Acute heart failure (AHF) is a life-threatening condition with high morbidity and mortality. Even though this pathology has been extensively researched, there are still challenges in establishing an accurate and early diagnosis, determining the long- and short-term prognosis and choosing a targeted therapeutic strategy. The use of reliable biomarkers to support clinical judgment has been shown to improve the management of AHF patients. Despite a large pool of interesting candidate biomarkers, endothelin-1 (ET-1) appears to be involved in multiple aspects of AHF pathogenesis that include neurohormonal activation, cardiac remodeling, endothelial dysfunction, inflammation, atherosclerosis and alteration of the renal function.
- endothelin-1
- acute heart failure
- biomarkers
1. Introduction
2. The Endothelin System: Morphofunctional Considerations
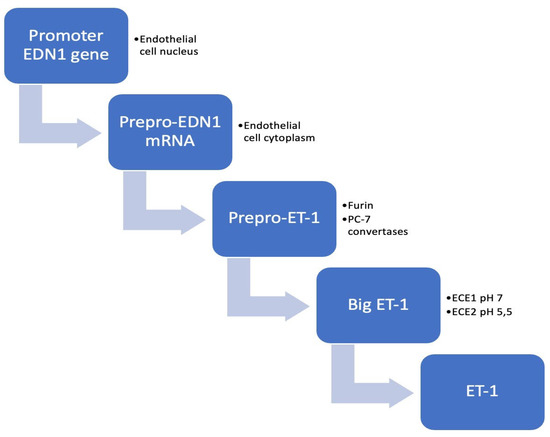
The endothelium has an essential role in protecting the arterial wall by releasing nitric oxide (NO) and prostacyclin. One year after discovering that endothelium has the ability to constrict and relax, one proposed the hypothesis of an endothelium-derived constricting factor [17]. The discovery of ET-1 by Yahagisawa et al. in 1988 represented a fundamental landmark in the field of cardiovascular research. In 1990, two receptors of ET-1 were identified, type A (ETA) and type B (ETB), respectively, offering the foundation for designing Bosentan (an antagonist of the ET-1 receptor), which can be currently used in the treatment of patients with pulmonary arterial hypertension. Multiple molecular and pharmacological approaches have outlined ET-1 as the most potent vasoconstrictor identified in biological systems to date. Since its discovery, remarkable efforts have been made to thoroughly understand the pathophysiological implications of this peptide in the cardiovascular system [18,19,20][18][19][20]. The ET system consists of three interconnected peptides: ET-1, ET-2 and ET-3. The 21-amino-acid peptide ET-1 is the main isoform produced at cardiovascular level and it has been the most intensively studied [19]. ETs have multiple pathophysiological functions involved not only in cardiovascular disorders (HF, arterial hypertension, cardiac hypertrophy, atherosclerosis), but also in pulmonary and kidney pathologies. Moreover, the ET system is implicated in tumoral processes, wound healing, and neurohormonal activation [21]. ET-1 is a 21-amino-acid cyclic peptide with two disulfide bonds consisting of four cysteines. The N-terminal end of the peptide defines its binding affinity to the receptor, whereas at the C-terminal end is located in the amino acids that bind to the receptor [21] (Figure 1).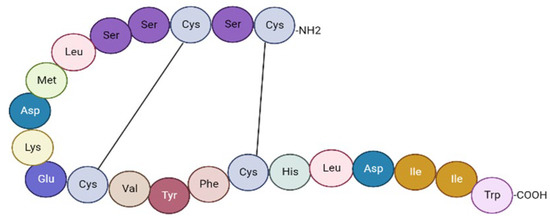
| ET-1 | ET-2 | ET-3 |
|---|---|---|
| Vascular smooth muscle cells | Gastrointestinal stromal cells | Gastrointestinal stromal cells |
| Endothelial cells | Kidney epithelial cells | Kidney epithelial cells |
| Cardiac myocytes | Neurons | |
| Kidney epithelial cells | Glia | |
| Inflammatory cells | ||
| Hepatocytes | ||
| Neurons |
References
- Mentz, R.J.; O’Connor, C.M. Pathophysiology and clinical evaluation of acute heart failure. Nat. Rev. Cardiol. 2016, 13, 28–35.
