Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Wendy Huang and Version 1 by Feyisayo Olabisi Adepoju.
Betulin is a natural triterpene, usually from birch bark, known for its potential wound-healing properties. Betulin has protective effects against cardiovascular and liver diseases, cancer, diabetes, oxidative stress, and inflammation. It reduces postprandial hyperglycemia by inhibiting α-amylase and α-glucosidase activity, combats tumor cells by inducing apoptosis and inhibiting metastatic proteins, and modulates chronic inflammation by blocking the expression of proinflammatory cytokines via modulation of the NFκB and MAPKs pathways.
- betulin
- cardiovascular disease
- diabetes
- cancer
- liver diseases
- inflammation
1. Introduction
Natural products have long been regarded as an attractive source of pharmacologically active substances, especially for infectious diseases and cancer. Despite the adoption of synthetic chemistry-based approaches in pharmaceutics, natural products continue to make significant contributions to the prevention and treatment of diseases. This is because of their diversity in nature, complexity, novelty, low toxicity, and broad efficacy. Beyond that, there is a need to uncover new compounds that can effectively treat diseases to improve therapeutic options [1]. Triterpenes are a class of natural compounds widely distributed in plants, with potential anti-inflammatory, hepatoprotective, antioxidant, anti-cancer, anti-viral, or cytotoxic properties. However, they usually account for less than 0.1% of plant organs’ dry weight.
Betulin, chemically known as lup-20(29)-ene-3β,28-diol, is a naturally occurring triterpene characterized by a five-membered ring and an isopropylidene group (Figure 1). Betulin and its other derivatives are found in various plant species, especially in the Betulaceae family, which is responsible for the silvery color of birch trees [2]. Factors such as species, geographical location, age, and climate have been shown to influence the dry weight content of betulin (10–45%) in plants. Considering the abundance of betulin in nature, other potentially bioactive forms are typically prepared using simple oxidation-reduction protocols, such as Jones oxidation and Pinnick oxidation.

Figure 1.
Image of birch trees which is the main source of betulin. The structural formula of betulin (
A
) and lupane pentacyclic skeleton of betulin (
B
).
According to the available literature, it is evident that betulin has a broad spectrum of biological activities (Figure 2), including anti-HIV, anti-fungal, anti-bacterial, anti-inflammatory, anti-tumor, anti-leishmania, and immune regulatory effects [1,3,4][1][3][4]. However, most of these studies have focused on derivatives of betulin. Recent studies have shown that betulin exerts significant pharmacological effects once its insolubility is resolved. From these studies, betulin is shown to exert multitarget activities in different organs and disease states. Betulin inhibits proinflammatory cytokines (IL-6, IL-1β, TNFα), HMGB-1, NFκB, and MAPK, which results in the reduction of lung and liver injuries in septic rats [5]. Even though the exact molecular mechanism of these activities is still unknown, studies have purported that the anti-inflammatory properties of betulin are at the heart of its different biological functions [5]. Many diseases, including infection, cancer, allergies, diabetes, asthma, arthritis, and atherosclerosis, are characterized by chronic inflammation [6,7][6][7]. Since inflammation is a key process in the development and occurrence of many chronic diseases, compounds with multitarget properties are the direction of the search for therapeutic drugs.
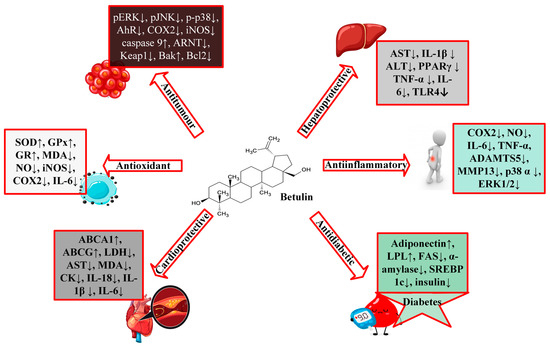
Figure 2.
A schematic representation of the biological activities of betulin.
