Acute respiratory distress syndrome (ARDS) is associated with a heterogeneous pattern of injury throughout the lung parenchyma that alters regional alveolar opening and collapse time constants. Such heterogeneity leads to atelectasis and repetitive alveolar collapse and expansion (RACE). The net effect is a progressive loss of lung volume with secondary ventilator-induced lung injury (VILI). Previous concepts of ARDS pathophysiology envisioned a two-compartment system: a small amount of normally aerated lung tissue in the non-dependent regions (termed “baby lung”); and a collapsed and edematous tissue in dependent regions. Based on such compartmentalization, two protective ventilation strategies have been developed: (1) a “protective lung approach” (PLA), designed to reduce overdistension in the remaining aerated compartment using a low tidal volume; and (2) an “open lung approach” (OLA), which first attempts to open the collapsed lung tissue over a short time frame (seconds or minutes) with an initial recruitment maneuver, and then stabilize newly recruited tissue using titrated positive end-expiratory pressure (PEEP).
1. Conceptual Approaches to Protective Lung Ventilation
Although the PLA is designed to reduce overdistension of the baby lung and is the current standard-of-care in ARDS, the mortality associated with the syndrome remains high
[1][2][3][4][3,4,5,6], as overdistension of the baby lung may not be the primary mechanism driving VILI. Previous studies have shown that normal aerated lung tissue is very resistant to high strain and overdistension-induced tissue damage
[5][6][7][8][9][10][11][12][13][37,38,39,40,41,42,43,44,45]. Moreover, the presence of repetitive alveolar collapse and expansion (RACE)
[14][46] and micro-atelectasis results in stress concentrators scattered throughout the lung
[15][16][17][18][19] (Videos S1 and S2) [8,9,10,47,48], which cannot be seen with conventional chest radiography or CT imaging
[20][49]. These phenomena have been termed “hidden micro-atelectasis”
[21][11], which, when present, may result in additional pathophysiologic complications. Indeed, it is well-known that persistent atelectasis is associated with multiple pathologies (
Table 1).
Table 1. Problems with the low tidal volume (LVt) Protective Lung Approach (PLA)
[22][27]. Although protecting normal tissue from over-distension and ‘resting’ the collapsed lung sounds protective, the lung is a dynamic organ designed to inflate and deflate continually. Long-term atelectasis (resting) generates multiple pathophysiologic problems including.
2. Protective Lung Approach
The PLA is
constrained to ventilating an injured lung with heterogeneously distributed regions of collapse since it does not attempt to reopen the lung. Such heterogeneity, combined with low V
T, further contributes to the progressive loss of aerated volume and drives the VILI Vortex (
Figure 13)
[40][18]. Laffey et al. investigated potentially adjustable ventilator settings associated with ARDS mortality in 2377 patients from 50 countries enrolled in the LUNG SAFE study
[2][4]. Ventilator settings associated with increased mortality were higher peak pressures, plateau pressures, PEEP, ∆P, and respiratory rate. By focusing on protocolized ventilator settings (i.e., V
T = 6 mL kg
−1, Pplat
< 30 cmH
2O), rather than the unique pathophysiology and disease processes of a given patient, the clinician may fail to individualize appropriate therapy. For example, low V
T applied to a patient with high C
RS has been shown to increase mortality
[23][29]. Parameters that take into account changes in lung physiology, such as Driving Pressure, have been shown to be better predictors of mortality.
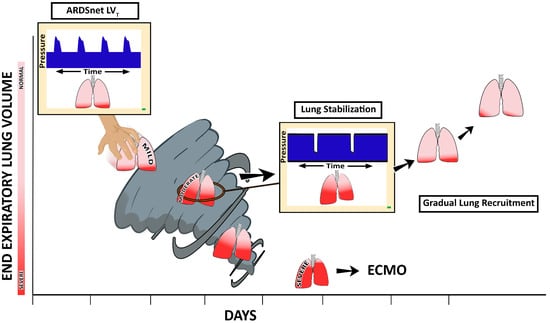
Figure 13. In patients with acute respiratory distress syndrome (ARDS), the evolution of ventilator-induced lung injury (VILI) can be described as an ever-shrinking normal ‘baby’ lung, resulting in a ‘VILI Vortex’. A ventilation strategy that does not prevent progressive lung collapse fuels the VILI Vortex. If unchecked, lung injury will progress into severe ARDS, at which point rescue methods such as extracorporeal membrane oxygenation (ECMO) may be necessary. In order to circumvent this VILI Vortex, methods to quickly stabilize and then gradually reopen collapsed lung tissue must be developed. It can be hypothesized that a stabilize the lung approach (SLA) that first stabilizes alveoli and then gradually reopens collapsed tissue can be accomplished using the time-controlled adaptive ventilation (TCAV) method to set and adjust the airway pressure release ventilation (APRV) mode. If our hypothesis is correct, this will be a paradigm shift in the way medicine is practiced from open-the-lung
first and stabilize-the-lung
second using the Open Lung Approach (OLA) to reversing this order of treatment (
stabilize and then gradually recruit) using the Stabilize Lung Approach. Reproduced from Reference
[41][64], under terms of the Creative Commons Attribution 4.0 International License.
