Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Marilena Mangiardi and Version 2 by Lindsay Dong.
Cerebral collateral circulation is a network of blood vessels which stabilizes blood flow and maintains cerebral perfusion whenever the main arteries fail to provide an adequate blood supply, as happens in ischemic stroke. These arterial networks are able to divert blood flow to hypoperfused cerebral areas. The extent of the collateral circulation determines the volume of the salvageable tissue, the so-called “penumbra”. Clinically, this is associated with greater efficacy of reperfusion therapies (thrombolysis and thrombectomy) in terms of better short- and long-term functional outcomes, lower incidence of hemorrhagic transformation and of malignant oedema, and smaller cerebral infarctions.
- acute ischemic stroke
- collateral circulations
- penumbra
- thrombectomy
- intravenous thrombolysis
- anterior brain circulation
1. Introduction
In recent times, researchers have bestowed significant attention upon the role of collateral circulation in acute ischemic stroke due to its correlation with patient outcomes. It has been demonstrated that stroke patients with inadequate collateral circulation experience a four-fold higher rate of deterioration compared to others [1]. The presence of robust collateral networks can be considered an independent predictor of improved functional outcomes at the 90-day mark [2].
The significance of collateral circulation in the brain stems from the core/penumbra concept, which refers to the observation that a gradient of hypoperfusion is observed during acute ischemic stroke. This gradient ranges from a central region of severe hypoperfusion to peripheral areas with mild hypoperfusion. Visualizing this phenomenon, three concentric zones can be envisioned: a “core” region characterized by severe hypoperfusion, a “penumbra” area where neural cells are dysfunctional yet salvageable with adequate perfusion restoration through intravenous thrombolysis (IVT) and/or mechanical thrombectomy (MT), and an “oligemia” zone where hypoperfusion is mild and recovery is expected even without treatment.
Notably, it has been observed that tissue subjected to severe hypoperfusion has a shorter survival period, and the penumbra tissue will inevitably progress to irreversible damage if perfusion is not restored. The collateral circulation determines the fate of the penumbra, although it cannot indefinitely salvage it [3]. An inverse correlation exists among total infarct volume, collateral grade (indicating the extent of vascularity), and clinical outcome (assessed via the modified Rankin scale at discharge). Due to variations in vascular arrangement, collaterals afford greater protection to the cortex compared to deep tissues [4]. Additionally, for every 10 mL increase in pretreatment ischemic core volume, the odds of achieving a favorable functional outcome diminish by 20–30% [5].
The recognition that the core expands over time in the absence of restored perfusion has had a profound impact, leading to the development of revascularization procedures that are currently the only therapeutic strategies capable of altering the prognosis of stroke patients. The timing of intervention directly influences the impact on functional outcomes and the reduction of associated complications, such as hemorrhagic transformation [6]. Indeed, the most recent guidelines recommend thrombolysis within 4.5 h without advanced imaging (CT perfusion) or within 9 h with CT perfusion, as well as thrombectomy within 6 h without CT perfusion or within 24 h if CT perfusion is available [7]. Although IVT is currently recommended prior to MT, there is a debate about whether IVT is necessary for patients directly arriving in the stroke center with thrombectomy expertise.
2. Anatomy and Neurophysiology
2.1. The Anatomy of Collateral Anterior and Posterior Circulation
The cerebral collateral circulation denotes the intricate network of blood vessels responsible for supplying the brain parenchyma with an adequate blood and oxygen supply in situations where the main arteries fail to function properly. Within the anterior circulation, a distinction can be made between primary collaterals, represented by the components comprising the circle of Willis, and secondary collaterals, encompassing the ophthalmic artery and leptomeningeal vessels. Collateral vessels can be established between intra- and extra-cranial vessels, including: (1) the branches of the external carotid artery, the cavernous segment of the internal carotid artery, and ophthalmic artery, and (2) the dural anastomosis connecting the distal branches of the middle and occipital meningeal arteries with cerebral arteries. Additionally, collaterals between intracranial arteries include: (1) basal collaterals involving communication through the vessels forming the circle of Willis (circulus arteriosus), (2) superficial collaterals consisting of pial communication through the leptomeningeal artery, and (3) intraparenchymal collaterals encompassing small precapillary anastomoses between the branches of the perforating arteries [4].2.2. The Role of Posterior Circulation
