated. For the time the mouse spent in the reward quadrant, the stimulus (20 Hz for 3 sec) was repeated every 10s. (
B
)
Left
, AAV5-hSyn-ArchT-GFP was injected in the DCN in order to selectively inhibit cerebellar axons through bilateral optical fibers implantation into the VTA.
Right
, Social preference was examined using a three-chambered social task. The tested mouse was allowed to approach a “stimulus” mouse confined to one side chamber (social side) or a novel object placed on the other side (non-social side). On the first day (training day), the mouse was allowed to freely explore all three chambers. On the subsequent day, the cerebellar axons innervating the VTA were optically inhibited for the time the mouse stayed by the social side. The optogenetic stimulation was immediately ceased when the mouse exited the social side.
Table 1. Viral constructs used in optogenetic manipulation of non-motor behavior.
Viral constructs used in optogenetic manipulation of non-motor behavior.
Author Contributions: Writing-original draft, F.P., I.M., L.M.; visualization, F.P.; writing-review and editing, L.M.; Funding acquisition, E.D. and L.M.
Funding: This work has received funding from: the European Union’s Horizon 2020 Framework Programme for Research and Innovation under the Specific Grant Agreement No. 785907 (Human Brain Project SGA2) to ED; Blue-Sky Research Grant of the University of Pavia (Università degli Studi di Pavia; BSR77992) to LM.
Conflicts of interest: The authors declare no conflict of interest. The funders had no role in the design of the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
|
Serotype |
Promoter |
Expression (Cell type) |
Opsin |
Cerebellar Region |
Behavior |
Behavioral Outcomes |
|
|
AAV1 |
hSyn |
Neuron-specific |
ChR2 |
DCN |
Reward |
Increased place preference [61] |
|
|
AAV5 |
CAG |
All cells |
ArchT |
DCN |
Social behavior |
Altered social preference [61] |
|
|
|
Pcp2-Cre* |
Purkinje cells |
ChR2 |
Cortex (simplex) |
Epilepsy |
Reduction in hippocampal seizure duration and seizure-induced inhibition[69] |
|
|
AAV9 |
VGluT-Cre* |
Glutamatergic |
ChR2 |
DCN (fastigial) |
Epilepsy |
Reduction in hippocampal seizure duration [67] |
|
|
AAV2 |
hSyn |
Neuron-specific |
ChR2 |
DCN (dentate) |
Epilepsy |
Reduction in thalamocortical oscillations [68] |
|
|
AAV2/9 |
Pcp2-Cre* |
Purkinje cells |
ChR2 |
Cortex (Crus I) |
Working memory |
Reduction in performance accuracy [62] |
|
|
Lentivirus |
L7 |
Purkinje cells |
eNpHR3.0 |
Cortex (uvula) |
Postural alterations |
Reduction in the extent of blood pressure recovery [70] |
|
|
AAV |
CamKII |
Glutamatergic |
ChR2 |
DCN (dentate) |
Schizophrenia |
Increase in prefrontal activity [65] |
|
|
Serotype |
Promoter |
Expression (Cell type) |
Opsin |
Cerebellar Region |
Behavior |
Behavioral Outcomes |
|
|
AAV1 |
hSyn |
Neuron-specific |
ChR2 |
DCN |
Reward |
Increased place preference [61] |
|
|
AAV5 |
CAG |
All cells |
ArchT |
DCN |
Social behavior |
Altered social preference [61] |
|
|
|
Pcp2-Cre* |
Purkinje cells |
ChR2 |
Cortex (simplex) |
Epilepsy |
Reduction in hippocampal seizure duration and seizure-induced inhibition[69] |
|
|
AAV9 |
VGluT-Cre* |
Glutamatergic |
ChR2 |
DCN (fastigial) |
Epilepsy |
Reduction in hippocampal seizure duration [67] |
|
|
AAV2 |
hSyn |
Neuron-specific |
ChR2 |
DCN (dentate) |
Epilepsy |
Reduction in thalamocortical oscillations [68] |
|
|
AAV2/9 |
Pcp2-Cre* |
Purkinje cells |
ChR2 |
Cortex (Crus I) |
Working memory |
Reduction in performance accuracy [62] |
|
|
Lentivirus |
L7 |
Purkinje cells |
eNpHR3.0 |
Cortex (uvula) |
Postural alterations |
Reduction in the extent of blood pressure recovery [70] |
|
|
AAV |
CamKII |
Glutamatergic |
ChR2 |
DCN (dentate) |
Schizophrenia |
Increase in prefrontal activity [65] |
|
Transgenic lines that have been used in mouse studies are denoted by an asterisk (*).
