Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 3 by Jessie Wu.
Skin regeneration after an injury is very vital, but this process can be impeded by several factors. Regenerative medicine is a developing biomedical field with the potential to decrease the need for an organ transplant.
- biopolymers
- applications
1. Properties of Collagen in Biomedical Applications
There are major qualities that must be considered when choosing a suitable material for skin regenerative and wound dressing applications. The biomaterials that were originally employed in the arena of biomedicine include ceramics and metals due to their non-immunogenic effect, but the materials such as polymers have been reported to be appropriate because of their interesting properties [1]. Biopolymers can interact with the cells, stimulating the formation of new tissues and promoting regeneration [2][3][4]. Collagen is one of the biopolymers that is often employed in skin regeneration and wound healing because of its several attractive features. The molecular structure of collagen is presented in Figure 1. Collagen is mostly obtained from porcine and cattle slaughterhouse trashes, but fishery by-products have also become a significant substitute source for collagen recently [5]. Among the 29 types of collagens that are currently known, type I collagen is the greatest, most abundant, and can be obtained from various mammalian connective tissues, such as the skin, cornea, and tendon [6][7]. Type I collagen shows the characteristic structure with two α1-chains and one α2-chain. The well-known derivative of collagen is gelatin, which is composed of the same repetition of amino acids arrangement as collagen of Gly-X-Y, where X and Y are proline and hydroxyproline, respectively [8].
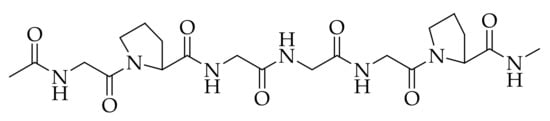
Figure 1. Chemical structure of collagen.
Collagen is broadly employed in wound dressing and tissue engineering products because of its low antigenicity, good biocompatibility, hemostatic properties, capability to promote cellular proliferation and adhesion, and reduced cytotoxicity [9]. Various collagen wound dressing products are available in the market, some of them are summarized in Table 1. Nevertheless, most commercial collagen dressings suffer from some limitations. Some studies have demonstrated that collagen materials that are fabricated in the form of gels, films, or powder offer haemorrhage control [10]. Collagen-based porous scaffolds possess the ability to absorb large volumes of exudates, maintain moisture for the injury, thus promoting an enhanced wound healing process [11]. Collagen has been proven to be biocompatible both in vitro and in vivo, especially porcine- and bovine-derived collagen. Collagen derived from marine has resulted in biocompatible wound dressing materials that can be developed in the shape of nanofibers, sheets, hydrogels, sponges, membranes, and films [11].
Collagen-based materials that are used as porous scaffolds for the migration of cells also offer mechanical and structural support and promote the development of new tissues [12]. The collagen biomatrix imitates the natural ECM collagen and stabilizes the cellular and vascular constituents in the injury by decreasing matrix metalloproteinases (MMP) levels that are characteristically imbalanced in chronic lesions while offering structural support for the repair of tissues [13]. The modes in which the collagen wound dressing enhances the wound healing process include the capacity to connect to the GFs, control functions of the cells, enable intracellular transmission, and act as a physical structure to help tissue restoration in both chronic and acute injuries [14]. In wound healing, collagen plays a significant role in controlling inflammatory response to injury followed by repairs. It also influences the protein synthesis in the ECM, the release of growth factors and inflammatory cytokines, and the remodeling of the ECM [13][14]. Polymer-based materials are formulated by combining collagen with other polymers such as poly (ε-caprolactone) (PCL), poly (lactide-co-glycolide) (PLGA), poly (ethylene glycol) (PEG), polyglycolide (PGA), polylactic acid (PLA), and polyvinyl alcohol (PVA) [15]. These materials are also used as carrier matrices useful for accelerated wound healing mechanisms in skin injury. These materials are regularly utilized as drug delivery systems for antibiotics, GFs, essential oils, nutrients, and vitamins to further improve their wound healing activity [16][17][18].
Nanofibers formulated from various techniques, especially the electrospinning method, are often utilized as drug delivery systems making them ideal wound dressings and skin regeneration scaffolds.