Despite contributing only approximately one third of the overall cerebral blood flow, the posterior cerebral circulation remains responsible for numerous vital functions within the nervous system. It arises from the paired vertebral arteries and a singular basilar artery to provide blood supply to the inferior thalamus, temporal and occipital lobes, cerebellum, and brainstem. The vascular reserve within the vertebro-basilar circulation includes bidirectional flow through the anterior inferior cerebellar artery, posterior inferior cerebellar artery), and cerebellar leptomeningeal vessels [6]. The leptomeningeal connections between the arteries of the cerebellum resemble those found in the cerebral pial network. These connections can reverse the blood flow through the tributaries of the basilar artery. Blood flow direction can be reversed due to hemodynamic connections involving the posterior communicating artery (PComA), the first segment of the posterior cerebral artery (PCA), and the carotid circulation. In cases of basilar artery occlusion, the PComAs reverse the blood flow through the basilar artery’s bifurcation, the PCA, and the superior cerebellar artery (SCA) located in the quadrigeminal plate [7].2.3. Collateral Circulation Risk Factors and Genetic Liability
The extent of collaterals at baseline seems to be dependent on genetic factors [8][9]. Aging plays an important role too, as several studies have shown a rarefaction of cerebral arterioles, a decrease in capillary density and a decrease in the number of venules and arteriole-to-arteriole anastomoses in aged humans and animal models [9][10]. It was observed that the rarefaction of collaterals leads to a failure of collaterals recruitment with an impaired pial vessel dynamic (a reduced red blood cell velocity) which worsens tissue perfusion [10][11][11,12]. Moreover, with age progression, there appears to be a decrease in vessel diameter, an increased vessel tortuosity, and, consequently, increased vascular resistances and larger infarct volumes. Aging also seems to inhibit vascular remodeling. These findings may be related to a decreased expression of VEGF and to a reduced synthesis of NO [12][13]. Sex differences also seem to play a role: it has been observed that women have a better collateral status compared to men, but the reasons for a such difference remain unclear. Studies in animals have found no difference between male and female subjects in terms of structure and function for what concerns the leptomeningeal anastomoses and the circle of Willis [13][14]. Nevertheless, women were found to have higher mortality and longer hospitalizations after a stroke compared to men. Vascular risk factors, such as diabetes and hypertension, have been indicated as responsible for impaired cerebral autoregulation and have been associated with larger infarct volumes [14][15]. Epidemiological studies have shown that women have a higher prevalence of hypertension and atrial fibrillation, but a lower prevalence of heart disease, peripheral vascular disease, smoking, and alcohol use [15][16]. Hypertension was indeed found to be associated with poor collateral status and little salvageable tissue [16][17]. Smoking, chronic kidney disease, and metabolic syndrome have also been associated with poor collaterals [17][18].2.4. The Role of Cerebral Hypoperfusion
The normal cerebral blood flow (CBF) is typically around 50–60 mL/100 g/min. However, during hypoperfusion, when the CBF drops to a range of 10 to 20 mL/100 g/min, cellular functions are interrupted, but the cells remain structurally intact. Once the CBF falls below this threshold, the cells begin to undergo death within a matter of minutes [18][19]. Consequently, collaterals assume a vital role in substituting for the main arteries when they fail to deliver an adequate blood supply and maintain sufficient levels of CBF and cerebral blood volume (CBV). Such circumstances may arise from sudden occlusion of the blood vessels or as a consequence of chronic blood flow reduction.2.5. Collateral Circulation and Neuromodulation
Many growth factors are indeed upregulated after ischemia: TGF-alfa, TGF-beta- TNF-alfa- IL-8, and, most importantly, VEGF. However, there are some doubts that the newly formed vessels are truly functional because, even though these molecular mechanisms lead to the formation of new microvessels, this process seems to be aimed at macrophage infiltration and at the removal of cellular debris from the necrotic tissue. In addition to this, the newly formed microvessels are maintained for a limited time only and their regression can be observed already after 30 days since reperfusion. In any case, data seem to indicate that they are beneficial to neurovascular repair [19][20][24,25]. In contrast to angiogenesis, which is the formation of new capillaries by sprouting or “de novo” and is triggered by oxygen deprivation, arteriogenesis is the remodeling of existing arterio-arteriolar anastomoses toward fully functional arteries. Arteriogenesis is initiated by fluid shear stress (FSS) following sudden occlusion or progressive stenosis in an arterial vessel. This process encompasses various interconnected events, including endothelial activation, monocytic cell recruitment, promotion of inflammatory processes, secretion of growth factors and cytokines, extracellular matrix digestion, and proliferation of smooth muscle cells, all contributing to the outward remodeling of collateral vessels [21][26]. FSS refers to the tangential frictional forces exerted by the flow of blood, that is a viscous fluid, against the vessel wall. Arterial occlusion leads to a decrease in distal vascular pressure, resulting in increased flow through pre-existing collaterals due to the pressure gradient. Initially, the increased FSS on the endothelium triggers a cascade of signaling events. As the collaterals grow in diameter, FSS decreases, and collateral flow decreases, establishing a self-regulating mechanism. Endothelial cells lining the blood vessel lumen have the ability to sense changes in flow