- Malacarne, V. Nuova Esposizione della Vera Struttura del Cervelletto Umano; Briolo, Ed. Torino (Italy), 1776; pp. 129.
- Flourens, P. Recherchers experimentales sur le proprietes et les functions du systeme nerveux dans les animaux vertebres; Crevot: Paris, 1824.
- Schmahmann, J.D.; Sherman, J.C. The cerebellar cognitive affective syndrome. Brain 1998, 121 ( Pt 4), 561-579, doi:10.1093/brain/121.4.561.
- Schmahmann, J.D. Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci 1998, 2, 362-371, doi:10.1016/s1364-6613(98)01218-2.
- Timmann, D. [Contribution of the cerebellum to cognition]. Fortschr Neurol Psychiatr 2012, 80, 44-52, doi:10.1055/s-0031-1282022.
- Baillieux, H.; De Smet, H.J.; Paquier, P.F.; De Deyn, P.P.; Mariën, P. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg 2008, 110, 763-773, doi:10.1016/j.clineuro.2008.05.013.
- Gottwald, B.; Wilde, B.; Mihajlovic, Z.; Mehdorn, H.M. Evidence for distinct cognitive deficits after focal cerebellar lesions. J Neurol Neurosurg Psychiatry 2004, 75, 1524-1531, doi:10.1136/jnnp.2003.018093.
- Schmahmann, J.D. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 2004, 16, 367-378, doi:10.1176/appi.neuropsych.16.3.367.
- Manto, M. Cerebellar motor syndrome from children to the elderly. Handb Clin Neurol 2018, 154, 151-166, doi:10.1016/B978-0-444-63956-1.00009-6.
- Prestori, F.; Mapelli, L.; D'Angelo, E. Diverse Neuron Properties and Complex Network Dynamics in the Cerebellar Cortical Inhibitory Circuit. Front Mol Neurosci 2019, 12, 267, doi:10.3389/fnmol.2019.00267.
- D'Angelo, E.; Solinas, S.; Mapelli, J.; Gandolfi, D.; Mapelli, L.; Prestori, F. The cerebellar Golgi cell and spatiotemporal organization of granular layer activity. Front Neural Circuits 2013, 7, 93, doi:10.3389/fncir.2013.00093.
- Mapelli, L.; Solinas, S.; D'Angelo, E. Integration and regulation of glomerular inhibition in the cerebellar granular layer circuit. Front Cell Neurosci 2014, 8, 55, doi:10.3389/fncel.2014.00055.
- Apps, R.; Hawkes, R.; Aoki, S.; Bengtsson, F.; Brown, A.M.; Chen, G.; Ebner, T.J.; Isope, P.; Jörntell, H.; Lackey, E.P., et al. Cerebellar Modules and Their Role as Operational Cerebellar Processing Units: A Consensus paper [corrected]. Cerebellum 2018, 17, 654-682, doi:10.1007/s12311-018-0952-3.
- Leto, K.; Arancillo, M.; Becker, E.B.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.B.; Dusart, I.; Haldipur, P.; Hatten, M.E., et al. Consensus Paper: Cerebellar Development. Cerebellum 2016, 15, 789-828, doi:10.1007/s12311-015-0724-2.