Table 1. Summary of commercially available collagen-based wound dressings.
| Form of Dressing | Composition | Product Name | Advantage | Limitations | Wounds Suitable for | Ref |
|---|---|---|---|---|---|---|
| Gel | Collagen | CelleraRX | Maintain moisture for wound bed | Bovine sources, and require secondary wound dressing | Partial and Full-thickness injuries including traumatic wounds, surgical wounds, diabetic ulcers, and burns |
[19] |
| Gel | Collagen Polypeptides | Stimulen | Provide moisture for wound bed | Bovine source, and require secondary wound dressing | Full- and partial-thickness wounds including pressure ulcers, partial-thickness burns, abrasions | [20] |
| Pad | Collagen fleece, gentamicin salts |
Septocoll E | Activate platelets | skin responses |
Full and partial thickness injuries including infected wounds, and bleeding lesion |
[21] |
| Pad | Collagen, carboxymethylcellulose, sodium alginate, AgCl | ColActive Plus Ag |
Hinders the function of MMPs |
Bovine sources, require secondary wound dressing | Full and partial thickness wounds including burns, dehisced surgical incisions abrasions, diabetic, venous, or pressure ulcers |
[22] |
| Pad | Collagen and Ca alginate | Fibracol Plus |
Maintain moisture for wound bed | Require secondary wound dressing | Full and partial thickness wounds including burns, dehisced surgical incisions abrasions, diabetic, venous, or pressure ulcers |
[23] |
| Pad | Bovine collagen, and Manuka Honey |
Puracol | No extra debridement Required |
Bovine source and expensive. | Full and partial thickness injuries including dehisced surgical incisions abrasions, burns, diabetic, venous, or pressure ulcers |
[24] |
| Pad | Type I equine Collagen | Biopad | Free from collagen degradation products |
Equine source, time consuming, high cost |
Full and partial thickness wounds including dehisced surgical incisions abrasions, diabetic, venous, or pressure ulcers |
[25] |
| Pad | Bovine collagen and oxidized cellulose | Promogran | Hemostatic activity | Bovine source, not to be utilized in third-degree burns. | Full and partial thickness wounds including abrasions, bleeding wounds, venous or diabetic ulcers, pressure wounds |
[26] |
| Powder | Collagen | Catrix | Decrease bleeding, Biodegradable |
Bovine source, require secondary wound dressing | Full and Partial-thickness wounds including cuts, abrasions, irritations, pressure, diabetic ulcers radiation dermatitis, burns |
[27] |
| Membrane | porcine dermal collagen, nylon, silicon |
Biobrane | Flexibility | Bovine source, require secondary wound dressing | Partial-thickness burn wounds | [28] |
| Cellular matrix |
Collagen, polycarbonate membrane |
Apligraf | Resorbable | Not suitable for infected injuries, bovine source, and expensive. | Full and partial thickness injuries including venous leg ulcers, diabetic foot ulcers |
[29] |
| Cellular matrix |
Type I collagen | Orcel | Full resorbable | Not suitable for infected wounds, bovine source, high cost | Full-thickness injuries including burns | [30] |
2. Collagen-Based Nanofibers in Skin Regeneration and Wound Dressing
Wound care is gradually becoming a major public health concern globally. Most wound dressings are not effective in promoting skin regeneration. Hence, several studies are currently focused on the development of effective and novel dressing materials that can advance the wound healing process [31]. Collagen-based materials can be formulated in the form of nanofibrous scaffolds using various techniques (e.g., melt-blowing, self-assembly, template synthesis, phase separation, electrospinning, etc.) [32]. Among them all, the electrospinning technique is the most employed method in the preparation of nanofibers because of its cost-effectiveness and simplicity [33][34]. The electrospinning apparatus is composed of three parts: voltage system, spinneret system, and collecting system (Figure 2) [35][36]. The electrospinning technique utilizes high voltage electric fields to create nanofibers with diameters in various nanometers or micrometres. The spinneret system assists in the production of the near-field, and the coaxial electrospinning development has been advanced to deposit nanofibers in a controllable, direct, and continuous manner [37]. The electrospun nanofibers that possess high porosity and large surface-to-volume ratio have been beneficial in several areas, particularly in biomedical applications such as tissue regeneration, wound healing, and drug delivery systems [38][39][40][41][42]. Nanofibers are beneficial in regenerative medicine and wound healing because of their capability to imitate the ECM and stimulate the proliferation and migration of cells [43][44][45]. The features of electrospun nanofibers that make them useful in the field of wound healing are high porosity, good gaseous permeation, good cellular adhesion, good swelling capacity, and the ability to offer moisture for the acceleration of skin regeneration and wound healing process [46][47].