2. Protective Effects of Betulin on Cardiovascular Diseases
Cardiovascular disease (CVD) is a group of conditions with several root causes that involve the heart and blood vessels, such as heart failure, cardiomyopathy, arrhythmia, ischemic heart disease, congenital heart disease, etc.; however, the underlying mechanisms vary depending on the disease. An estimated 17.9 million people died of cardiovascular disease in 2016, accounting for 31% of all deaths worldwide and the largest cause of death globally. According to the American Heart Association, cardiomyopathy is a heterogeneous group of cardiac muscle diseases, usually with inappropriate ventricular hypertrophy or dilation, with varying causes and can either be genetic or acquired, i.e., develop from other disease conditions [40][8].
It was found that betulin inhibited SREBP activation by significantly decreasing endoplasmic reticulum stress markers (BIP, CHOP, and PDI) in murine H9c2 cardiomyoblast cells, markedly improving cardiac morphological characteristics and alleviating pathological cardiac conditions (such as degenerating muscle fibers, vasculitis and infiltrating immune cells); lowering cardiac lipid levels (acetyl CoA carboxylase (ACC), FAS, and LDL); and increasing cardiac levels of ABCA1 [41][9].
A recent study using C57BL/KsJ-db/db mice and H9c2 cells discovered that betulin significantly decreased the ST-segment of the electrocardiogram and the area of myocardial infarction; improved myocardial function and cardiac pathological changes; upregulated Sirt1 expression while downregulating ASC, IL-1β, caspase-1, NLRP3, p-NFκB, CD68, and Gr-1 [42,43][10][11]. Moreover, oral betulin treatment (30 mg/kg per day for 14 weeks) to LDLR-knockout mice resulted in decreased lesions in the aortic arch and thoracic aorta and increased stability of atherosclerotic plaques [44][12]. Atherosclerosis is a major contributor to cardiovascular disease, and in HFD-apoE−/− mice, betulin (20 and 40 mg/kg) inhibited atherosclerotic lesions and enhanced cholesterol efflux by overexpressing the levels of ATP-binding cassette transporters, ABCA1 and ABCG1. The reseauthorchers further showed that betulin-enhanced ABCA1 expression in THP-1 and RAW264.7 cells was mediated by repression of SREBPs and inhibition of its target genes (HMG-CR, FAS, LDLR) [45][13].
3. Protective Effects of Betulin on Diabetes
About 537 million people globally are living with diabetes which is expected to increase to about 783 million in 2045 (www.idf.org; accessed on 22 May 2023). Diabetes mellitus is a long-term metabolic disorder featuring hyperglycemia, hyperlipidemia, and dysfunctional insulin secretion [43][11] The disease condition is accompanied by severe and debilitating comorbidities, including microvascular diseases: diabetic nephropathy, neuropathy, and retinopathy, as well as macrovascular diseases, such as coronary heart disease and peripheral vascular diseases. Triterpenoids, particularly the lupane-type, may be a promising therapeutic drug for diabetes because of their diverse biological actions, which include effects on glucose uptake and absorption, diabetic vascular dysfunction, and insulin secretion [46][14].
According to Wen et al., betulin administered to male C57BL/KsJ-db/db mice for 12 weeks at 20 and 40 mg/kg doses significantly decreased blood sugar, serum insulin, total triglyceride, and total cholesterol levels [43][11]. Other researchers have demonstrated that betulin restores insulin resistance by improving glucose tolerance, inhibiting lipid peroxidation in the hippocampus, modifying basal learning performance, reducing inflammatory cytokines (IL-6, IL-1β, TNFα), and inhibiting NFκB signaling axis in diabetic rats [47][15]. In a different investigation, betulin potentiated insulin-stimulated glucose absorption by increasing PPAR-γ activity in 3T3-L1 adipocytes [22][16] and significantly decreased glucose levels time-dependently in healthy and Alloxan-induced diabetic rabbits (0.2 g/kg), hence, exhibiting hypoglycemic effects [48][17]. Additionally, an in silico study using betulin isolated from Ruellia tuberosa L. was shown to be a non-competitive α-amylase inhibitor [49,50][18][19].
