Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Yingyi Han.
Given the heightened awareness of PFASs globally, it is especially critical to identify the sources of PFAS pollution in edible oils. Having a thorough knowledge of the causes of PFAS pollution in edible oils can assist in precisely pinpointing the sources of contamination and taking the necessary steps to decrease PFAS contamination and thereby safeguard human health. By recognising the sources of contamination, it is possible to enhance the effectiveness of pollution control, avoid superfluous investments, save resources, and advance sustainable environmental progress.
- perfluoroalkyl substances
- edible oil
1. PFAS Accumulation in Oil Crops
As previously mentioned, PFASs are easily accumulated in protein-rich food matrices. Most of the contamination of oil crops occurred during their growth stage, which will severely affect the safety of edible plant oil [32][1]. Edible plant oil can be derived from a variety of oil crops such as rapeseed, soybean, peanut, and sesame. Additionally, the protein concentration in soybeans and peanuts was approximately 40% and 28%, respectively, which was even more than that in milk [60][2]. The PFAS life cycle begins with the primary producer and progresses to the commercial user, consumer, and eventually disposal, all of which involve the release of chemicals into the atmosphere and water and the storage of PFASs in soils for a prolonged period of time [61][3]. The presence of PFASs in soils has been reported worldwide [62[4][5][6],63,64], while PFOA constituted the main component of PFASs in soil and plants due to its high solubility in water [65][7]. The accumulation of PFASs could lead to acute toxic effects on growth and development in plant communities [66][8]. It was reported that soybean and rape can absorb PFOS-K and PFOA from soil by root and transfer them to the stem and leaf; the concentrations of PFOS-K and PFOA in root, stem, and leaf were positively correlated with the concentrations in soil [67][9], which is consistent with the published result that PFASs could be absorbed and accumulated via plant root from soil and water [65][7]. Moreover, Tian et al. also confirmed that airborne PFASs and homologs presented in vapour and particulate form could be adsorbed into plants by aerial parts such as foliage and bark [68][10]. Furthermore, the transport/distribution of PFASs by the plant from the root to above-ground tissues is mostly related to the chain length; the longer-chain PFASs prefer to accumulate in the root, while the shorter-chain compounds prefer to transport to other tissues [65][7]. As mentioned above, oil crops absorb PFASs from the environment (soil, water, and atmosphere) readily and eventually transfer them to oil products [69][11].
2. PFAS Accumulation in Animal Edible Oil Raw Materials
In addition to edible plant oils, edible oils of animal origin are also widely used because of their rich nutrients, such as lard, butter, fish oil, cod liver oil, etc. However, studies conducted both in the field and in the laboratory have revealed that certain PFASs can accumulate in the bodies of predators in wildlife, water, and land environments, as well as in humans [70][12]. As opposed to lipophilic-bioaccumulative POPs, PFASs are involved in a protein-associated bioaccumulative pathway [71][13]. PFASs can attach to proteins in the serum of the blood, resulting in high concentrations of these chemicals in both the blood and the blood meals [72][14]. Therefore, PFASs could enter the food chain through various pathways, mainly through PFAS contamination in animal living environments and feed additives.
Aquatic edible animals from a river-estuary-sea environment that were affected by the fluorochemical industry have been widely reported around the world [73,74,75][15][16][17]. The research conducted in China to investigate the PFCA levels in the edible tissues of 40 aquatic species from the river-estuary-sea environment, affected by a large fluorochemical industrial area, revealed that PFOA was the major contaminant, with concentrations as high as 2161 ng g−1 wet weight (found in the freshwater winkle) [75][17]. Of the 200 North East Arctic cod liver samples tested for 16 PFASs, PFOS was present in the majority of the samples (72%) at concentrations above the limit of quantitation (LOQ) (1.5 μg kg−1 wet weight), with the highest level detected being 21.8 μg kg−1 wet weight [76][18]. This is in good accordance with the fact that PFOS and PFCAs are prone to accumulate in the liver [77,78][19][20]. However, the PFSAs in cod liver oil are not under supervision, and little is known about them. PFOA and PFOS were also detected in fish samples collected from a local supermarket in Sweden at concentrations of 4.15 pg g−1 and 55.3 pg g−1, respectively [79][21].
