Natural gas is a fossil fuel-based energy resource that is abundant in nature. It is a mixture of various components, predominantly methane. Natural gas has wide applications in the industries, residential buildings for heating, electricity generation, as a transportation fuel, and for various commercial purposes. The use of natural gas for electric power generation offers several advantages compared to other fossil fuels.
- capital investment cost
- operation expenditure
- economic analysis
- natural gas conversion
- environmental sustainability
- natural gas reforming
1. Introduction
Natural gas has high fuel efficiency and its cleaner compare to coal and petroleum [1][9]. Hence, less CO2 is emitted when natural gas is utilized for power generation compared to coal and petroleum [2][10]. Consequently, the global consumption of natural gas for power generation has increased over the years [3][11]. Due to its vital role in the global energy mix, the International Energy Outlook in 2016 projected that the global consumption of natural gas will rise to 203 TcF in 2040 [3][11]. Compared to other primary energy sources, natural gas accounted for a large proportion of global primary energy consumption [4][12].
Besides being used as a primary source of energy, the value chain of natural gas can be improved by converting it to various chemical products for sustainable use [5][6][5,13]. To achieve this, methane, a vital component of natural gas, is often used as a feedstock to produce value-added chemicals and chemical intermediates such as methanol and syngas [7][8][9][14,15,16]. Additionally, a large proportion of global hydrogen consumption is produced from the conversion of methane by the steam reforming technique [10][17]. The conversion of methane to value-added products can be achieved using various technological pathways such as reforming, fermentative and photocatalytic processes [11][12][18,19]. An extensive review of the nano-catalytic conversion of natural gas to liquid fuels and petrochemical feedstocks has been reported by Gharibi et al. [13][20]. The authors reported that natural gas can be converted directed or indirectly to various products using different types of nano-catalysts. The direct conversions include pyrolysis, methane dehydro-aromatization, and direct oxidative conversions. While the indirect methods involve the reforming of methane using different types of oxidants such as carbon dioxide, oxygen, and steam. A review reported by Gür [14][21] revealed the prospects of efficient electricity generation by the conversion of methane in solid oxide fuel cells. The study revealed that the electrochemical conversion of methane in solid oxide fuel offers a great potential for efficient generation of electricity [15][22]. The efficient conversion of natural gas to value-added products is key to its sustainable utilization. However, it is expedient to consider the economic viability and environmental sustainability of some of the emerging conversion technologies. Although there are several review papers on the conversion of natural gas to various products, none of these studies focused on the economic analysis and environmental implications of the technological processes used for natural gas conversion.
2. Natural Gas Conversion Technologies
The conversion of natural gas can be done using various technological routes broadly classify as thermochemical, biochemical, and photochemical in Figure 1. The thermochemical route entails the use of thermal energy and catalytic materials for the conversion of natural gas to various products. These methods include methane reforming using steam, carbon dioxide, and oxygen. Recently, research focus is shifting to the advanced form of reforming such as tri-reforming of methane, chemical looping reforming, and photocatalytic reforming [16][17][18][19][20][21][23,24,25,26,27,28]. Amongst the thermochemical processes, the steam methane reforming is the most established and presently being employed for a large proportion of global hydrogen production [10][22][23][17,29,30]. Other emerging reforming technologies using carbon dioxide and oxygen are seriously receiving research attention due to their perceived advantages over the well-established steam methane reforming [24][31]. One of such advantages is the prospect of the methane dry reforming to help mitigate greenhouse gases through the utilization of carbon dioxide. Besides, it is a potential technological route for producing syngas used as chemical intermediates for Fischer Tropsch synthesis.
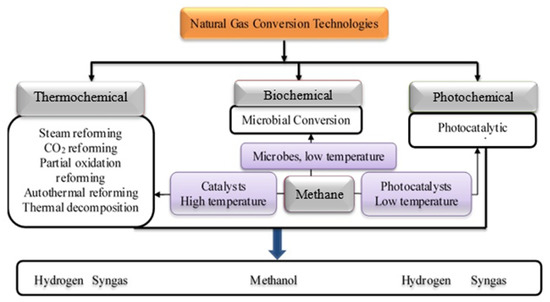
Figure 1. Schematic representation of natural gas conversion technologies.
