1. Introduction
Chitin is abundantly present in the cell walls of
P. xanthii, either in epiphytic structures such as primary and secondary hyphae or in endophytic fungal cells, the haustoria. Due to
P. xanthii’s obligate biotrophic lifestyle, the fungus needs to cope with PTI (PAMP-triggered immunity), a defense reaction brought on by fungal contact with the plant cells, the immunity elicited by chitin oligomers being particularly intense. For a successful infection, it is believed that an arsenal of
P. xanthii effectors are required during penetration to downregulate chitin-mediated PTI signaling. Some of these effectors have been found in model biotrophs like
B. graminis f. sp.
hordei,
Cladosporium fulvum, and
U. maydis [1][121].
In previous studies, the epiphytic transcriptome of
P. xanthii was sequenced and annotated, determining the presence of 138 proteins, including 53
Podosphaera effector candidates (PECs). These proteins were identified based on the presence of a predicted signal peptide and the lack of functional annotations
[2][108]. Subsequently, host-induced gene silencing (HIGS) was used to uncover the genes involved during the initial plant–pathogen interactions
[3][118]. Several PEC-encoding genes were chosen to be investigated, revealing that six of them—PEC007, PEC009, PEC019, PEC032, PEC034, and PEC054—are necessary for
P. xanthii pathogenicity, as evidenced by a decreased fungal growth and an increased hydrogen peroxide production by host cells. Additionally, the biochemical activity of three of these effectors, PEC019, PEC032, and PEC054, was demonstrated, supporting the computational predictions and revealing new roles for powdery mildew effectors, including phospholipid-binding protein (PEC019/PxPLBP1), a-mannosidase (PEC032/PxMLE1), and cellulose-binding protein (PEC054/PxCLBE1). More intriguingly, these effectors are extensively dispersed in phytopathogenic fungi, according to BLAST searches, pointing to new targets for fungal effectors, including host–cell glycosylation, host–cell plasma membranes, and damage-associated molecular pattern-triggered immunity
[4][119]. Woth respect to the way that
P. xanthii avoids chitin recognition to deal with chitin resistance, this pathogen has developed several strategies such as binding, breaking, or modifying immunogenic oligomers, as described below and represented in
Figure 1.
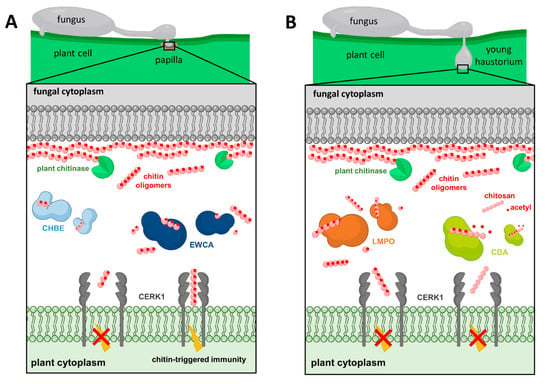
Figure 1. P. xanthii’s strategies to subvert chitin signaling. (A) EWCAs and CHBE are released by the fungus at the penetration site to break down or bind immunogenic chitin oligomers, respectively, blocking the dimerization of CERK1 and thus suppressing the activation of chitin-triggered immunity. (B) During the development of the haustorium, LMPO catalyzes chito-oligosaccharides into smaller molecules, whereas CDA converts cell wall chitin fragments into chitosan, thus evading chitin perception by the host plant.