Several other authors have corroborated the excellent inhibitory activity of betulin on α-amylase and α-glucosidase [34,51,52,53][20][21][22][23]. Inhibitors of alpha-amylase and alpha-glucosidase have been therapeutically shown to improve post-prandial hyperglycemia in diabetic patients by delaying the rate of glucose metabolism [51,54][21][24] In another study, C57BL/6J mice fed a high-fat diet demonstrated that betulin treatment improved insulin sensitivity and glucose tolerance. The work further reported that betulin inhibited SREBP expression, downregulated SREBP-2 target genes (FAS, ACC, and SREBP-1c), and significantly increased adiponectin, LPL, and PPAR-γ expression in white adipose tissue, where the overexpression of these genes was thought to be antidiabetic and anti-inflammatory [44][12]. Impaired wound healing is a major risk factor associated with diabetes mellitus. An in vitro model of fibroblasts and keratinocytes obtained from both diabetic and non-diabetic donors evaluated for betulin-enhancing wound-healing effects led to enhanced mRNA levels of proinflammatory cytokines, chemokines, and mediators crucial for wound healing such as IL-6, TNF, IL-8 and RANTES [55][25]. Betulin demonstrated a range of advantageous benefits as an SREBP inhibitor in different experimental models, indicating betulin could be a promising therapeutic target to treat metabolic illnesses, particularly diabetes mellitus, and atherosclerosis.
4. Protective Effects of Betulin on Cancer
Nowadays, various therapies are employed clinically to improve cancer, including drugs or drug combinations, such as cisplatin, doxorubicin, etoposide, temozolomide, 5-fluorouracil, gefitinib, sorafenib; however, these drugs have various undesirable effects that greatly limit their applications (https://www.cancerresearchuk.org/about-cancer/treatment/chemotherapy/side-effects; https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs (accessed on 22 May 2023)). Hence, the need to discover new anti-cancer drugs or drug combinations with less toxicity and side effects. Understanding the association between naturally occurring bioactive compounds and known cellular targets is critical for developing effective cancer therapy strategies. There exists a considerable body of literature on many natural substances inducing intrinsic (mitochondrial) and extrinsic (Fas/FasL) apoptosis in cancer cells [56,57,58,59][26][27][28][29]. Other mechanisms of action are demonstrated by downregulating the expression of angiogenic and metastatic proteins (matrix metalloproteinases, MMPs, and VEGF) and by inhibiting several inflammatory mediators, including IL-6, iNOS, IL-8, COX2, IFN-γ, and TNF-α [60,61,62,63,64][30][31][32][33][34].
Natural substances or secondary metabolites widely dispersed in various organisms can exhibit anticancer effects or enhance the effectiveness of common chemotherapies. Studies on the anticancer activities of betulin are well documented (Table 1); it is also well acknowledged that betulin affected these activities through several mechanisms such as: (1) causing mitochondrial damage that results in cytochrome c release and apoptosis induction, (2) inducing apoptosis via caspase 9 and 3 activation pathway, (3) induction of autophagy, (4) over-expressing of PKC-δ, (5) inducing the death receptors via caspase 8 activity, (6) cell cycle arrest.
Numerous investigations have corroborated that betulin was effective in inhibiting the growth of prostate, breast, colorectal, and lung cancer cell lines [65,66][35][36]. A study on the human cell lines: epidermoid carcinoma (A431), cervix cancer (HeLa), and breast adenocarcinoma (MCF-7) demonstrated that betulin (13.28 µg/mL) extracted from Betula pendula exhibited 81.39, 70.30, and 35.54% inhibition, respectively [18][37]. In another in vitro research by Dehelean and co., betulin inhibited the growth of cancer cell lines in a dose-dependent manner (IC50 values: HeLa, 6.67 µM; A431, 6.76 µM; MCF7, 8.32 µM) by exhibiting a gradual nuclear condensation, fragmentation, and contraction, characteristics of cell apoptosis. For further confirmation, they performed an in vivo study using a chick chorioallantoic membrane, in which betulin demonstrated anti-angiogenic activity by reducing newly generated capillaries, particularly in the mesenchyme, without modification to the stromal architecture [67][38]. Additionally, a study on human gastric cancer (SGC7901 cells) revealed that betulin prevented cell proliferation and clonogenic growth of gastric cancer cells via activation of intrinsic apoptotic signaling axis by downregulating anti-apoptosis proteins XIAP and Bcl-2 [68][39].