The consumption of terrestrial animals, such as dairy and meat products, is a major source of edible animal-based oils. The contamination of PFASs in milk (butter-making raw materials) and meat (animal oil-making raw materials) was also frequently reported [80,81][22][23]. PFOS and PFOA were detected in milk samples from Greece. Concentrations of PFOS varied from <LOQ to 730 pg g−1 and PFOA from <LOQ to 570 pg g−1 (LOQ: 500 pg g−1) [82][24]. Analysis of milk samples from Turkey indicated that PFOA was not detected at levels above the reported LOQ of 38 pg g−1, yet PFOS was present at concentrations between 544 and 828 pg g−1 [83][25]. Researchers studied the concentrations of seven PFCAs and three PFSAs in milk and milk products from Poland. The most commonly detected was PFOA, followed by PFBA and perfluorohexane sulfonate, on par with perfluorooctane sulfonate. PFBA was the most prominent PFAS present in the studied food items, and it had an average concentration of 13.34 ng g−1 [84][26]. PFOS and PFOA were 100% detected in cow milk, butter, beef, and chicken meat samples; the highest PFOA and PFOS concentrations were found in butter at 9.4 pg g−1 and 114 pg g−1 [79][21].
PFASs have also been found in feed and animal-derived food, as well as in the transfer of PFASs through the “feed-to-food” chain [85,86][27][28]. Hence, PFAS contamination in animal feed should be taken into consideration. Ninety-two commercial fishmeal samples from the most important fishmeal-producing countries were collected for evaluating PFAS levels, and the results showed that Σ16 PFCAs ranged from 6.29 to 84.5, 1.42–52.0, 2.47–45.3, 1.06–42.1, and 1.02–38.8 ng g−1 in the U.S., China, Europe, South America, and Southeast Asia, respectively. Noteworthy, the presence of short-chain PFCAs (e.g., PFBA and PFBS) in fishmeal was found for the first time in the study [85][27]. Researchers from the same group also collected the most commonly used animal protein supplement feeds (blood meal, meat meal, feather meal, soybean meal, and dried grains with solubles), with concentrations of Σ16 PFCAs ranging from undetectable to 37.1 ng g−1 dw (dry weight) (average: 7.23 ng g−1 dw) [72][14]. The investigation of the occurrence of PFASs in cow feed samples from nine Chinese provinces revealed concentrations in the range of 0.99–144 ng g−1 dw (7.68 ng g−1 dw) and the PFBA dominating 34.0% of PFASs in feed [87][29]. In eighteen different lab animal feeding materials, PFOS, PFHxS, PFOA, and short-chain PFCAs (C < 6) had the highest detection levels and frequencies across all samples. PFAS levels found in feed were as high as 215.6 μg kg−1 dw [88][30]. It is of great concern that feed exposure to PFASs has not drawn enough attention. Meanwhile, investigations conducted recently have revealed an increase in the amount of short-chain PFCAs and PFSAs found in the environment, which may be due to the restriction of long-chain PFASs, causing a reduction in the use of long-chain PFASs and a heightened focus on short-chain PFASs.
3. Contamination of PFASs during Edible Oil Production
Most common edible oils are plant-derived through intricate manufacturing steps, which increases the risk of PFAS contamination. The primary emphasis here is on the PFAS pollution that occurs during plant-derived oil production. The production of edible oil mainly includes three parts: extraction, refining, and filtering [89][31], as shown in Figure 1. High temperatures can cause the breakdown of PFAS-containing materials, releasing more of these chemicals into the solvent, which in turn can dissolve in numerous organic solvents and ultimately into the product. Therefore, PFASs are easily introduced during extraction and refining processes involving high temperatures and organic solvents. The unrefined oil produced by mechanical pressing or solvent extraction of oil crops is called crude oil, which is not edible. During the pressing process, physical processes such as high-temperature baking and crushing may increase the concentration of environmental pollutants in oil products caused by the grinding machine being contaminated with PFAS-made detergent and lubricant, as well as PFASs from the plastic interface of the grinding machine. The solvent extraction is to extract the oil raw material by soaking it in organic solvent oil, which could also introduce PFASs [90][32]. It has been suggested that the higher concentration of C8-chain-length PFASs detected in the crude oil may be caused by the degradation of the precursor material into stable C8-chain-length PFASs and by the contamination of the crude oil by the processing apparatus [91][33].