shear stress and transmit signals to smooth muscle cells in the tunica media and fibroblasts in the tunica adventitia. Microarray data have identified a potential role for the shear stress-sensitive gene transient receptor potential cation channel subfamily V member 4 (Trpv4), a calcium channel found in endothelial cells. Pharmacological activation of Trpv4 has been shown to strongly enhance cerebral arteriogenesis and collateral flow, while blocking Trpv channels reduces collateral flow [22][23][27,28]. The signal generated by FSS is likely transmitted through nitric oxide (NO), which can diffuse through the basement membrane separating the endothelial cells from the smooth muscle cells and can interact with prostacyclin. Although the initiation of arteriogenesis does not necessarily require the presence of hypoxia-inducible factors (HIFs), hypoxia activates signaling pathways that lead to the production of various molecules, including VEGF and inflammatory cytokines. HIF-1 also promotes the secretion of integrin β2, which, in turn, induces the adhesion and infiltration of leukocytes and vascular progenitor cells into hypoxic sites [24][25][29,30]. The deletion of one allele of the Phd2 gene not only induces a shift in macrophages towards an M2 phenotype but also enhances arteriogenesis following hindlimb ischemia. These infiltrating macrophages play a crucial role in producing numerous angiogenic growth factors, including MCP-1, VEGF, FGF, GM-CSF, HGF, TNF-α, TGF-β, and PDGF, which are essential for the development and maturation of collaterals. Arteriogenesis shares certain similarities with an active inflammatory process.2.6. The Role of the “Core” and the “Penumbra”
In recent years the collateral circulation has received much attention from researchers because it has been shown to be related to the outcome of patients with stroke. Stroke patients with diminished collaterals have a rate of worsening four times higher and a better collateral status can be considered an independent predictor of an improved functional outcome [26][38]. A good collateral circulation was associated also with better functional recovery at 90 days [27][39]. The importance of the brain collateral circulation arises from the core/penumbra concept: the observation that, in the stroke setting, it is possible to observe a gradient of hypoperfusion, ranging from an area of severe hypoperfusion to the most peripherical regions where hypoperfusion is mild. One may thus imagine three concentric zones: a “core” where hypoperfusion is severe, the “penumbra” where neural cells are dysfunctional but still salvageable if adequate perfusion is restored, and the “oligemia” where hypoperfusion is mild. An inverse correlation exists between total infarct volume, collateral grade (a measure of the extent of vascularity as measured at the Sylvian fissure and the cerebral convexity), and clinical outcome (expressed as modified Rankin scale at discharge) and because of the different vascular arrangement, collaterals will protect the cortex more than the deep tissues. Every 10 mL increase in pretreatment ischemic core volume reduces the odds of favorable functional outcomes by 20–30% [28][41]. The observation that the core grows over time if perfusion is not restored had a dramatic clinical impact and allowed trials to be run which ultimately led to the revascularization procedures: IVT and MT which are the only therapeutic strategies that can alter the prognosis of stroke patients. The impact on functional outcomes is directly related to the timing of the intervention, resulting in both greater treatment efficacy and a reduction in complications associated with these therapies, including hemorrhagic infarction. Multiple studies have acknowledged the significant role of collateral circulation in determining prognosis in stroke patients. This role proves valuable both in the diagnostic phase, aiding in the selection of optimal therapeutic approaches, and in the post-revascularization phase, serving as a prognostic indicator. One study suggests that collateral circulation status should be considered alongside symptom onset time when making therapeutic decisions, particularly for thrombolysis. The most crucial factor in therapy selection for stroke patients is the ASPECT (Alberta Stroke Program Early CT) score, which represents the extent of infarct expansion observed in CT brain imaging and serves as a prognostic determinant of ischemic stroke. A low ASPECT score, indicative of a large ischemic lesion, is an absolute contraindication for both thrombolysis and thrombectomy. However, several studies suggest that including collateral imaging in CT angiography (CTA) along with the ASPECT score can enhance prognostic accuracy and improve therapeutic decision-making [5].3. Imaging
3.1. Manual Assessment
The most widely recognized grading system is the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) collateral scale based on DSA, which assesses the cerebral collateral status as follows:-
grade 0: no collaterals to the ischemic site are visible;
-
grade 1: slow collaterals to the periphery of the ischemic site with persistence of some of the defect;
-
grade 2: rapid collaterals to the periphery of ischemic site with persistence of some of the defect and to a portion of the ischemic territory;
-
grade 3: collaterals with slow but complete angiographic blood flow to the ischemic bed in the early venous phase;
-
grade 4: collaterals with rapid and complete angiographic blood flow to the ischemic bed.
-
the method proposed by Miteff et al.: grading collateral flow distal to MCA occlusion.