It was also discovered that betulin significantly inhibited the viability of several human cell lines, including cervix cancer (HeLa), lung cancer (A549), liver cancer (HepG2), and breast cancer (MCF-7) with IC50 values ranging from 10–15 µg/mL and exhibited moderate antitumor activity in hepatoma (SK-HEP-1), prostate cancer (PC-3), and lung cancer (NCI-H460) with IC50 values of 20–60 µg/mL. The study further revealed that betulin induced apoptosis by activating caspase 9 and 3/7 but not caspase 8 in HeLa cells [69][40]. Mullauer et al. evaluated the effects of betulinic acid and betulin in combination with cholesterol on Jurkat T leukemia cells and described that the combination of betulin and cholesterol was effective in killing cancer cells in vitro [70][41]. Mitochondrial damage is responsible for betulin-cholesterol-induced apoptosis in Jurkat cells, according to the reseauthorchers. This damage leads to apoptosis and the release of cytochrome c, which is completely independent of Bcl-2 [70][41]. Another study on the antiproliferative effects of betulin on several cancer cells showed that betulin inhibited the growth of nervous system tumor cells (SK-N-AS, C6, and TE671), peripheral tissues (HT-29, T47D, FTC238, and A549), blood malignancies (RPMI8226, and Jurkat IE.6) and primary culture (HPOC, HPCC, and HPGBM). Additionally, betulin effectively reduced the migration of glioma (C6), lung cancer (A549), and medulloblastoma (TE671) cells and considerably caused apoptotic cell death in A549 cells at a low dose of 5 µM [71][42]. This raises the possibility that betulin can be used as a chemopreventive agent for patients with a higher risk of developing metastases in lung cancer, given that a non-toxic concentration of betulin can substantially inhibit the migration of multiple tumor cells and that the same dose can significantly inhibit the proliferation of tumor cells [71][42].
Oral administration of betulin abated lung metastasis of CT26 cells in Balb/c mice via cell cycle arrest, autophagy, and apoptosis through the regulation of the AMPK, PI3K/Akt/mTOR, and MAPK signaling pathways [72][43]. Other studies have also demonstrated that betulin nanoemulsion has a relative anti-angiogenic effect, low cytotoxicity, and inhibition of VEGF expression at the chorioallantoic membrane vascular level in chick embryos and demonstrated inhibition of skin tumor appearance and promotion by histological findings [73][44]. With an IC50 of 8 µM, betulin considerably slowed the growth of SK-N-SH cells in a more recent investigation on neuroblastoma. Additionally, it increased PKC-δ activity, which in turn activated caspases 3, 8, and 9, triggering endogenous apoptotic pathways in SK-N-SH cells that are mediated by mitochondria [74][45]. Similarly, betulin showed chemopreventive effects against cadmium-induced cytotoxicity in HepG2 cells. Betulin prevents apoptotic processes by inhibiting ROS production, cadmium-induced upregulation of Fas, caspase-8-dependent Bid activation, and subsequent inhibition of the mitochondrial pathway [75][46].
Given the number of studies conducted on betulin as a chemotherapeutic agent, some other studies have considered the use of betulin as a combination therapy. Co-treatment of human renal carcinoma cells (RCC4) with betulin (10 μM) and etoposide (10 μM) synergistically increases the levels of cleaved PARP and decreases MDR1 [76][47]. In another study, a combination treatment of betulin and cisplatin caused 50% inhibition of H460 cells at concentrations less than 5 μM as compared to the individual drugs [27][48] Likewise, another study on the co-treatment of hepatocellular carcinoma tumors with betulin and sorafenib revealed that betulin prevented the resistance of HCC cells to sorafenib [77][49]. Despite all of these recent positive studies on the chemotherapeutic properties of betulin, earlier studies on the cytotoxic effects of betulin have shown that it has no or limited cytotoxic effects on cancer cell lines [78,79][50][51].