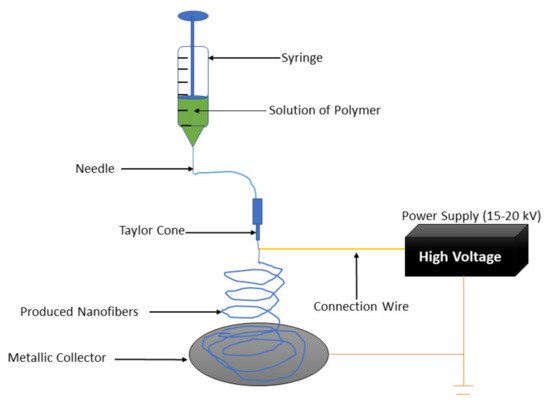
Figure 2. Electrospinning setup.
2.1. Advantages and Disadvantages of the Electrospun for Skin Regeneration and Wound Dressing
The electrospinning method allows the production of interconnected networks from fibers of nano-scale diameter and they are similar to the native structure of the natural ECM, hence they promote the normal functions of the cells, such as proliferation [31]. The flexibility and easy combination of drugs and other bioactive molecules, such as nanoparticles, antimicrobials, growth factors, and anti-inflammatory agents into the nanofibers is another significant advantage of the electrospinning method [48]. Electrospun wound dressings can provide flexibility and patient compliance. Wound dressings fabricated by electrospinning of biodegradable polymers improve patient comfort and compliance with no need for frequent changing of the dressing [49]. The biodegradable electrospun wound dressings also induce healing and enhance cell growth due to their high compatibility with tissues and blood. The degradation rate of the wound dressings can be tuned with the rate of tissue regeneration. Therefore, the aforementioned advantages make electrospun nanofibers promising materials for enhanced skin regeneration and wound healing [48][49]. However, the use of organic solvents and the limited control of pore structures is a limitation [50]. Although electrospun fibers frequently have high porosity, it is difficult to control due to dependence on the fiber diameter. Moreover, it might also limit cell penetration into the scaffold in some cases [51]. The higher voltage of electrospun might lead to more solution deposition. Thus, the properties of both the solution and the process parameters should be considered during the optimization of the electrospinning process [52][53].
2.2. Application of Electrospun Collagen-Based Nanofibers in Skin Regeneration and Wound Dressing
Electrospinning is a flexible and simple technique used to formulate fibers with diameters that range from micrometers to a nanometer. The polymers that have been electrospun are more than 200. The electrospinning technique has become one of the most prevalent scaffold fabrications to make nanofiber mesh for tissue engineering applications [54]. Collagen is the most abundant ECM protein in the human body; it has been electrospun to fabricate biomimetic scaffolds that imitate the architecture of native human tissues. Electrospun collagen nanofiber mesh has a high surface area to volume ratio, porosity, tunable diameter and tissue formation and also excellent biological activity to regulate cell function [54]. Currently, a lot of attention has been on fabricating biopolymer-based nanofibrous structures through the electrospinning process. The electrospinning technique is known for its low-cost and tunable method for generating ultra-fine fibers with some exceptional properties [31]. Owing to flexibility in choosing the raw materials and the possibility to tune the ultimate properties, the electrospinning method has been broadly employed for biomedical materials such as wound dressings, tissue engineering scaffolds and drug delivery systems [31]. Electrospun nanofibers can influence and interact with the damaged tissue and its biological environment according to their physical and chemical characteristics, as well as through additional linked bioactive molecules [55]. The use of crosslinkers on electrospun collagen may lead to appropriate scaffold stability and resistance to degradation in vitro and in vivo [56]. Nevertheless, more improvements in terms of their mechanical properties, the optimization of biological response, and reductions in the degradation rate are needed. Thus, several methods have been explored to combine collagen with other synthetic or natural polymers or additives through co-electrospinning, blending, and electrospinning alternating layers of the constituents or coating the electrospun fibers [56].