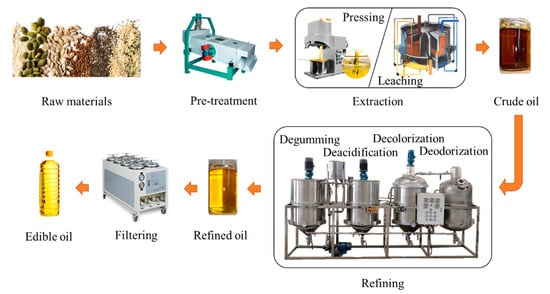
Figure 1.
Edible oil production process.
The refining process of edible oil mainly includes degumming, deacidification, decolorization and deodorization (Figure 1) [92,93][34][35]. PFASs are used in the production of various chemicals that are commonly used in the edible oil industry, such as surfactants and emulsifiers. Surfactants and emulsifiers are usually added to the oil during the refining process to improve its texture, taste, and appearance [94,95,96][36][37][38]. Furthermore, both deacidification and decolorization are performed at higher temperatures, which may also increase the possibility that PFASs dissolve in edible oil products.
The lack of research on the introduction of PFAS in the manufacturing of edible oils makes additional investigations necessary.
4. Migration from Oil Contact Materials to Edible Oil
A broad range of PFASs are used in paper and plastic, and they are commonly used to package high-fat content and convenience foods nowadays due to their non-hydrophilic and non-lipophilic properties [97][39]. Direct contact between the food contact materials and food could facilitate the migration of these PFASs into food products [98,99,100][40][41][42]. The migration of PFASs from contact materials to edible oil products occurs in the following two ways. First, oil-contact packaging, storage, and transport processing [90,101,102][32][43][44], particularly the use of plastic containers, can allow contaminants to leach into oil products depending on the contact time with the packaging materials [103,104][45][46]. Second, the product oil is usually ingested after being heated in contact with fluorocarbon resin-coated frying pans, baking utensils, and non-stick baking papers [31][47]. Research revealed that the concentration of PFOA in empty cooking pans increased up to 75 µg kg−1 after heat treatment [90][32]. The presence of PFOA and PFOS was determined in polytetrafluoroethylene (PTFE)-coated non-stick cookware sold in Turkey [105][48]. It was noted that FTOHs could migrate from paper bowls to oil, with migration efficiencies ranging from 0.04 to 2.28%; however, the efficiency of migration decreased as the carbon chain length of the FTOHs increased [106][49]. Therefore, routine monitoring and risk assessment of PFAS in oil are necessary.
It is essential to take the PFAS life cycle into account. These compounds are long-lasting in the environment, entering it through production and manufacturing processes, consumer use, and disposal. Once introduced to the environment, PFASs can remain in the atmosphere, aquifers, and the ground and can become concentrated in the tissues of living organisms, which could potentially be used as sources of edible oil. Edible oils are unavoidably contaminated with PFAS as a result of the use of fluorinated consumer products at the manufacturing, processing, and packaging stages, thus raising the probability of human exposure. The flow of PFASs from the primary producer to the product oil and the human body is shown in Figure 2.
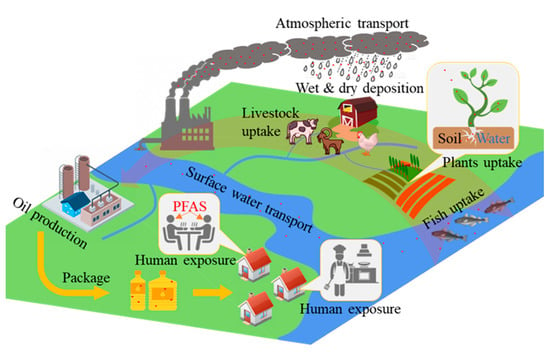
Figure 2.
The life cycle of PFASs in edible oil from the primary producer to human exposure.
References
- Zhou, Y.; Zhao, W.; Lai, Y.; Zhang, B.; Zhang, D. Edible plant oil: Global status, health issues, and perspectives. Front. Plant Sci. 2020, 11, 1315.