Table 1.
Molecular mechanisms of betulin in tumor cells in different preclinical studies.
| Experimental Model | Dose/Concentration | Pharmacological Indicator | Molecular Mechanism | References | |
|---|---|---|---|---|---|
| Gastric SGC7901 cells | - | IC50 13 µg/mL | ROS ↑ Caspase 3 ↑ cleaved PARP ↑ Smac ↑ cytochrome c ↑ Bax ↑ Bak ↑ Bcl-2 ↓ XIAP ↓ Caspase 9 ↑ Bcl-xL * c-IAP1 * c-IAP2 * | Mitochondrial pathway | [68][39] |
| Human hepatoma HeLa cells | - | IC50 10 µg/mL | caspase9 ↑ caspase3/7 ↑ caspase 8 * cytochrome c ↑ Smac ↑ Bax ↑ Bak ↑ | Mitochondria pathway | [69][40] |
| Human lung adenocarcinomaA549 cells | - | 20 µM | enoyl-CoA hydratase ↓ PCBP 1 ↓ isoform 1 of 3-hydroxyacyl-CoA dehydrogenase type 2 ↓ malate dehydrogenase ↑ HSP 90-alpha 2 ↓ aconitate hydratase ↑ arginine/serine-rich splicing factor 1 ↑ | None | [65][35] |
| HepG2 cells | - | 10 µg/mL | Caspase 3 ↑ Caspase 9 ↑ | None | [80][52] |
| Murine CT26 human HCT116 | BALB/c mice injected intravenously with CT26 cells | 0–8 μM 5 and 10 mg/kg for 14 days |
Bcl-2 ↓ CyclinD1/CDK4 ↓ Bax ↑ cleaved caspase-3, -9, and -PARP ↑ LC3-II ↑ beclin ↑ p-ERK ↓ p-p38 ↓ Bcl-xL ↓ p-JNK ↓ | AMPK activation Blockage of the MAPK signaling pathway Inhibition of Pi3k/Akt/mTOR signaling pathway |
[72][43] |
| - | Female rats DMBA (25 mg/kg b.wt. s.c injection) |
20 mg/kg/b.wt. in corn oil (1 mL) | TBARS ↓ LOOH ↓ CAT ↑ SOD ↑ GPx ↑ Vit C ↑ Vit E ↑ GSH ↑ AhR ↓ ARnT ↓ CYP1A1 ↓ Keap1 ↓ HO-1 ↑ | Inhibition of MAPK proteins Activation of AhR/Nrf2 signaling axis | [81][53] |
| Human renal carcinoma cells (RCC4) | - | 10 and 25 μM | cleaved caspase3/7 ↑ cleaved caspase 8 ↑ cleaved PARP ↑ TRAIL R1/DR4 and R2/DR5 ↑ TNFR1 ↑ Bax ↑ XIAP ↓ PUMA ↑ Bcl-2 ↓ cleaved caspase 9 ↑ |
Activated mitochondrial apoptotic signaling and inhibited NFκB pathway | [76][47] |
| Non-small lung cancer cells (H460) | - | 11 and 30 μM | p53 ↓ Bcl-2L1 ↓ MMP2/9 ↓ BAK ↑ BAX ↑ caspase 3 ↑ caspase 6 ↑ caspase 9 ↑ caspase 8 ↓ HRK ↑ VEGF ↓ COX2 ↓ osteopontin ↓ | Mitochondrial intrinsic pathway | [27][48] |
| Human colon cancer cells (HCT116 and HT29) | - | 10 μg/mL | cleaved caspase 9 ↑ cleaved caspase 3 ↑ cytochrome c ↑ Bim ↑ | Induction of NOXA | [82][54] |
| Renal cell carcinoma (786-O and Caki-2) | - | 5 μM | p-S6 ↓ p-4EBP1 ↓ PKM2 ↓ HK2 ↓ | Modulation of mTOR signaling pathway | [83][55] |
| Human osteosarcoma cell (HOS and MG-63) | - | 0–20 μM | cleaved caspase 3 ↑ cleaved PARP ↑ p-mTOR ↓ cleaved caspase 9 ↑ p-4E-BP1 ↓ LC3-II ↑ cleaved caspase 8 ↑ p-Akt ↑ | Inhibition of mTOR signaling Activating autophagy |
[84][56] |
| - | Male Wistar Rat (DMH 20 mg/kg b.wt. s.c.) | 20 mg/kg b.wt for 16 weeks | GSH ↑ GPx ↑ SOD ↑ CAT ↑ IL-1β ↓ CYP450 ↓ CYT-b5 ↓ GST ↑ GR ↑ COX-2 ↓ iNOS ↓ TNF-α ↓ PCNA ↓ cyclin D1 ↓ IL-6 ↓ | None | [85][57] |
| human ovarian carcinoma cells (OVCAR-3) | - | 0–120 μM | Cyclin-D1 ↓ Bad ↑ Bax ↑ Bcl-2 ↓ Bcl-xL ↓ Cyclin-B1 ↑ Cyclin-E1 ↑ | modulating mTOR/Pi3k/Akt signaling pathway | [86][58] |
N.B: ↑—upregulate/increase, ↓—downregulate/decrease, * signifies no change poly(rC)-binding protein 1 (PCBP-1), heat shock protein 90-alpha 2.