Deng et al. electrospun type I collagen to produce scaffolds that are similar to the native ECM within the dermis [57]. They are frequently used in skin regenerative medicine and wound regeneration. In terms of in situ crosslinked collagen-chitosan nanofibers, they have been used to improve epithelialization and angiogenesis in a rat scald model [57]. Some studies have reported the efficacy of collagen nanofibrous scaffolds in proliferation, normal human keratinocyte attachment and early-stage wound healing. A microscopic observation using identical full-thickness rectangular back injuries using a Sprague–Dawley rat as an animal model revealed early-stage wound healing in the collagen nanofiber scaffold cluster that was quicker than in the control cluster [58]. The wound surface of the control cluster was enclosed with fibrous tissue debris, along with a layer with a dense infiltration of leukocytes and an accumulation of proliferating fibroblasts. In contrast, in the collagen nanofiber scaffolds cluster, there were no surface tissue debris and fibroblast proliferation, indicating the effectiveness of collagen nanofibrous scaffolds in enhancing early-stage wound healing [58]. Pilehvar-Soltanahmadi et al. reported electrospun gelatin scaffolds as promising scaffolds for wound healing applications. Though gelatin has poor mechanical strength, it is used in combination with other materials to improve the mechanical and biological properties [58]. Electrospun collagen is a desired material for tissue engineering due to its biocompatibility and architectural versatility. The changes in the structure that happen through processing may contribute to high degradation rates that are not appropriate for numerous biomedical applications [59]. Electrospun collagen scaffolds quickly degrade in aqueous environments. Physical and chemical crosslinking of electrospun collagen improved both the mechanical properties and scaffold stability [59]. Augustine et al. developed electrospun scaffolds with antibacterial activity to inhibit wound infections [60]. The antibacterial nanofibers are commonly fabricated by incorporating antibacterial agents during electrospinning. Diverse antimicrobial agents such as metallic, nanoparticles, antibiotics, and natural extracts derived products have been loaded into electrospun nanofibers to improve their antibacterial activities. Metallic nanomaterials such as AgNPs are known as effective agents for the management of wound infections. Nanoscale particles with a high surface to volume ratio promote the antibacterial activity of electrospun wound dressings [60][61].
3. Integrity of Collagen during the Fabrication of Nanofibers and Standardization of Raw Collagen
Collagen obtained from different sources differs in their physicochemical properties slightly [62]. Collagens obtained from frog skin, bird feet, shark skin, and sea urchin has a molecular structure that is different from those obtained from domestic animals [63][64][65]. Furthermore, their thermal property, peptide constitution, amino acid composition, and content of glycosaminoglycan are significantly different from collagen isolated from land animals [63]. Collagen is also isolated from yeast, plants, bacteria, etc., and is known as recombinant human collagen [66]. However, it is expensive and isolated in poor yield but overcomes the risk of transmission of diseases and batch-to-batch variations [67]. The commonly electrospun collagen types are collagen I-IV. II, III and IV. Several factors make electrospun collagen nanofibers superior compared to nanofibers prepared from other polymers such as their capability to mimic the native tissues, their poor immunogenicity, capability to activate the host immune response, and excellent biocompatibility. However, their shortcomings are poor mechanical properties which can be improved by cross-linking with synthetic polymers [63]. The sources of collagen affect the properties of the nanofibers. Most of the studies on collagen-based nanofibers reported the use of type I bovine skin collagen to develop electrospun collagen nanofibers. The molecular weight of collagen has been reported to influence the formation of nanofibers. Low molecular atelocollagen did not form fibers [68]. Similar findings were reported by Zeugolis et al. in which the source of collagen influenced their capability to form fibers and also the properties of the formed fibers [69]. Collagen type I isolated from human placenta used in the design of nanofibers resulted in less uniform fibers and a larger range of diameter [70]. Choosing an ideal solvent for collagen electrospinning for the formation of the fibers without compromising the integrity of collagen is crucial. Solvents such as 1,1,1,3,3,3-hexafluoro-2-propanol, acetic acid, 2,2,2-trifluoroethanol, and phosphate buffered saline/ethanol have been commonly used for the preparation of collagen nanofibers. Using 1,1,1,3,3,3-hexafluoro-2-propanol promoted collagen fiber formation but denatured it [63]. It can also result in gelatin fibers because the denatured form of collagen is gelatin which is obtained when the triple helical structure is denatured [71]. Using acetic acid resulted in fibers with a more triple helical structure when compared to using 1,1,1,3,3,3-hexafluoro-2-propanol for the development of electrospun nanofibers [72]. Using glacial acetic acid in combination with DMSO in a 93:7 ratio produced collagen nanofibers with retained features of native collagen [73].References
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. Food Hydrocolloids The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633.