2. Degradation of Chitin Oligomers
Chitinases are well-known fungal proteins that play a role in cell wall remodeling during growth as well as competitive interactions with other fungi
[5][122]. Chitinases and chitinase-like proteins have been found or computationally predicted in numerous powdery mildew genomes
[6][7][8][9][83,87,123,124]. A recent study of
P. xanthii revealed the involvement of a new family of secreted proteins with unclear functions
[2][10][57,108]. According to RNAi silencing assays, protein modeling, protein–ligand predictions, enzymatic assays, and protein localization studies, these proteins have been characterized as possessing intrinsic chitinase activities, enabling them to break down immunogenic chitin oligomers released from fungal cell walls by plants chitinases at pathogen penetration sites. These effectors disrupt the integrity of fungal structures and modify the chitin-derived signals perceived by the plant’s immune system, preventing the activation of chitin-triggered immunity
[10][57]. As a result, these proteins have been designated as effectors with chitinase activity (EWCAs) (
Figure 1A) which differ mostly in their C-terminal regions that, interestingly, contain a low-complexity region (LCR) abundant in alanine residues linked to gene growth and diversification
[11][125]. The reduced levels of chitin signals by EWCA activity leads to the attenuation of immune responses, as was determined after using various staining techniques to visualize callose deposits, which is indicative of cell wall strengthening, and hydrogen peroxide, which is a marker of ROS accumulation
[12][13][41,51]. The silenced samples showed greater accumulation of callose deposits compared to the control group, which only exhibited weak yellow fluorescence in penetrated cells and small yellow spots at penetration points
[10][57]. Similarly, there was a significant increase in the accumulation of brown precipitates, indicative of H
2O
2 production, in the plant cells of the silenced samples processed with the 3,3′-diaminobenzidine according to the DAB uptake method
[3][10][57,118]. In terms of fungal development, the control group had large colonies with a high number of widely spaced haustoria per colony. In contrast, the silenced colonies were smaller and had a low number of haustoria, but the haustoria were more densely packed. In addition, fungal biomass quantification using qPCR showed a clear reduction in fungal growth after silencing the EWCA genes. Overall, the study demonstrated that different effector candidates within the EWCA gene family have varying effects on plant responses, suggesting functional diversity among these genes
[10][57]. Additionally, similar genes were discovered in the genomes of numerous fungal pathogens, indicating that these effectors play an important role in fungal pathogenesis
[10][57].
The
P. xanthii haustorial transcriptome has also made it possible to discover the effector candidate PHEC27213, later known as PxLPMO1, which is the most highly expressed, haustorium-specific, putative secreted protein
[14][110]. Different computational predictions showed that PxHEC27213′s protein folding was similar to a lytic polysaccharide monooxygenase (LPMO) (
Figure 1B) that included a conserved histidine brace, but it had low sequence similarity with LPMO proteins and displayed a putative chitin-binding domain that differed from the canonical carbohydrate-binding module
[15][58]. However, binding and enzymatic experiments revealed that this protein functions as an LPMO and could bind and catalyze colloidal chitin and chito-oligosaccharides generated by plant endochitinases during the growth of haustoria, evading chitin perception by the host plant and permitting the development of haustoria inside plant epidermal cells. Furthermore, to validate its role, an
Agrobacterium tumefaciens-mediated host-induced gene silencing (ATM-HIGS) assay was conducted. The efficacy of ATM-HIGS was confirmed by the decreased transcript levels of PxLPMO1 during gene silencing experiments. After silencing PxLPMO1, the development of
P. xanthii was significantly altered and delayed compared to the negative control. Additionally, there was a strong accumulation of H
2O
2, indicating the activation of chitin-triggered immunity in PxLPMO1-silenced tissues. Fungal growth quantification through haustorial counts and qPCR showed a drastic reduction in fungal development in PxLPMO1-silenced melon cotyledons
[15][58]. To further confirm the role of PxLPMO1 in preventing chitin-triggered immunity, additional RNAi silencing experiments were conducted, including co-silencing of the melon chitin receptor kinase gene
CmCERK1. Co-silencing
PxLPMO1 and
CmCERK1 restored the normal phenotype, with normal
P. xanthii development and a low level of reactive epidermal cells. Fungal growth quantification and hydrogen peroxide production analyses supported these observations. Overall, the plant’s response to this fungal attack involved the activation of chitin-triggered immunity, suggesting that PxLPMO1 plays a crucial role in preventing the activation of the immune system
[15][58].
3. Chitin Deacetylation
P. xanthii has developed another strategy to overcome chitin detection by the conversion of cell wall chitin into chitosan by chitin deacetylase (CDA;
Figure 1B). Given the vast conservation of CDA in fungi, it is expected that deacetylation of chitin oligomers to avoid host recognition by chitin receptors is a widespread and conserved approach of plant pathogenic fungi to host survival
[16][46]. This enzyme catalyzes the hydrolysis of the N-acetamido group in the N-acetylglucosamine units of chitin to produce chitosan, a poor substrate for chitinases and a molecule with a significantly lower elicitor activity than chitin
[17][18][14,126]. In a previous study, the role of chitin deacetylase (CDA) in the pathosystem
P. xanthii melon and its interaction with the plant’s immune response were investigated. Two transcripts of the
P. xanthii CDA gene (
PxCDA1 and
PxCDA2) were identified, and RNAi silencing experiments were conducted to reduce their expression
[19][59]. The silencing of these transcripts resulted in a significant decrease in fungal growth and a concomitant increase in hydrogen peroxide production, suggesting the rapid activation of plant defense mechanisms against the pathogen. To further confirm the activation of chitin-triggered immunity, the same experiments were conducted with simultaneously silencing of the
PxCDA gene and the plant chitin elicitor receptor kinase gene
CmCERK1. In co-silenced tissues, fungal growth was fully restored and the production of hydrogen peroxide was considerably reduced. This confirms that the response activated in the plant after
PxCDA silencing is indeed chitin-triggered immunity, and the interaction between CDA and the plant’s immune receptors is important for disease development
[19][59]. Moreover, treatment with carboxylic acids, such as ethylenediaminetetraacetic acid (EDTA), a well-known CDA inhibitor, effectively suppressed powdery mildew disease, showing that CDA is a promising target for controlling phytopathogenic fungi and EDTA could be a starting molecule for fungicide design. In addition, EDTA treatment demonstrated efficacy in controlling other fungal diseases in strawberry and orange fruits caused by
B. cinerea and
Penicillium digitatum, respectively
[19][59]. However, high concentrations of EDTA had a phytotoxic effect, hampering host responses and allowing fungal growth on the compromised tissue. In this regard, in a recent study, novel fungicidal compounds with the potential to inhibit CDA were identified using a computer-aided drug design approach called molecular topology
[20][127]. These results indicate that CDA plays a major role in powdery mildew virulence and suggest that interfering with mechanisms of inhibition of chitin-triggered immunity could be a novel strategy for powdery mildew control
[19][59].