The various reforming processes have been investigated using transition metal catalysts such as Ni, Co, Pd, Pt Cu, Ru, and Rh [25][26][27][28][32,33,34,35]. Among the various catalysts, Ni-based catalysts have been reported to displayed high activity for the steam reforming reaction [29][36]. Besides, it has a very low cost compared to other metal catalysts. However, it is very prone to catalyst deactivation by sintering and carbon deposition [30][37]. The stability of the Ni-catalysts during the reforming reaction can be improved using promoters. The use of noble metals in small composition has been reported to be effective in improving the stability of Ni-catalysts during reforming reaction. Rategarpanah et al. [31][38] reported the influence of noble metal on the activity of Ni-Cu/MgO-Al2O3 catalysts in the thermo-catalytic conversion of methane. The unpromoted Ni-Cu/MgO-Al2O3 was found to be unstable during the thermo-catalytic methane conversion. The addition of the noble metal promoters created a synergistic effect with the Ni-Cu/MgO-Al2O3, thereby preventing the sintering and deactivation of the catalysts and enhancing its stability with time on stream. Besides the use of promoters, a well-synthesized support material could significantly improve the activity and stability of Ni catalysts as demonstrated by Kumar et al. [17][24]. The stability of Ni-catalyst synthesized on Al2O3, ZrO2, TiO2, SBA-15, MgO, and CeO2-ZrO2 was investigated in tri-reforming of methane at 800 °C [17][24]. The Ni/SBA-15 was found to display the highest stability throughout the 10 h time on stream. The high stability of the Ni/SBA-15 can be attributed to the confinement of the Ni-species within the pores of the SBA-15 thereby preventing sintering and deactivation [32][39].
The use of advanced reforming processes such as coupling chemical looping reforming incorporated with CO2 splitting reactions has been reported [33][40]. This advanced reforming process could offer an advantage of mitigating CO2 emissions in a cyclic two-step syngas production. Additionally, the integration of solar power units with chemical looping reforming has been proven to help meet the high energy required for the reaction [34][41]. The use of a ceria oxygen carrier for solar enhanced chemical looping methane reforming was reported to increase syngas selectivity, methane conversion, and reactor performance [34][41]. In a similar study, Wang et al. [35][42] employed the use of CeO2-ZrO2-CuO oxygen carriers to catalyze syngas production by chemical looping reforming. The addition of Cu and Zr to the CeO2 leads to the distortion of the lattice thereby resulting in better oxygen mobility.
The biochemical conversion entails the use of microorganisms (methane-oxidizing organisms) to convert methane to methanol. The methanotrophic bacterium is commonly used for the conversion of methane to methanol. Studies have shown that the biochemical methane conversion to methanol offers the advantages of high conversion efficiency. More so, the biochemical process is more environmentally friendly compared to the thermochemical process. Besides, the presence of toxic impurities in the natural gas composition could inhibit catalytic activity during thermochemical methane conversion. On the contrary, the presence of impurities does not impede the biochemical process. Henard et al. [36][43] revealed that methanotrophic bacterium has a high capability of bio-converting methane to lactate, an industrial chemical platform. One of the major constraints with the bioconversion process is the low yield of the products. Moreover, there is often the challenge of insufficient genetic tractability of microorganisms that can be used to convert methane.
The use of abundant solar energy resources could help in overcoming the high thermal energy required for the thermochemical process. The photochemical conversion process enables the conversion of methane to hydrogen and syngas using a photocatalyst that can be excited under visible or ultraviolet irradiation. The photocatalytic reaction often occurs at a very low temperature compared to the thermochemical conversion process. Shoji et al. [37][44] demonstrated that strontium titanate supported rhodium nanoparticles (Rh/STO) was efficient in the photocatalytic conversion of natural gas to syngas. The Rh/STO photocatalyst exhibited high efficiency in promoting the conversion of methane under ultraviolet light irradiation. An H2:CO ratio close to 1 was obtained from photocatalytic reforming the methane, making it suitable as a technological route to produce oxygenated fuel by FTS using cobalt-based catalysts. Comparatively, each of the natural gas conversion processes has its pros and cons which can be evaluated using the life cycle assessment and life cycle cost analysis. The review of selected published articles on the various technological routes used for converting natural gas to value-added chemicals and chemical intermediated based on their life cycle assessment and life cycle cost analysis are presented in the next section.
Reference (we'll rearrange the references after you submitted it)
- Thiruvengadam, A.; Besch, M.; Padmanaban, V.; Pradhan, S.; Demirgok, B. Natural gas vehicles in heavy-duty transportation-A review. Energy Policy 2018, 122, 253–259.