-
the method proposed by Maas et al.: assessing collaterals at the Sylvian sulcus and cerebral convexity as well as the collateral pathways via the circle of Willis;
-
the method proposed by Tan et al.: grading collaterals in the MCA territory;
-
the regional leptomeningeal collateral (rLMC) score: assessing collaterals in MCA cortical regions, parasagittal ACA territory, the basal ganglia and the Sylvian sulcus.

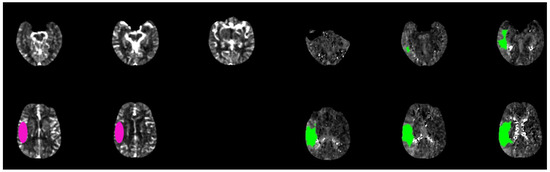
Figure 1. Brain CT-scan perfusion analyzed with rapid software in a patient with acute ischemic stroke show a favorable mismatch core (pink circle) vs. penumbra (green circle) with the following parameters: CBF < 30% volume: 15 mL; Tmax > 6.0 s volume: 160 mL; mismatch volume: 145 mL; mismatch ratio: 10, 6.
3.2. Automatic Assessment
A recently introduced technique for assessing collateral status is a color-coded mapping method called “ColorViz” (GE Healthcare Fast Stroke). This post-processing tool enables rapid and clear evaluation by maintaining the temporal resolution of multiphase CTA (mCTA) and generating a single image that combines three different cerebral vascular phases using a time variant color map. The color-coded mCTA summation maps are generated using the Fast Stroke software (GE Healthcare, Milwaukee, WI, USA) on a workstation. This software processes the complete set of CT stroke protocol images to create a single color-coded map known as ColorViz. The vessels depicted on the map are assigned different colors based on the arrival time of the contrast medium using a per-person adaptive threshold technique. Specifically, the vessels are assigned the following colors: (a) red (indicating maximal enhancement during the arterial phase), (b) green (representing the early venous phase), and (c) blue (representing the late venous phase). The post-processing procedure is straightforward and fully automated.
A score of three (“good”) is given when a well-preserved or slightly reduced representation of the collateral circles is observed on the affected side, with the predominant color being red. A score of two (“intermediate”) is assigned when a similar or reduced extension of collateral circulation is observed compared to the healthy side, and the predominant color is green (indicating slightly slowed circulation). Lastly, a score of one (“poor”) is assigned when the most prevalent color is blue (indicating significantly slowed circulation compared to the contralateral side) or when the extension of collateral circles is markedly reduced, even in the presence of a few green or red vessels (Figure 2) [31][53].
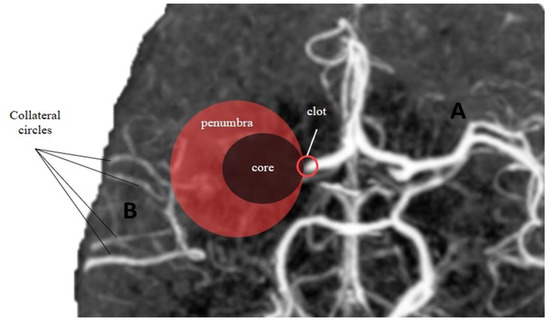

Figure 2. Core-penumbra ratio under different collateral circles condition. (A) Good collateral circles result in a large core-penumbra mismatch. (B) Poor collateral circles lead to a big core with small core-penumbra mismatch.
4. Summary
The collateral network comprises arterial vessels that provide support to cerebral circulation in hypoperfused areas affected by ischemic stroke. The establishment of this network is crucial for preserving the ischemic penumbra and salvaging viable tissue. Numerous studies in the recent literature have demonstrated a direct correlation between collateral circulation and both clinical and therapeutic outcomes in patients with ischemic stroke.
To improve the accuracy of identifying hypoperfused areas amenable to salvage through collateral circulation, various sophisticated radiological software tools have been developed. One such tool is “ColorViz,” which provides immediate post-processing visualization of cerebral vessels using colored maps. The vessels enhancing maximally during the arterial phase are assigned the color red, while the early and late venous phases are represented by green and blue colors, respectively. The presence of well-represented collateral circles is indicated by a predominant red color in the maps. This graphical representation allows for easy interpretation and facilitates the assignment of a quantitative score to assess the extent of collateral circulation.
Despite this, there is no one or more standardized and reproducible software available that can be applied in clinical practice on all kinds of patients. In particular, a computer tool that allows us to accurately highlight the presence and extent of collateral circulation in a quantitative way is desirable; it could be useful to select those patients with onset of ischemic stroke symptoms beyond the classic time windows. This would allow a careful selection of patients to be treated, but above all to be “not treated”, as the latter are those at greater risk of complications and post-stroke mortality.