- Yang, L.; Jin, F.; Zhang, P.; Zhang, Y.; Wang, J.; Shao, H.; Jin, M.; Wang, S.; Zheng, L.; Wang, J. Simultaneous determination of perfluorinated compounds in edible oil by gel-permeation chromatography combined with dispersive solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2015, 63, 8364–8371.
- Evich, M.G.; Davis, M.J.B.; Mccord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065.
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Recent progress and challenges on the removal of per- and poly-fluoroalkyl substances (pfas) from contaminated soil and water. Environ. Sci. Pollut. Res. 2022, 29, 58405–58428.
- Zhang, D.Q.; Wang, M.; He, Q.; Niu, X.; Liang, Y. Distribution of perfluoroalkyl substances (pfass) in aquatic plant-based systems: From soil adsorption and plant uptake to effects on microbial community. Environ. Pollut. 2020, 257, 113575.
- Zhi, Y.; Liu, J. Sorption and desorption of anionic, cationic and zwitterionic polyfluoroalkyl substances by soil organic matter and pyrogenic carbonaceous materials. Chem. Eng. J. 2018, 346, 682–691.
- Xu, B.; Qiu, W.; Du, J.; Wan, Z.; Zhou, J.L.; Chen, H.; Liu, R.; Magnuson, J.T.; Zheng, C. Translocation, bioaccumulation, and distribution of perfluoroalkyl and polyfluoroalkyl substances (pfass) in plants. iScience 2022, 25, 104061.
- Huang, D.; Xiao, R.; Du, L.; Zhang, G.; Yin, L.; Deng, R.; Wang, G. Phytoremediation of poly- and perfluoroalkyl substances: A review on aquatic plants, influencing factors, and phytotoxicity. J. Hazard. Mater. 2021, 418, 126314.
- Yang, H.; Zhao, Y.; Tang, Y.; Gong, H.; Guo, F.; Sun, W.; Liu, S.; Tan, H.; Chen, F. Antioxidant defence system is responsible for the toxicological interactions of mixtures: A case study on pfos and pfoa in daphnia magna. Sci. Total Environ. 2019, 667, 435–443.
- Tian, Y.; Yao, Y.; Chang, S.; Zhao, Z.; Zhao, Y.; Yuan, X.; Wu, F.; Sun, H. Occurrence and phase distribution of neutral and ionizable per- and polyfluoroalkyl substances (pfass) in the atmosphere and plant leaves around landfills: A case study in tianjin, china. Environ. Sci. Technol. 2018, 52, 1301–1310.
- Yang, H.; Li, G.; Rao, Z.; Guo, F.; Li, Z.; Xie, F.; Tan, H. Occurrence and incidence of 18 per- and polyfluoroalkyl compounds in edible oils commonly consumed in guiyang, china. Food Addit. Contam. Part A-Chem. 2017, 34, 1573–1583.
- Chen, Y.; Fu, J.; Ye, T.; Li, X.; Gao, K.; Xue, Q.; Lv, J.; Zhang, A.; Fu, J. Occurrence, profiles, and ecotoxicity of poly- and perfluoroalkyl substances and their alternatives in global apex predators: A critical review. J. Environ. Sci. 2021, 109, 219–236.
- Ng, C.A.; Hungerbuehler, K. Exploring the use of molecular docking to identify bioaccumulative perfluorinated alkyl acids (pfaas). Environ. Sci. Technol. 2015, 49, 12306–12314.
- Li, X.; Gao, K.; Dong, S.; Liu, X.; Fu, K.; Wang, P.; Zhang, A.; Su, X.; Fu, J. Length-specific occurrence and profile of perfluoroalkyl acids (pfaas) in animal protein feeds. J. Hazard. Mater. 2019, 373, 224–231.
- Barhoumi, B.; Sander, S.G.; Driss, M.R.; Tolosa, I. Survey of legacy and emerging per- and polyfluorinated alkyl substances in mediterranean seafood from a north african ecosystem. Environ. Pollut. 2022, 292, 118398.
- Miranda, D.D.A.; Peaslee, G.F.; Zachritz, A.M.; Lamberti, G.A. A worldwide evaluation of trophic magnification of per- and polyfluoroalkyl substances in aquatic ecosystems. Integr. Environ. Assess. Manag. 2022, 18, 1500–1512.
