Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by XIAOZHEN DIAO and Version 2 by Wendy Huang.
The blood-brain barrier (BBB) is a dynamic barrier separating neurocytes and brain tissues from blood that is extremely sealed and strictly regulated by transporters such as aquaporin-4 (AQP-4), glucose transporter (GLUT), and specialized tight junctional complexes (TJCs) including tight junctions (TJs), adherens junctions (AJs), and Zonulae occludens (ZOs). With specifically selective transcellular and paracellular permeability, the BBB maintains a homeostatic microenvironment to protect the central nervous system (CNS).
- blood-brain barrier
- tight junctions
- transporters
- aquaporin-4
- bioactive compounds
1. Introduction
So far, the BBB is well-known as a firmly guarded “gate” of the cerebral microenvironment against not only pathogens but also drugs or bioactive molecules, in which case great efforts have been devoted to drug delivery strategies to improve their permeability through the BBB [1]. However, its role as a therapeutic target of neurological disorders and diseases came into our horizon with the further investigation into structural or functional proteins of the BBB, especially the discovery of AQP-4 as a membrane “water channel” by Peter Agre et al., rewarded in 2003 with the Nobel Prize. Evidence of the correlation between the BBB disruption or dysfunction and CNS diseases also supports the perspective [2][3][4][2,3,4]. Although it seems to be a chance for us to seek new therapeutic strategies and develop novel drugs or bioactive compounds, limited achievements have been found until now. Moreover, due to their high safety and bioavailability compared to chemical drugs, natural bioactive compounds are among the most sought-after spots in the treatment of CNS diseases.
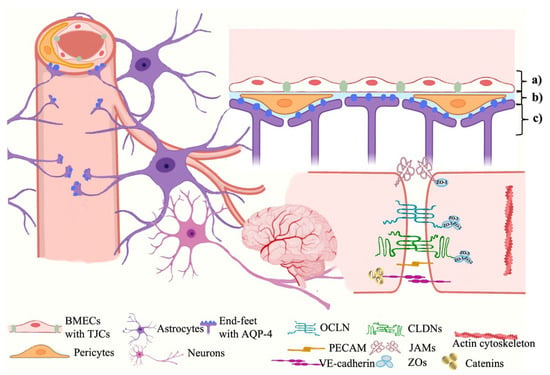
The BBB is composed of three spatial membranes spatially from the capillary to the parenchyma, which include: (a) the endothelial membrane constructed by the cytoplasmic membranes and tight junctional complexes (TJCs) between brain microvascular endothelial cells (BMECs), (b) the basement membrane formed by a network of extracellular matrix proteins secreted by BMECs, pericytes, and glial endfeet, and (c) the glia limitans formed by the endfeet of astrocytes (ACs) surrounding the capillary [5][6] (Shown in Figure 1). These membranes are correlated by the coordinated communication networks so-called neurovascular units (NVUs), which are composed of endothelial cells and other CNS cell types (such as pericytes, astrocytes, microglia, and leukocytes) to form barrier characteristics and function together [6][7].
 The surrounding astrocytes extend their neurites to form perivascular end-feet that attach to the surface of capillaries [16][18]. Astrocytes are highly polarized cells which express various transport proteins such as aquaporin-4 (AQP-4), glucose transported type 1(GLUT1), big current potassium (BK) channels [17][18][19,20]. Therefore, astrocytes not only maintain BBB function but also regulate cerebral blood flow to support neuronal metabolism in bidirectional neurovascular coupling, which is another essential participant in the regulation of the microenvironment in the central nervous circulatory system (CNS), along with the BBB [19][21]. Furthermore, astrocytes participate in the clearance of the wastes produced from brain [20][22]. AQP-4 which is highly concentrated on the cell surfaces of the astrocytic endfeet play a role in the maintenance of cerebral water homeostasis and neural conduction, and it is implicated in the development and resolution of edema in the pathophysiology of stroke [21][23]. Each monomer of AQP-4 existing as tetramers with four independent pores is composed of two similar halves integrating in opposite orientations by their internal pseudo two-fold symmetry [22][24]. (Shown in Figure 2).