- Majid, Q.A.; Fricker, A.T.R.; Gregory, D.A.; Davidenko, N.; Hernandez, C.O.; Jabbour, R.J.; Owen, T.J.; Basnett, P.; Lukasiewicz, B.; Stevens, M.; et al. Natural Biomaterials for Cardiac Tissue Engineering: A Highly Biocompatible Solution. Front. Cardiovasc. Med. 2020, 7, 192.
- Grabska-Zielińska, S.; Sionkowska, A.; Coelho, C.C.; Monteiro, F.J. Silk Fibroin/collagen/chitosan scaffolds cross-linked by a glyoxal solution as biomaterials toward bone tissue regeneration. Materials 2020, 15, 3433.
- Abdulghani, S.; Mitchell, G.R. Biomaterials for in situ tissue regeneration: A review. Biomolecules 2019, 9, 750.
- Ricard-Blum, S. The collagen family. Cold Spring Harb Perspect. Biol. 2011, 3, 004978.
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168.
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887.
- Marina, A.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200.
- Amirrah, I.N.; Farhanulhakim, M.; Razip, M.; Tabata, Y.; Bt, R.; Idrus, H.; Nordin, A.; Fauzi, M.B. Antibacterial-Integrated Collagen Wound Dressing for Diabetes-Related Foot Ulcers: An Evidence-Based Review of Clinical Studies. Polymers 2020, 12, 2168.
- Agrawal, P.; Soni, S.; Mittal, G.; Bhatnagar, A. Role of polymeric biomaterials as wound healing agents. Int. J. Low Extrem. Wounds 2014, 13, 180–190.
- Gaspar-pintiliescu, A.; Stanciuc, A.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865.
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833.
- Gould, L.J. Topical Collagen-Based Biomaterials for Chronic Wounds: Rationale and Clinical Application. Adv. Wound Care 2016, 5, 19–31.
- Brett, D. A Review of Collagen and Collagen-based Wound Dressings. Wound Repair Regen. 2018, 20, 347–356.
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42.
- Johnson, N.; Wang, Y. Drug Delivery Systems for Wound Healing. Curr. Pharm. Biotechnol. 2015, 16, 621–629.
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Üstündağ Okur, N.; Siafaka, P.I. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684.
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2019, 127, 138–166.
- Newman, M.I.; Baratta, L.G.; Swartz, K. Activated, type I collagen (CellerateRx) and its effectiveness in healing recalcitrant diabetic wounds: A case presentation. Adv. Ski. Wound Care 2008, 21, 370–374.
- Elgharably, H.; Roy, S.; Khanna, S.; Abas, M.; Das, G.P.; Das, A.; Mohammed, K.; Sen, C.K. A modified collagen gel enhances healing outcome in a pre-clinical swine model of excisional wounds. Wound Repair Regen. 2013, 21, 473–481.
- Gruessner, U.; Clemens, M.; Pahlplatz, P.V.; Sperling, P.; Witte, J.; Rosen, H.R. Improvement of perineal wound healing by local administration of gentamicin-impregnated collagen fleeces after abdominoperineal excision of rectal cancer. Am. J. Surg. 2001, 182, 502–509.
- ColActive® Plus Ag (Collagen Matrix Dressing with Silver). Available online: https://www.woundsource.com/product/colactive-plus-ag-collagen-matrix-dressing-silver#:~:text=ColActive%C2%AE%20Plus%20Ag%20(Collagen%20Matrix%20Dressing%20with%20Silver),for%20the%20management%20of%20full-%20and%20partial-thickness%20wounds (accessed on 12 July 2021).
- Colenci, R.; Abbade, L.P.F. Fundamental aspects of the local approach to cutaneous ulcers. An. Bras. Dermatol. 2018, 93, 859–870.
- Capella-Monsonís, H.; Tilbury, M.A.; Wall, J.G.; Zeugolis, D.I. Porcine mesothelium matrix as a biomaterial for wound healing applications. Mater. Today Bio. 2020, 7, 100057.
- Turner, N.J.; Badylak, S.F. The use of biologic scaffolds in the treatment of chronic nonhealing wounds. Adv. Wound Care 2015, 4, 490–500.
- da Silva, C.V.; Pereira, V.J.; Costa, G.M.N.; Cabral-Albuquerque, E.C.M.; Vieira de Melo, S.A.B.; de Sousa, H.C.; Dias, A.M.A.; Braga, M.E.M. Supercritical solvent impregnation/deposition of spilanthol-enriched extracts into a commercial collagen/cellulose-based wound dressing. J. Supercrit. Fluids 2018, 133, 503–511.