Furthermore, betulin was shown to inhibit the expression of some inflammatory factors that augment and sustain a tumorigenic environment, including IL-6 and TNF-α, in the colon tissues of experimental and control animals [85][57]. Other studies further demonstrated that betulin treatment inactivated the activity of protein kinase B (Akt)/mTOR signaling, which enhances other proapoptotic mediators [72,83,84,86][43][55][56][58].
5. Protective Effects of Betulin on Liver Diseases
There are hundreds of liver diseases caused by viruses, toxins, genetic factors, alcohol, obesity, unknown causes, and other factors. Liver damage is a clinical manifestation that poses a life-threatening risk associated with a high mortality rate. Chronic diseases such as diabetes can also progressively affect the liver, thereby impairing liver function. Ample evidence has shown the beneficial effect of betulin on hepatic damage, with its spectrum of activities ranging from inhibition of SREBP-1 to upregulation of Sirt1, to reduction of NFκB signaling, etc. Since sustained excessive alcohol use can deteriorate the liver’s condition, researchers have modeled this disease pathology by inducing steatohepatitis via ethanol administration. Previous work showed that betulin (100 mg/kg b.wt.) attenuated steatosis, inflammation, and fibrosis in the liver of rats fed chronically with ethanol. The study also showed that betulin treatment could significantly improve histopathological signs of steatohepatitis, reduce liver function markers (AST and ALT) and liver and serum triglyceride levels, and restore redox imbalance by increasing GSH content and decreasing superoxide anions and TBARs concentration [87,88][59][60]. Similarly, another study demonstrated that betulin exerts hepatoprotective effects on alcohol-induced liver damage in mice through the Sirt1/LKB1/AMPK signaling pathway by inhibiting ethanol-induced activation of SREBP-1 and NFκB [89][61]. Szuster-Ciesielska et al. reported that betulin treatment inhibited the production of superoxide anions, procollagen type I, MMP-2, TIMP-1, and -2, α-SMA, and cells’ motility in acetaldehyde-induced toxicity in rat hepatic stellate cells (CFSC-2G cells) [90][62]. Furthermore, another study revealed that betulin decreased serum levels of ALT, AST, and triglycerides; inhibited EtOH-induced acidophilic necrosis; ameliorated liver histopathological changes; significantly decreased activity of CYP2E1 and expression of SREBP-1; markedly lessened the expression of TLR4; and improved phosphorylation of STAT3 in vitro and in vivo [91][63]. Elsewhere, betulin, betulinic acid, and oleanolic acid (1 μM each) were investigated for their hepatoprotective activity against ethanol-induced cytotoxicity in HepG2 cells, with betulin being the most active protectant of HepG2 cells against ethanol-induced cytotoxicity [92][64]. Another study proposed that betulin limited fibrosis formation and attenuated cisplatin-stimulated hepatic damage in rats by targeting apoptosis and NEK7-independent NLRP3 inflammasome pathways [93,94][65][66]. In addition, betulin mitigated increased levels of inflammatory factors, including IL-1α, IL-1β, IL-6, IL-18, TNF-α, TGF-β, and MMP2 in ethanol-induced liver stellate cells and chronic EtOH-treated rodents [87,88,94,95][59][60][66][67]. The hepatoprotective effects of betulin as well as its potential molecular pathways are briefly elucidated in Table 2, showing the concentration and dose of administration of betulin.Table 2.