- Marzban, H.; Del Bigio, M.R.; Alizadeh, J.; Ghavami, S.; Zachariah, R.M.; Rastegar, M. Cellular commitment in the developing cerebellum. Front Cell Neurosci 2014, 8, 450, doi:10.3389/fncel.2014.00450.
- Kim, C.K.; Adhikari, A.; Deisseroth, K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci 2017, 18, 222-235, doi:10.1038/nrn.2017.15.
- Proville, R.D.; Spolidoro, M.; Guyon, N.; Dugue, G.P.; Selimi, F.; Isope, P.; Popa, D.; Lena, C. Cerebellum involvement in cortical sensorimotor circuits for the control of voluntary movements. Nat Neurosci 2014, 17, 1233-1239, doi:10.1038/nn.3773.
- Kelly, R.M.; Strick, P.L. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 2003, 23, 8432-8444.
- Albergaria, C.; Silva, N.T.; Pritchett, D.L.; Carey, M.R. Locomotor activity modulates associative learning in mouse cerebellum. Nat Neurosci 2018, 21, 725-735, doi:10.1038/s41593-018-0129-x.
- Gao, Z.; Proietti-Onori, M.; Lin, Z.; Ten Brinke, M.M.; Boele, H.J.; Potters, J.W.; Ruigrok, T.J.; Hoebeek, F.E.; De Zeeuw, C.I. Excitatory Cerebellar Nucleocortical Circuit Provides Internal Amplification during Associative Conditioning. Neuron 2016, 89, 645-657, doi:10.1016/j.neuron.2016.01.008.
- El-Shamayleh, Y.; Kojima, Y.; Soetedjo, R.; Horwitz, G.D. Selective Optogenetic Control of Purkinje Cells in Monkey Cerebellum. Neuron 2017, 95, 51-62.e54, doi:10.1016/j.neuron.2017.06.002.
- Sarnaik, R.; Raman, I.M. Control of voluntary and optogenetically perturbed locomotion by spike rate and timing of neurons of the mouse cerebellar nuclei. In eLife, 2018; Vol. 7.
- Payne, H.L.; French, R.L.; Guo, C.C.; Nguyen-Vu, T.B.; Manninen, T.; Raymond, J.L. Cerebellar Purkinje cells control eye movements with a rapid rate code that is invariant to spike irregularity. Elife 2019, 8, doi:10.7554/eLife.37102.
- Jelitai, M.; Puggioni, P.; Ishikawa, T.; Rinaldi, A.; Duguid, I. Dendritic excitation-inhibition balance shapes cerebellar output during motor behaviour. Nat Commun 2016, 7, 13722, doi:10.1038/ncomms13722.
- Chen, C.H.; Fremont, R.; Arteaga-Bracho, E.E.; Khodakhah, K. Short latency cerebellar modulation of the basal ganglia. Nat Neurosci 2014, 17, 1767-1775, doi:10.1038/nn.3868.
- Van Overwalle, F.; D'Aes, T.; Marien, P. Social cognition and the cerebellum: A meta-analytic connectivity analysis. Hum Brain Mapp 2015, 36, 5137-5154, doi:10.1002/hbm.23002.
- Moulton, E.A.; Elman, I.; Becerra, L.R.; Goldstein, R.Z.; Borsook, D. The cerebellum and addiction: insights gained from neuroimaging research. Addict Biol 2014, 19, 317-331, doi:10.1111/adb.12101.
- Schmahmann, J.D.; Caplan, D. Cognition, emotion and the cerebellum. Brain 2006, 129, 290-292, doi:10.1093/brain/awh729.
- D'Angelo, E. The cerebellum gets social. Science 2019, 363, 229, doi:10.1126/science.aaw2571.
- Courchesne, E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol 1997, 7, 269-278.
- Soda, T.; Mapelli, L.; Locatelli, F.; Botta, L.; Goldfarb, M.; Prestori, F.; D'Angelo, E. Hyperexcitability and Hyperplasticity Disrupt Cerebellar Signal Transfer in the IB2 KO Mouse Model of Autism. J Neurosci 2019, 39, 2383-2397, doi:10.1523/jneurosci.1985-18.2019.