4. Binding of Chitin Oligomers
In previous studies, effector genes containing LysM domains were found to be absent in the genomes of powdery mildew fungi
[21], which was surprising, as suppression of chitin-triggered immunity should be essential for the survival in the host of these biotrophic fungi. In particular, this fact has been recently confirmed for
P. xanthii as a result of analyses of three independent genomes
[22][23][24][111,112,128], raising the question of whether a similar function to that of the LysM effectors may be performed by other proteins in these fungi. Perhaps the answer to this question lies in a recent study
[25][60].
PxCDA3, a remarkably brief CDA transcript, was identified in
P. xanthii. This transcript appeared to encode a shortened form of CDA because of an alternative splicing of the
PxCDA gene, which retained the carbohydrate-binding module but lost most of the chitin deacetylase activity domain, suggesting a potential ability to bind chitin oligomers. Experiments with the recombinant protein demonstrated its capacity to bind to chitin oligomers and prevent the activation of chitin signaling, and localization studies using fluorescent fusion proteins showed that PxCDA1 is restricted to the fungal cell wall, while PxCDA3 is found in plant papillae. These are structures that form at pathogen penetration sites, containing high levels of chitin due to the activity of plant chitinases that break down chitin fragments released by the pathogen
[10][57]. This suggesting PxCDA3′s role in scavenging chitin and preventing the activation of chitin-triggered immunity due to its chitin-binding capabilities similar to those of
C. fulvum Ecp6 or
M. oryzae Slp1 proteins
[13][17][21][25][26][7,14,21,51,60]. Protein coding by PxCDA3 was proposed as a new fungal chitin-binding effector and designated CHBE (
Figure 1A). This protein accumulates when plant chitinases are highly active during the early stages of infection, as
P. xanthii uses different effectors at precise times and locations to disarm chitin signaling. In addition, the presence of cysteine residues in PxCHBE was significant. Cysteine residues can form disulfide bridges, which provide stability and protection against plant proteases
[27][129]. This feature is characteristic of secreted proteins and allows PxCHBE to withstand the hostile environment in the plant and effectively perform its function. The plant’s immune response is a complex interplay of various defense mechanisms, including chitinases, antimicrobial peptides, ROS, and hormonal signaling pathways
[28][27]. However, pathogens like
P. xanthii evolving effector proteins such as PxCHBE to evade or defeat these immune responses allows them to establish successful infections, suppressing the activation of chitin-triggered immunity and promoting the growth and development of the fungus within the plant tissues. The authors also suggest that alternative splicing may be an evolutionary pathway for the emergence of new virulence factors in fungi, and in this case, powdery mildew fungi have evolved chitin-binding proteins that are involved in the manipulation of chitin-triggered immunity via chitin sequestration. These findings reinforce the idea that the evolution of molecular mechanisms to disarm the activation of chitin-triggered immunity is required for the successful colonization of plant habitats by fungi, especially haustorium-forming fungal pathogens.
Overall, the discovery of these effectors has provided insights into the complex interplay between fungal pathogens and plant immune systems. They play a critical role in suppressing chitin-triggered immunity by degrading, modifying, or binding to chitin molecules and intervening with chitin-derived immune signals. Understanding the mechanisms underlying the plant response to these fungal effectors is essential for developing strategies to enhance plant resistance against fungal pathogens and improve crop protection in agriculture.