- Wang, P.; Lu, Y.; Su, H.; Su, C.; Johnson, A.C.; Yu, L.; Jenkins, A. Managing health risks of perfluoroalkyl acids in aquatic food from a river-estuary-sea environment affected by fluorochemical industry. Environ. Int. 2020, 138, 105621.
- Valdersnes, S.; Nilsen, B.M.; Breivik, J.F.; Borge, A.; Maage, A. Geographical trends of pfas in cod livers along the norwegian coast. PLoS ONE 2017, 12, e177947.
- Groffen, T.; Wepener, V.; Malherbe, W.; Bervoets, L. Distribution of perfluorinated compounds (pfass) in the aquatic environment of the industrially polluted vaal river, south africa. Sci. Total Environ. 2018, 627, 1334–1344.
- Wang, G.; Lu, J.; Xing, Z.; Li, S.; Liu, Z.; Tong, Y. Occurrence, distribution, and risk assessment of perfluoroalkyl acids (pfaas) in muscle and liver of cattle in xinjiang, china. Int. J. Environ. Res. Public Health 2017, 14, 970.
- Sadia, M.; Yeung, L.W.Y.; Fiedler, H. Trace level analyses of selected perfluoroalkyl acids in food: Method development and data generation. Environ. Pollut. 2020, 263, 113721.
- Death, C.; Bell, C.; Champness, D.; Milne, C.; Reichman, S.; Hagen, T. Per- and polyfluoroalkyl substances (pfas) in livestock and game species: A review. Sci. Total Environ. 2021, 774, 144795.
- Pasecnaja, E.; Bartkevics, V.; Zacs, D. Occurrence of selected per- and polyfluorinated alkyl substances (pfass) in food available on the european market-a review on levels and human exposure assessment. Chemosphere 2022, 287, 132378.
- Kedikoglou, K.; Costopoulou, D.; Vassiliadou, I.; Leondiadis, L. Preliminary assessment of general population exposure to perfluoroalkyl substances through diet in Greece. Environ. Res. 2019, 177, 108617.
- Sungur, Ş.; Köroğlu, M.; Turgut, F. Determination of perfluorooctanoic acid (pfoa) and perfluorooctane sulfonic acid (pfos) in food and beverages. Int. J. Environ. Anal. Chem. 2018, 98, 360–368.
- Sznajder-Katarzyńska, K.; Surma, M.; Wiczkowski, W.; Cieślik, E. The perfluoroalkyl substance (pfas) contamination level in milk and milk products in poland. Int. Dairy J. 2019, 96, 73–84.
- Li, X.; Dong, S.; Zhang, W.; Fan, X.; Wang, R.; Wang, P.; Su, X. The occurrence of perfluoroalkyl acids in an important feed material (fishmeal) and its potential risk through the farm-to-fork pathway to humans. J. Hazard. Mater. 2019, 367, 559–567.
- Li, X.; Liu, Y.; Yin, Y.; Wang, P.; Su, X. Occurrence of some legacy and emerging contaminants in feed and food and their ranking priorities for human exposure. Chemosphere 2023, 321, 138117.
- Liu, Y.; Zhang, Q.; Li, Y.; Hao, Y.; Li, J.; Zhang, L.; Wang, P.; Yin, Y.; Zhang, S.; Li, T.; et al. Occurrence of per- and polyfluoroalkyl substances (pfass) in raw milk and feed from nine chinese provinces and human exposure risk assessment. Chemosphere 2022, 300, 134521.
- Choi, Y.J.; Lee, L.S.; Hoskins, T.D.; Gharehveran, M.M.; Sepúlveda, M.S. Occurrence and implications of per and polyfluoroalkyl substances in animal feeds used in laboratory toxicity testing. Sci. Total Environ. 2023, 867, 161583.
- Van Doosselaere, P. Production of oils. In Edible Oil Processing, 2nd ed.; Hamm, W., Hamilton, R.J., Calliauw, G., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 55–96.
- Begley, T.H.; White, K.; Honigfort, P.; Twaroski, M.L.; Neches, R.; Walker, R.A. Perfluorochemicals: Potential sources of and migration from food packaging. Food Addit Contam 2005, 22, 1023–1031.