The surrounding astrocytes extend their neurites to form perivascular end-feet that attach to the surface of capillaries [16][18]. Astrocytes are highly polarized cells which express various transport proteins such as aquaporin-4 (AQP-4), glucose transported type 1(GLUT1), big current potassium (BK) channels [17][18][19,20]. Therefore, astrocytes not only maintain BBB function but also regulate cerebral blood flow to support neuronal metabolism in bidirectional neurovascular coupling, which is another essential participant in the regulation of the microenvironment in the central nervous circulatory system (CNS), along with the BBB [19][21]. Furthermore, astrocytes participate in the clearance of the wastes produced from brain [20][22]. AQP-4 which is highly concentrated on the cell surfaces of the astrocytic endfeet play a role in the maintenance of cerebral water homeostasis and neural conduction, and it is implicated in the development and resolution of edema in the pathophysiology of stroke [21][23]. Each monomer of AQP-4 existing as tetramers with four independent pores is composed of two similar halves integrating in opposite orientations by their internal pseudo two-fold symmetry [22][24]. (Shown in Figure 2).
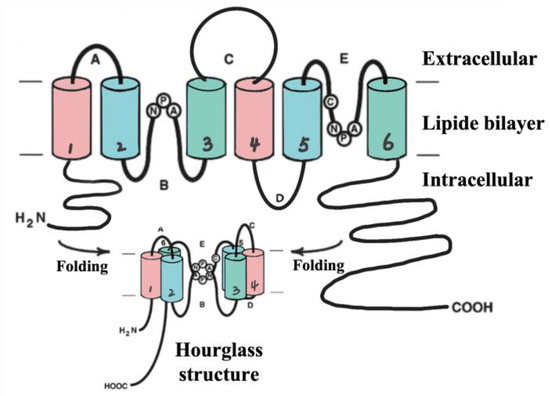

Figure 1. The BBB ultrastructure. The BBB is mainly constructed by three different membranes including (a) the endothelial membrane, (b) the basement membrane, and (c) the glia limitans, which involves various neurovascular unit (NVU) cells, structural and functional proteins of TJCs, and transporters, etc. BMECs, brain microvascular endothelial cells; TJCs, tight junctional complexes; AQP-4, aquaporin-4; OCLN, occludin; CLDNs, claudins; PECAM, platelet endothelial cell adhesion molecule; JAMs, junctional adhesion molecules; ZOs, zonulae occludens.
2. BMECs and BBB Tight Junctional Complexes
The junctional structures between BMECs form a physical barrier to provide highly selective permeability for the ions and molecules to maintain the homeostasis of the neural microenvironment and resist invasion and attack by endogenous or exogenous pathogens, which are known as tight junctional complexes (TJCs). TJCs fill up the intercellular interval between adjacent cells by TJs, AJs, and ZOs to construct the adjustable channels which only allow water or ions to pass through [5][6]. TJs consist of over forty kinds of proteins, mainly including claudins (CLDNs), occludin (OCLN), and junctional adhesion molecules (JAMs) [4]. So far, there are twenty known isoforms of CLDNs (CLDN-1~20) but only four of them (CLDN-1, 3, 5, 12) have been identified in the BBB [7][8], among which CLDN-3 and CLDN-5 own the highest expressions [8][9][10][9,10,11] and play vital roles in maintaining the TJs integrity [7][8]. Each CLDN monomer comprises four transmembrane helical bundles and forms two extracellular loops by folding, which are the ECL1 (determines structural compactness and selective permeability) and ECL2 (involved in intermolecular interactions) domains [11][12]. The expressions of OCLN and JAMs (only JAM-1 and 3 are expressed in the BBB) also influence the structure and function of the TJs [12][13]. AJs beneath TJs construct an adhesion belt across the intercellular crevice by vascular endothelial cadherin (VE-cadherin), connecting to actin cytoskeleton via catenins (α, β and γ) to form the E-cad/cat complex which provides cell-cell adhesion and structural support for TJs [13][14]. Although there has been no evidence for its determination of paracellular permeability so far, AJs’ structural destruction contributes to the disruption of TJs [14][15]. ZOs, mainly including ZO-1/2/3, link the TJs’ proteins to intracellular actin and cytoskeleton by cingulin, which play an important role to further inhibit the penetration of polar solutes from the plasma into the cerebral extracellular fluid via intercellular diffusion [15][16].3. Astrocytes and Aquaporin-4

Figure 2. AQP-4 structure (hourglass structure). AQP-4 is comprised of four subunits (~28 kDa), each of which contains six transmembrane domains including helix 1~6 (H1~6). Loop B (between H2 and H3) and loop E (between H2 and H3) contribute to the hourglass structure after folding to provide the water channel.