3. Open Lung Approach
By contrast, the OLA is designed to reopen collapsed lung tissue using higher PEEP, with or without recruitment maneuvers (RMs) to release the constraints of ventilating a heterogeneous lung
[42][43][44][45][14,15,16,17]. However, OLA failed to reduce ARDS-related mortality below that of the original ARDSnet low V
T strategy
[22][27]. High-frequency oscillatory ventilation (HFOV) may also be viewed as an OLA strategy achieved by an entirely different ventilator method, but thus far has not shown superiority to the original ARDSnet protocol
[46][47][48][65,66,67]. Several possible explanations have been proposed for these results, including (1) maintenance of an open lung with mechanical ventilation does not necessarily protect it from VILI. This is not likely since physiologic studies have shown that an open lung is highly resistant to injury during mechanical ventilation
[49][68]; (2) the ventilation methods used to open the lung did so incompletely or transiently and, therefore, heterogeneity remained
[50][69]; (3) high distending pressures during HFOV may result in hemodynamic compromise
[51][70]; and (4) the maldistribution of oscillatory flow in a heterogeneously injured lung, resulting in regions of high parenchymal strain
[52][71].
Thus, from multiple physiological perspectives, the efficacy of the LV
T PLA method is problematic. The outcome data support the physiologic-based assumptions, with many statistical and meta-analyses showing that the LV
T ventilation strategy has not further lowered mortality in patients with established ARDS
[1][2][4][53][54][55][56][3,4,6,72,73,74,75] below that in the ARDSnet study published 23 years ago
[22][27].
Data from previous OLA clinical trials support the hypothesis that the recruitment methods did not effectively open the lung, since there was no clear evidence of long-term lung recruitment based on lung imaging, arterial blood gases, or estimates of C
RS. These negative trials have led some to suggest that the OLA should be abandoned, although the likely reason for failure was that the goals of the strategy were not achieved
[57][76]. If regional RACE and micro-atelectasis result in stress concentrators that propagate VILI, then the most effective way to reduce VILI is to both reopen and stabilize the lung
[5][8][9][18][19][21][58][11,37,40,41,47,48,77]. Danti et al. demonstrated that the combination of PEEP and RM duration plays a role in the mortality associated with ARDS. They found that: (1) higher PEEP without an RM was superior to lower PEEP; (2) higher PEEP with a prolonged RM was inferior to higher PEEP alone; and (3) higher PEEP with a brief RM was superior to higher PEEP alone. These data reveal the complex relationship between PEEP and RMs and are another explanation for why the OLA has not further reduced mortality in ARDS
[59][78].
4. The Acutely Injured Lung Becomes Time- and Pressure-Dependent
ARDS causes the lung to become both
time- and
pressure-dependent. This means that it will take more time to recruit collapsed alveoli and less time for them to collapse at any given airway pressure. Changes in the time constants associated with alveolar opening and collapse result in greater susceptibility to progressive atelectasis and reductions in EELV, which decreases C
RS and increases ∆P for any given V
T [2][60][61][62][63][64][4,31,32,33,34,35]. The OLA attempts to eliminate atelectasis and normalize EELV; however, as discussed above, the OLA has not further reduced ARDS-related mortality
[59][78]. Combined, these studies suggest that a better ventilation strategy must be developed to break the constraints of ventilating a heterogeneously injured lung (
Figure 13)
[41][64].
5. Stabilize Lung Approach
It can be postulated that the ventilation strategy necessary to break the constraints of ventilating a heterogeneously injured lung must (i) first stabilize the lung by halting RACE, since atelectrauma is a primary VILI mechanism, and then (ii) reopen the lung gradually so that it may heal in a more natural, inflated state (even though it is being supported by an unnatural mechanical ventilator)
[40][18]. Unfortunately, identifying such a ventilation strategy has been elusive. If instability and collapse are the engines that drive the VILI Vortex, the goal of any protective ventilation strategy should be to eliminate instability as soon as possible. When atelectrauma is minimized, collapsed (but potentially recruitable) tissue may be reinflated slowly and safely over hours or even days. (
Figure 13)
[15][16][17][21][8,9,10,11].
Time-controlled adaptive ventilation (TCAV) is a strategy designed to stabilize lung tissue at risk for RACE, then gradually reopen collapsed tissue (
Figure 13). TCAV utilizes a continuous positive airway pressure (CPAP) that is periodically interrupted by brief Release Phases
(Figure 2B). The extended time at inspiration combined with an inflate and break ratchet method will gradually reopen collapsed tissue. The duration of this Release Phase is very brief and acts as a break to prevent newly recruited tissue from re-collapsing. The precise timing of the Release Phase necessary to prevent RACE is personalized and adaptive, based on evolving changes in C
RS [65][66][79,80]. TCAV is the most investigated method for setting the parameters of airway pressure release ventilation (APRV), and has been comprehensively studied and reviewed by multiple investigators (e.g., TCAVnetwork.org)
[67][68][69][70][71][72][73][74][81,82,83,84,85,86,87,88]. TCAV is a personalized approach to ventilation and differs from other methods to set APRV parameters, which may not stabilize and open the lung as effectively
[8][75][40,89].