- Badura, A.; Verpeut, J.L.; Metzger, J.W.; Pereira, T.D.; Pisano, T.J.; Deverett, B.; Bakshinskaya, D.E.; Wang, S.S. Normal cognitive and social development require posterior cerebellar activity. Elife 2018, 7, doi:10.7554/eLife.36401.
- Wang, S.S.; Kloth, A.D.; Badura, A. The cerebellum, sensitive periods, and autism. Neuron 2014, 83, 518-532, doi:10.1016/j.neuron.2014.07.016.
- Andreasen, N.C.; Pierson, R. The role of the cerebellum in schizophrenia. Biol Psychiatry 2008, 64, 81-88, doi:10.1016/j.biopsych.2008.01.003.
- Picard, H.; Amado, I.; Mouchet-Mages, S.; Olie, J.P.; Krebs, M.O. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull 2008, 34, 155-172, doi:10.1093/schbul/sbm049.
- Giza, J.; Urbanski, M.J.; Prestori, F.; Bandyopadhyay, B.; Yam, A.; Friedrich, V.; Kelley, K.; D'Angelo, E.; Goldfarb, M. Behavioral and cerebellar transmission deficits in mice lacking the autism-linked gene islet brain-2. J Neurosci 2010, 30, 14805-14816, doi:10.1523/JNEUROSCI.1161-10.2010.
- Whyatt, C.; Craig, C. Sensory-motor problems in Autism. Front Integr Neurosci 2013, 7, 51, doi:10.3389/fnint.2013.00051.
- Schmahmann, J.D.; Guell, X.; Stoodley, C.J.; Halko, M.A. The Theory and Neuroscience of Cerebellar Cognition. Annu Rev Neurosci 2019, 42, 337-364, doi:10.1146/annurev-neuro-070918-050258.
- Strick, P.L.; Dum, R.P.; Fiez, J.A. Cerebellum and nonmotor function. Annu Rev Neurosci 2009, 32, 413-434, doi:10.1146/annurev.neuro.31.060407.125606.
- Dum, R.P.; Strick, P.L. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol 2003, 89, 634-639, doi:10.1152/jn.00626.2002.
- Steele, C.J.; Anwander, A.; Bazin, P.L.; Trampel, R.; Schaefer, A.; Turner, R.; Ramnani, N.; Villringer, A. Human Cerebellar Sub-millimeter Diffusion Imaging Reveals the Motor and Non-motor Topography of the Dentate Nucleus. Cereb Cortex 2017, 27, 4537-4548, doi:10.1093/cercor/bhw258.
- Bernard, J.A.; Peltier, S.J.; Benson, B.L.; Wiggins, J.L.; Jaeggi, S.M.; Buschkuehl, M.; Jonides, J.; Monk, C.S.; Seidler, R.D. Dissociable Functional Networks of the Human Dentate Nucleus. In Cereb Cortex, 2014; Vol. 24, pp. 2151-2159.
- Magnotta, V.A.; Adix, M.L.; Caprahan, A.; Lim, K.; Gollub, R.; Andreasen, N.C. Investigating connectivity between the cerebellum and thalamus in schizophrenia using diffusion tensor tractography: a pilot study. Psychiatry Res 2008, 163, 193-200, doi:10.1016/j.pscychresns.2007.10.005.
- Gornati, S.V.; Schafer, C.B.; Eelkman Rooda, O.H.J.; Nigg, A.L.; De Zeeuw, C.I.; Hoebeek, F.E. Differentiating Cerebellar Impact on Thalamic Nuclei. Cell Rep 2018, 23, 2690-2704, doi:10.1016/j.celrep.2018.04.098.
- Parnaudeau, S.; O'Neill, P.K.; Bolkan, S.S.; Ward, R.D.; Abbas, A.I.; Roth, B.L.; Balsam, P.D.; Gordon, J.A.; Kellendonk, C. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron 2013, 77, 1151-1162, doi:10.1016/j.neuron.2013.01.038.