- Yang, L. Study on Analysis Methodology of Perfluorinated Compounds in Edible Oils and Its Application. Master’s Thesis, China Agricultural University, Beijing, China, 2015.
- De Greyt, W. Edible oil refining: Current and future technologies. In Edible Oil Processing, 2nd ed.; Hamm, W., Hamilton, R.J., Calliauw, G., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 127–151.
- Vaisali, C.; Charanyaa, S.; Belur, P.D.; Regupathi, I. Refining of edible oils: A critical appraisal of current and potential technologies. Int. J. Food Sci. Technol. 2015, 50, 13–23.
- Babin, H.; Dickinson, E.; Chisholm, H.; Beckett, S. Interactions in dispersions of sugar particles in food oils: Influence of emulsifier. Food Hydrocoll. 2005, 19, 513–520.
- Johansson, D.; Bergenståhl, B. The influence of food emulsifiers on fat and sugar dispersions in oils. Iii. Water content, purity of oils. J. Am. Oil Chem. Soc. 1992, 69, 728–733.
- Tereshchuk, L.V.; Starovoytova, K.V.; Ivashina, O.A. Practical aspects of the use of emulsifiers in manufacturing emulsion fat-and-oil products. Food Raw Mater. 2018, 6, 30–39.
- Xu, Y.; Noonan, G.O.; Begley, T.H. Migration of perfluoroalkyl acids from food packaging to food simulants. Food Addit. Contam. Part A-Chem. 2013, 30, 899–908.
- Begley, T.H.; Hsu, W.; Noonan, G.; Diachenko, G. Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Addit. Contam. Part A-Chem. 2008, 25, 384–390.
- Lerch, M.; Fengler, R.; Mbog, G.; Nguyen, K.H.; Granby, K. Food simulants and real food-what do we know about the migration of pfas from paper based food contact materials? Food Packag. Shelf Life 2023, 35, 100992.
- Zabaleta, I.; Blanco-Zubiaguirre, L.; Baharli, E.N.; Olivares, M.; Prieto, A.; Zuloaga, O.; Elizalde, M.P. Occurrence of per- and polyfluorinated compounds in paper and board packaging materials and migration to food simulants and foodstuffs. Food Chem. 2020, 321, 126746.
- Poothong, S.; Boontanon, S.K.; Boontanon, N. Determination of perfluorooctane sulfonate and perfluorooctanoic acid in food packaging using liquid chromatography coupled with tandem mass spectrometry. J. Hazard. Mater. 2012, 205–206, 139–143.
- Yuan, J.; Ye, L.; Zhang, J.; Du, X.; Ma, A.; Pan, J. Nonaqueous electroextraction with tunable selectivity for direct, fast, and exhaustive enrichment of per- and polyfluoroalkyl acids from oils and food contact materials. Anal. Chem. 2022, 94, 15663–15670.
- Guillén, M.D.; Sopelana, P.; Palencia, G. Polycyclic aromatic hydrocarbons and olive pomace oil. J. Agric. Food Chem. 2004, 52, 2123–2132.
- Zhou, R.; Jiang, J.; Mao, T.; Zhao, Y.; Lu, Y. Multiresidue analysis of environmental pollutants in edible vegetable oils by gas chromatography-tandem mass spectrometry. Food Chem. 2016, 207, 43–50.
- Choi, H.; Bae, I.; Choi, J.C.; Park, S.; Kim, M. Perfluorinated compounds in food simulants after migration from fluorocarbon resin-coated frying pans, baking utensils, and non-stick baking papers on the korean market. Food Addit. Contam. Part B-Surveill. 2018, 11, 264–272.
- Toptancı, I.; Ketenoglu, O.; Kıralan, M. Assessment of the migration of perfluorinated compounds and primary aromatic amines from ptfe-coated non-stick cookware marketed in turkey. Environ. Sci. Pollut. Res. 2022, 29, 38535–38549.
- Yuan, G.; Peng, H.; Huang, C.; Hu, J. Ubiquitous occurrence of fluorotelomer alcohols in eco-friendly paper-made food-contact materials and their implication for human exposure. Environ. Sci. Technol. 2016, 50, 942–950.
More