- Gilbert, A.Q.; Sovacool, B.K. Benchmarking natural gas and coal-fired electricity generation in the United States. Energy 2017, 134, 622–628.
- International Energy Agency. World Energy Outlook 2016; International Energy Agency: Paris, France, 2016.
- International Energy Agency. World Energy Outlook 2019; International Energy Agency: Paris, France, 2019.
- Carmona, M.; Feria, J.; Golpe, A.A.; Iglesias, J. Energy consumption in the US reconsidered. Evidence across sources and economic sectors. Renew. Sustain. Energy Rev. 2017, 77, 1055–1068.
- Ayodele, B.V.; Hossain, S.S.; Lam, S.S.; Osazuwa, O.U.; Khan, M.R.; Cheng, C.K. Syngas production from CO2 reforming of methane over neodymium sesquioxide supported cobalt catalyst. J. Nat. Gas Sci. Eng. 2016, 34, 873–885.
- Ayodele, B.V.; Bin Mohd Yassin, M.Y.; Naim, R.; Abdullah, S. Hydrogen production by thermo-catalytic conversion of methane over lanthanum strontium cobalt ferrite (LSCF) and αAl2O3 supported Ni catalysts. J. Energy Inst. 2019, 92, 892–903.
- Ayodele, B.V.; Khan, M.R.; Cheng, C.K. Greenhouse gases mitigation by CO2 reforming of methane to hydrogen-rich syngas using praseodymium oxide supported cobalt catalyst. Clean Technol. Environ. Policy 2016, 1–13.
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrogen Energy 2014, 39, 16983–17000.
- Ayodele, B.V.; Khan, M.R.; Lam, S.S.; Cheng, C.K. Production of CO-rich hydrogen from methane dry reforming over lanthania-supported cobalt catalyst: Kinetic and mechanistic studies. Int. J. Hydrogen Energy 2016, 41, 4603–4615.
- Dagle, R.A.; Dagle, V.; Bearden, M.D.; Holladay, J.D.; Krause, T.R.; Ahmed, S. An Overview of Natural Gas Conversion Technologies for Co-Production of Hydrogen and Value-Added Solid Carbon Products; (No PNNL-26726, ANL-17/11); Pacific Northwest Natl Lab(PNNL): Richland, WA USA; Argonne Natl Lab(ANL): DuPage, IL, USA, 2017; Volume 65.
- Gharibi, M.; Zangeneh, F.T.; Yaripour, F.; Sahebdelfar, S. Nanocatalysts for conversion of natural gas to liquid fuels and petrochemical feedstocks. Appl. Catal. A Gen. 2012, 443, 8–26.
- Gür, T.M. Comprehensive review of methane conversion in solid oxide fuel cells: Prospects for efficient electricity generation from natural gas. Prog. Energy Combust. Sci. 2016, 54, 1–64.
- Pirker, G.; Wimmer, A. Sustainable power generation with large gas engines. Energy Convers. Manag. 2017, 149, 1048–1065.
- Alipour-Dehkordi, A.; Khademi, M.H. Use of a micro-porous membrane multi-tubular fixed-bed reactor for tri-reforming of methane to syngas: CO2, H2O or O2 side-feeding. Int. J. Hydrogen Energy 2019, 44, 32066–32079.
- Kathe, M.; Fryer, C.; Sandvik, P.; Kong, F.; Zhang, Y.; Empfield, A.; Fan, L.S. Modularization strategy for syngas generation in chemical looping methane reforming systems with CO2 as feedstock. AIChE J. 2017, 63, 3343–3360.
- Kim, S.; Crandall, B.S.; Lance, M.J.; Cordonnier, N.; Lauterbach, J.; Sasmaz, E. Activity and stability of NiCe@SiO2 multi–yolk–shell nanotube catalyst for tri-reforming of methane. Appl. Catal. B Environ. 2019, 259, 118037.
- Tahir, M.; Tahir, B.; Zakaria, Z.Y.; Muhammad, A. Enhanced photocatalytic carbon dioxide reforming of methane to fuels over nickel and montmorillonite supported TiO2 nanocomposite under UV-light using monolith photoreactor. J. Clean. Prod. 2019, 213, 451–461.