- Ferguson, B.R.; Gao, W.J. Thalamic Control of Cognition and Social Behavior Via Regulation of Gamma-Aminobutyric Acidergic Signaling and Excitation/Inhibition Balance in the Medial Prefrontal Cortex. Biol Psychiatry 2018, 83, 657-669, doi:10.1016/j.biopsych.2017.11.033.
- Sieveritz, B.; Garcia-Munoz, M.; Arbuthnott, G.W. Thalamic afferents to prefrontal cortices from ventral motor nuclei in decision-making. Eur J Neurosci 2019, 49, 646-657, doi:10.1111/ejn.14215.
- Collins, D.P.; Anastasiades, P.G.; Marlin, J.J.; Carter, A.G. Reciprocal Circuits Linking the Prefrontal Cortex with Dorsal and Ventral Thalamic Nuclei. Neuron 2018, 98, 366-379.e364, doi:10.1016/j.neuron.2018.03.024.
- Rogers, T.D.; Dickson, P.E.; McKimm, E.; Heck, D.H.; Goldowitz, D.; Blaha, C.D.; Mittleman, G. Reorganization of circuits underlying cerebellar modulation of prefrontal cortical dopamine in mouse models of autism spectrum disorder. Cerebellum 2013, 12, 547-556, doi:10.1007/s12311-013-0462-2.
- Thierry, A.M.; Tassin, J.P.; Blanc, G.; Glowinski, J. Selective activation of mesocortical DA system by stress. Nature 1976, 263, 242-244, doi:10.1038/263242a0.
- Brozoski, T.J.; Brown, R.M.; Rosvold, H.E.; Goldman, P.S. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 1979, 205, 929-932, doi:10.1126/science.112679.
- Chudasama, Y.; Robbins, T.W. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology 2004, 29, 1628-1636, doi:10.1038/sj.npp.1300490.
- Floresco, S.B. Prefrontal dopamine and behavioral flexibility: shifting from an "inverted-U" toward a family of functions. Front Neurosci 2013, 7, 62, doi:10.3389/fnins.2013.00062.
- Braver, T.S.; Krug, M.K.; Chiew, K.S.; Kool, W.; Westbrook, J.A.; Clement, N.J.; Adcock, R.A.; Barch, D.M.; Botvinick, M.M.; Carver, C.S., et al. Mechanisms of motivation-cognition interaction: challenges and opportunities. Cogn Affect Behav Neurosci 2014, 14, 443-472, doi:10.3758/s13415-014-0300-0.
- Westbrook, A.; Braver, T.S. Dopamine Does Double Duty in Motivating Cognitive Effort. Neuron 2016, 91, 708, doi:10.1016/j.neuron.2016.07.020.
- Howes, O.D.; Kapur, S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull 2009, 35, 549-562, doi:10.1093/schbul/sbp006.
- Ernst, M.; Zametkin, A.J.; Matochik, J.A.; Pascualvaca, D.; Cohen, R.M. Low medial prefrontal dopaminergic activity in autistic children. In Lancet, England, 1997; Vol. 350, p. 638.
- Nakamura, K.; Sekine, Y.; Ouchi, Y.; Tsujii, M.; Yoshikawa, E.; Futatsubashi, M.; Tsuchiya, K.J.; Sugihara, G.; Iwata, Y.; Suzuki, K., et al. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry 2010, 67, 59-68, doi:10.1001/archgenpsychiatry.2009.137.
- Mittleman, G.; Goldowitz, D.; Heck, D.; Blaha, C. Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse 2008, 62, 544-550, doi:10.1002/syn.20525.
- Rogers, T.D.; Dickson, P.E.; Heck, D.H.; Goldowitz, D.; Mittleman, G.; Blaha, C.D. Connecting the dots of the cerebro-cerebellar role in cognitive function: neuronal pathways for cerebellar modulation of dopamine release in the prefrontal cortex. Synapse 2011, 65, 1204-1212, doi:10.1002/syn.20960.