- Miftode, R.-S.; Costache, I.-I.; Cianga, P.; Petris, A.O.; Cianga, C.-M.; Maranduca, M.-A.; Miftode, I.-L.; Constantinescu, D.; Timpau, A.-S.; Crisan, A.; et al. The Influence of Socioeconomic Status on the Prognosis and Profile of Patients Admitted for Acute Heart Failure during COVID-19 Pandemic: Overestimated Aspects or a Multifaceted Hydra of Cardiovascular Risk Factors? Healthcare 2021, 9, 1700.
- Teerlink, J.R.; McMurray, J.J.; Bourge, R.C.; Cleland, J.G.; Cotter, G.; Jondeau, G.; Krum, H.; Metra, M.; O’Connor, C.M.; Parker, J.D.; et al. Tezosentan in patients with acute heart failure: Design of the Value of Endothelin Receptor Inhibition with Tezosentan in Acute heart failure Study (VERITAS). Am. Heart J. 2005, 150, 46–53.
- Tomasoni, D.; Lombardi, C.M.; Sbolli, M.; Cotter, G.; Metra, M. Acute heart failure: More questions than answers. Prog. Cardiovasc. Dis. 2020, 63, 599–606.
- Pourafkari, L.; Tajlil, A.; Nader, N.D. Biomarkers in diagnosing and treatment of acute heart failure. Biomark. Med. 2019, 13, 1235–1249.
- Arrigo, M.; Jessup, M.; Mullens, W.; Reza, N.; Shah, A.M.; Sliwa, K.; Mebazaa, A. Acute heart failure. Nat. Rev. Dis. Primers 2020, 6, 16.
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200.
- Sinnenberg, L.; Givertz, M.M. Acute heart failure. Trends Cardiovasc. Med. 2020, 30, 104–112.
- Wettersten, N. Biomarkers in Acute Heart Failure: Diagnosis, Prognosis, and Treatment. Int. J. Heart Fail. 2021, 3, 81–105.
- Maisel, A.S.; Choudhary, R. Biomarkers in acute heart failure-state of the art. Nat. Rev. Cardiol. 2012, 9, 478–490.
- Mallick, A.; Januzzi, J.L. Biomarkers in acute heart failure. Rev. Esp. Cardiol. 2015, 68, 514–525.
- Miftode, R.-S.; Constantinescu, D.; Cianga, C.-M.; Petris, A.-O.; Costache, I.-I.; Mitu, O.; Miftode, I.-L.; Mitu, I.; Timpau, A.-S.; Duca, S.-T.; et al. A Rising Star of the Multimarker Panel: Growth Differentiation Factor-15 Levels Are an Independent Predictor of Mortality in Acute Heart Failure Patients Admitted to an Emergency Clinical Hospital from Eastern Europe. Life 2022, 12, 1948.
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415.
- Jankowich, M.; Choudhary, G. Endothelin-1 levels and cardiovascular events. Trends Cardiovasc. Med. 2020, 30, 1–8.
- Matsubara, T.J.; Fujiu, K. Endothelin-1 and Atrial Cardiomyopathy. Int. Heart J. 2019, 60, 238–240.
- Omland, T. Targeting the endothelin system: A step towards a precision medicine approach in heart failure with preserved ejection fraction. Eur. Heart J. 2019, 40, 3718–3720.
- Ern Yeoh, S.; Docherty, K.F.; Campbell, R.T.; Jhund, P.S.; Hammarstedt, A.; Heerspink, H.J.L.; Jarolim, P.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; et al. Endothelin-1, Outcomes in Patients with Heart Failure and Reduced Ejection Fraction, and Effects of Dapagliflozin: Findings From DAPA-HF. Circulation 2023, 147, 1670–1683.
- Thorin, E.; Clozel, M. The cardiovascular physiology and pharmacology of endothelin-1. Adv. Pharmacol. 2010, 60, 1–26.
- Attinà, T.; Camidge, R.; Newby, D.E.; Webb, D.J. Endothelin antagonism in pulmonary hypertension, heart failure, and beyond. Heart 2005, 91, 825–831.
- Kostov, K. The Causal Relationship between Endothelin-1 and Hypertension: Focusing on Endothelial Dysfunction, Arterial Stiffness, Vascular Remodeling, and Blood Pressure Regulation. Life 2021, 11, 986.
- Khimji, A.K.; Rockey, D.C. Endothelin—Biology and disease. Cell. Signal. 2010, 22, 1615–1625.
- Böhm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18.
- Boerrigter, G.; Burnett, J.C. Endothelin in neurohormonal activation in heart failure. Coron. Artery Dis. 2003, 14, 495–500.