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S. Calcium promoted Fe/Al2O3 oxygen carrier for hydrogen production via cyclic chemical looping steam methane reforming process. Int. J. Hydrogen Energy 2015, 40, 16159–16168.
- Sastre, D.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Chemical insights on the activity of La1-xSrxFeO3 perovskites for chemical looping reforming of methane coupled with CO2-splitting. J. CO2 Util. 2019, 31, 16–26.
- Aboosadi, Z.A.; Yadecoury, M.F. Thermally Intensification of Steam Reforming Process by Use of Methane Tri-Reforming: A Review. Int. J. Chem. React. Eng. 2019, 17.
- Angeli, S.D.; Monteleone, G.; Giaconia, A.; Lemonidou, A.A. State-of-the-art catalysts for CH4 steam reforming at low temperature. Int. J. Hydrogen Energy 2014, 39, 1979–1997.
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611.
- Sidik, S.M.; Triwahyono, S.; Jalil, A.A.; Majid, Z.A.; Salamun, N.; Talib, N.B.; Abdullah, T.A. CO2 reforming of CH4 over Ni-Co/MSN for syngas production: Role of Co as a binder and optimization using RSM. Chem. Eng. J. 2016, 295, 1–10.
- Aman, D.; Radwan, D.; Ebaid, M.; Mikhail, S.; van Steen, E. Comparing nickel and cobalt perovskites for steam reforming of glycerol. Mol. Catal. 2018, 452, 60–67.
- Sadykov, V.A.; Gubanova, E.L.; Sazonova, N.N.; Pokrovskaya, S.A.; Chumakova, N.A.; Mezentseva, N.V.; Bobin, A.S.; Gulyaev, R.V.; Ishchenko, A.V.; Krieger, T.A.; et al. Dry reforming of methane over Pt/PrCeZrO catalyst: Kinetic and mechanistic features by transient studies and their modeling. Catal. Today 2011, 171, 140–149.
- Gokon, N.; Yamawaki, Y.; Nakazawa, D.; Kodama, T. Kinetics of methane reforming over Ru/γ-Al2O3-catalyzed metallic foam at 650–900 °C for solar receiver-absorbers. Int. J. Hydrogen Energy 2011, 36, 203–215.
- Abdullah, B.; Abd Ghani, N.A.; Vo, D.V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185.
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193.
- Rategarpanah, A.; Meshkani, F.; Wang, Y.; Arandiyan, H.; Rezaei, M. Thermocatalytic conversion of methane to highly pure hydrogen over Ni–Cu/MgO·Al2O3 catalysts: Influence of noble metals (Pt and Pd) on the catalytic activity and stability. Energy Convers. Manag. 2018, 166, 268–280.
- Kumar, R.; Kumar, K.; Choudary, N.V.; Pant, K.K. Effect of support materials on the performance of Ni-based catalysts in tri-reforming of methane. Fuel Process. Technol. 2019, 186, 40–52.
- Kang, D.; Lim, H.S.; Lee, J.W. Mesoporous Fe2O3–CeO2–Al2O3 Oxygen Carrier for Chemical Looping Dry Reforming with Subsequent Water Splitting. Ind. Eng. Chem. Res. 2020, 59, 15912–15920.
- Chuayboon, S.; Abanades, S.; Rodat, S. Solar chemical looping reforming of methane combined with isothermal H2O/CO2 splitting using ceria oxygen carrier for syngas production. J. Energy Chem. 2020, 41, 60–72.
- Wang, Y.; Zheng, Y.; Wang, Y.; Li, K.; Wang, Y.; Jiang, L.; Zhu, X.; Wei, Y.; Wang, H. Syngas production modified by oxygen vacancies over CeO2-ZrO2-CuO oxygen carrier via chemical looping reforming of methane. Appl. Surf. Sci. 2019, 481, 151–160.
- Henard, C.A.; Smith, H.; Dowe, N.; Kalyuzhnaya, M.G.; Pienkos, P.T.; Guarnieri, M.T. Bioconversion of methane to lactate by an obligate methanotrophic bacterium. Sci. Rep. 2016, 6, 21585.
- Shoji, S.; Peng, X.; Yamaguchi, A.; Watanabe, R.; Fukuhara, C.; Cho, Y.; Yamamoto, T.; Matsumura, S.; Yu, M.W.; Ishii, S.; et al. Photocatalytic uphill conversion of natural gas beyond the limitation of thermal reaction systems. Nat. Catal. 2020, 3, 148–153.