- Kalin, M.; Kuru, S.; Kismet, K.; Barlas, A.M.; Akgun, Y.A.; Astarci, H.M.; Ustun, H.; Ertas, E. The effectiveness of porcine dermal collagen (Permacol®) on wound healing in the rat model. Indian J. Surg. 2015, 77, 407–411.
- Harish, V.; Li, Z.; Maitz, P.K.M. The optimal timing of outpatient BiobraneTM application for superficial and mid dermal partial thickness burns: Evidence for the ‘12-hour rule. Burns 2019, 45, 936–941.
- Vlassova, N.; Lazarus, G. Use of Apligraf for a devascularized ulcer secondary to mastectomy and radiation. J. Am. Acad. Dermatol. 2011, 65, e132–e134.
- Still, J.; Glat, P.; Silverstein, P.; Griswold, J.; Mozingo, D. The use of a collagen sponge/ living cell compositematerial to treat donor sites in burn patients. Burns 2003, 29, 837–841.
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67.
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49.
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509.
- Liu, H.; Ding, X.; Zhou, G.; Li, P.; Wei, X.; Fan, Y. Electrospinning of nanofibers for tissue engineering applications. J. Nanomater. 2013, 2013, 3.
- Wen, Y.; Kok, M.D.R.; Tafoya, J.P.V.; Sobrido, A.B.J.; Bell, E.; Gostick, J.T.; Herou, S.; Schlee, P.; Titirici, M.M.; Brett, D.J.L.; et al. Electrospinning as a route to advanced carbon fibre materials for selected low-temperature electrochemical devices: A review. J. Energy Chem. 2020, 59, 492–529.
- Bavatharani, C.; Muthusankar, E.; Wabaidur, S.M.; Alothman, Z.A.; Alsheetan, K.M.; AL-Anazy, M.; Ragupathy, D. Electrospinning technique for production of polyaniline nanocomposites/nanofibres for multi-functional applications: A review. Synth. Met. 2021, 271, 116609.
- Alven, S.; Buyana, B.; Feketshane, Z.; Aderibige, B.A. Electrospun Nanofibers / Nanofibrous Scaffolds Loaded with Silver Nanoparticles as Effective Antibacterial Wound Dressing Materials. Pharmaceutics 2021, 13, 964.
- Alturki, A.M. Rationally design of electrospun polysaccharides polymeric nanofiber webs by various tools for biomedical applications: A review. Int. J. Biol. Macromol. 2021, 184, 648–665.
- Kamsani, N.H.; Haris, M.S.; Pandey, M.; Taher, M.; Rullah, K. Biomedical application of responsive ‘smart’ electrospun nanofibers in drug delivery system: A minireview. Arab. J. Chem. 2021, 14, 103199.
- Sabra, S.; Ragab, D.M.; Agwa, M.M.; Rohani, S. Recent advances in electrospun nanofibers for some biomedical applications. Eur. J. Pharm. Sci. 2020, 144, 105224.
- Kurakula, M.; Koteswara Rao, G.S.N. Moving polyvinyl pyrrolidone electrospun nanofibers and bioprinted scaffolds toward multidisciplinary biomedical applications. Eur. Polym. J. 2020, 136, 109919.
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr. Polym. 2020, 228, 115419.
- Cheng, G.; Dai, J.; Dai, J.; Wang, H.; Chen, S.; Liu, Y.; Liu, X.; Li, X.; Zhou, X.; Deng, H. Extracellular matrix imitation utilizing nanofibers-embedded biomimetic scaffolds for facilitating cartilage regeneration. Chem. Eng. J. 2021, 410, 128379.
- Arida, I.A.; Ali, I.H.; Nasr, M.; El-Sherbiny, I.M. Electrospun polymer-based nanofiber scaffolds for skin regeneration. J. Drug Deliv. Sci. Technol. 2021, 64, 102623.
- Zhang, X.; Li, L.; Ouyang, J.; Zhang, L.; Xue, J.; Zhang, H. Electroactive electrospun nanofibers for tissue engineering. Nano Today 2021, 39, 101196.
- Dias, J.R.; Granja, P.L.; Bártolo, P.J. Advances in electrospun skin substitutes. Prog. Mater. Sci. 2016, 84, 314–334.
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600.