- Carta, I.; Chen, C.H.; Schott, A.L.; Dorizan, S.; Khodakhah, K. Cerebellar modulation of the reward circuitry and social behavior. Science 2019, 363, doi:10.1126/science.aav0581.
- Deverett, B.; Kislin, M.; Tank, D.W.; Wang, S.S. Cerebellar disruption impairs working memory during evidence accumulation. Nat Commun 2019, 10, 3128, doi:10.1038/s41467-019-11050-x.
- Parker, K.L.; Narayanan, N.S.; Andreasen, N.C. The therapeutic potential of the cerebellum in schizophrenia. Front Syst Neurosci 2014, 8, 163, doi:10.3389/fnsys.2014.00163.
- Parker, K.L. Timing Tasks Synchronize Cerebellar and Frontal Ramping Activity and Theta Oscillations: Implications for Cerebellar Stimulation in Diseases of Impaired Cognition. Front Psychiatry 2015, 6, 190, doi:10.3389/fpsyt.2015.00190.
- Parker, K.L.; Kim, Y.C.; Kelley, R.M.; Nessler, A.J.; Chen, K.H.; Muller-Ewald, V.A.; Andreasen, N.C.; Narayanan, N.S. Delta-frequency stimulation of cerebellar projections can compensate for schizophrenia-related medial frontal dysfunction. Mol Psychiatry 2017, 22, 647-655, doi:10.1038/mp.2017.50.
- Krook-Magnuson, E.I.; Li, P.; Paluszkiewicz, S.M.; Huntsman, M.M. Tonically active inhibition selectively controls feedforward circuits in mouse barrel cortex. J Neurophysiol 2008, 100, 932-944, doi:10.1152/jn.01360.2007.
- Streng, M.L.; Krook-Magnuson, E. Excitation, but not inhibition, of the fastigial nucleus provides powerful control over temporal lobe seizures. J Physiol 2020, 598, 171-187, doi:10.1113/jp278747.
- Kros, L.; Eelkman Rooda, O.H.; Spanke, J.K.; Alva, P.; van Dongen, M.N.; Karapatis, A.; Tolner, E.A.; Strydis, C.; Davey, N.; Winkelman, B.H., et al. Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann Neurol 2015, 77, 1027-1049, doi:10.1002/ana.24399.
- Krook-Magnuson, E.; Szabo, G.G.; Armstrong, C.; Oijala, M.; Soltesz, I. Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. eNeuro 2014, 1, doi:10.1523/eneuro.0005-14.2014.
- Tsubota, T.; Ohashi, Y.; Tamura, K.; Miyashita, Y. Optogenetic inhibition of Purkinje cell activity reveals cerebellar control of blood pressure during postural alterations in anesthetized rats. Neuroscience 2012, 210, 137-144, doi:10.1016/j.neuroscience.2012.03.014.
- Miterko, L.N.; Baker, K.B.; Beckinghausen, J.; Bradnam, L.V.; Cheng, M.Y.; Cooperrider, J.; DeLong, M.R.; Gornati, S.V.; Hallett, M.; Heck, D.H., et al. Consensus Paper: Experimental Neurostimulation of the Cerebellum. Cerebellum 2019, 18, 1064-1097, doi:10.1007/s12311-019-01041-5.
- Baker, C.K.; Flannery, J.G. Innovative Optogenetic Strategies for Vision Restoration. Front Cell Neurosci 2018, 12, 316, doi:10.3389/fncel.2018.00316.
- Delbeke, J.; Hoffman, L.; Mols, K.; Braeken, D.; Prodanov, D. And Then There Was Light: Perspectives of Optogenetics for Deep Brain Stimulation and Neuromodulation. Front Neurosci 2017, 11, 663, doi:10.3389/fnins.2017.00663.
Transgenic lines that have been used in mouse studies are denoted by an asterisk (*)