- Ghorbani, S.; Eyni, H.; Tiraihi, T.; Asl, L.S.; Soleimani, M.; Atashi, A.; Beiranvand, S.P.; Warkiani, M.E. Combined effects of 3D bone marrow stem cell-seeded wet-electrospun poly lactic acid scaffolds on full-thickness skin wound healing. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 905–912.
- Dart, A.; Bhave, M.; Kingshott, P. Antimicrobial Peptide-Based Electrospun Fibers for Wound Healing Applications. Macromol. Biosci. 2019, 19, e1800488.
- Akhmetova, A.; Heinz, A. Electrospinning proteins for wound healing purposes: Opportunities and challenges. Pharmaceutics 2021, 13, 4.
- Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Polymeric nanofibers in tissue engineering. Tissue Eng. Part. B Rev. 2011, 17, 349–364.
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415.
- Pant, B.; Park, M.; Park, S.-J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019, 11, 305.
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun collagen nanofibers and their applications in skin tissue engineering. J. Tissue. Eng. Regen. Med. 2017, 14, 699–718.
- Wang, J.; Windbergs, M. Functional electrospun fibers for the treatment of human skin wounds. Eur. J. Pharm. Biopharm. 2017, 119, 283–299.
- Blackstone, B.N.; Gallentine, S.C.; Powell, H.M. Collagen-based electrospun materials for tissue engineering: A systematic review. Bioengineering 2021, 8, 39.
- Deng, A.; Yang, Y.; Du, S.; Yang, S. Electrospinning of in situ crosslinked recombinant human collagen peptide/chitosan nanofibers for wound healing. Biomater. Sci. 2018, 6, 2197–2208.
- Pilehvar-Soltanahmadi, Y.; Akbarzadeh, A.; Moazzez-Lalaklo, N.; Zarghami, N. An update on clinical applications of electrospun nanofibers for skin bioengineering. Artif. Cells. Nanomed. Biotechnol. 2016, 44, 1350–1364.
- Drexler, J.W.; Powell, H.M. Dehydrothermal crosslinking of electrospun collagen. Tissue Eng. Part. C. Methods 2011, 17, 9–17.
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020, 156, 153–170.
- Maleki, H.; Mathur, S.; Klein, A. Antibacterial Ag containing core-shell polyvinyl alcohol-poly (lactic acid) nanofibers for biomedical applications. Polym. Eng. Sci. 2020, 60, 1221–1230.
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, R.; et al. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials 2004, 25, 2831–2841.
- Lin, Y.K.; Liu, D.C. Comparison of physical–chemical properties of type I collagen from different species. Food Chem. 2006, 99, 244–251.
- Lin, K.; Liu, D.C. Effect of pepsin digestion at different temperatures and time on properties of telopeptide-poor collagen from bird feet. Food Chem. 2006, 94, 621–625.
- Li, H.; Liu, B.L.; Gao, L.Z.; Chen, H.L. Studies on bullfrog skin collagen. Food Chem. 2004, 84, 65–69.
- Wang, T.; Lew, J.; Premkumar, J.; Poh, C.L.; Naing, M.W. Production of recombinant collagen: State of the art and challenges. Eng. Biol. 2017, 1, 18–23.
- Willard, J.J.; Drexler, J.W.; Das, A.; Roy, S.; Shilo, S.; Shoseyov, O.; Powell, M.H. Plant-derived human collagen scaffolds for skin tissue engineering. Tissue Eng. Part. A 2013, 19, 1507–1518.
- Hofman, K.; Tucker, N.; Stanger, J.; Staiger, M.; Marshall, S.; Hall, B. Effects of the molecular format of collagen on characteristics of electrospun fibres. J. Mater. Sci. 2012, 47, 1148–1155.
- Zeugolis, D.; Li, B.; Lareu, R.R.; Chan, C.; Raghunath, M. Collagen solubility testing, a quality assurance step for reproducible electro-spun nano-fibre fabrication. A technical note. J. Biomater. Sci. Polym. Ed. 2008, 19, 1307–1317.
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238.
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.Y.L.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres—just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305.
- Yang, L.; Fitie, C.F.; van der Werf, K.O.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Mechanical properties of single electrospun collagen type I fibers. Biomaterials 2008, 29, 955–962.
- Elamparithi, A.; Punnoose, A.M.; Kuruvilla, S. Electrospun type 1 collagen matrices preserving native ultrastructure using benign binary solvent for cardiac tissue engineering. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1318–1325.
More