Hepatoprotective activity of betulin.
| Experimental Model | Dose/Concentration | Pharmacological Indicators | Mechanism of Action | References | |
|---|---|---|---|---|---|
| - | Wistar Rats (Ethanol-induced alcoholic steatohepatitis 4 g/kg for 8 weeks) | 50 and 100 mg/kg b.wt. | TG ↓ ALP ↓ AST ↓ ALT ↓ TNF-α ↓ IL-1β ↓ TGF-β ↓ TBARs ↓ GSH ↑ ROS ↓ | None | [88][60] |
| Hepatic stellate cells (LX-2 cells) ethanol 50 mM) | Male C57BL/6 mice (Ethanol 5 g/kg b.wt. 10 days) |
6.25–25 μM 20 and 50 mg/kg |
SREBP1 ↓ TG ↓ ALT ↓ AST ↓ p65 ↓ collagen-I ↓ α-SMA ↓ | Sirt1/LKB1/AMPK signaling pathway | [89][61] |
| AML-12 cells (Ethanol 50 mM) | Male C57BL/6 mice (5 g/kg b.wt. EtOH for 4 weeks) | 0–25 μM 20 and 50 mg/kg |
SREBP 1 ↓ Lipin1 ↓ Lipin2 ↑ ALT ↓ AST ↓ IL-1α ↓ TG ↓ PPAR-α ↑ FASN ↓ PPAR-γ ↓ IL-1β↓ IL-6 ↓ TNF-α ↓ IL-18 ↓ caspase-1 ↓ | blocking of P2X7/NLRP3 signaling pathway | [94][66] |
| Hepatic stellate cells (HSC-T6, EtOH 50 mM) | Male C57BL/6 mice (EtOH 5 mg/kg) |
12.5–25 μM 20 or 50 mg/kg |
ALT ↓ AST ↓ TG ↓ CYP2E1 ↓ SREBP-1c ↓ TLR4 ↓ p-STAT3 ↑ Collagen-I ↓ α-SMA ↓ | TLR4 and STAT3 pathway | [91][63] |
| Rat liver stellate cell CFSC-2G (EtOH 50 mM) |
- | 10 μM | procollagen I ↓ TNF-α ↓ MMP2 ↓ TGF-β ↓ TIMP1/2 ↓ α-SMA ↓ | inhibited NFκB and MAPKs signaling pathway | [95][67] |
| - | LPS/D-galactosamine-induced acute liver injury BALB/c mice | 2–8 mg/kg b.wt. | MPO ↓ AST ↓ ALT ↓ TNF-α ↓ IL-1β ↓ PPARγ ↑ | inhibiting the NFκB signaling pathway | [87][59] |
| Con A-stimulated splenocytes | Concanavalin A-challenged C57BL/6J mice | 0–32 μg/mL 20 mg/kg |
IFN-γ ↓ IL-4 ↓ IL-6 ↓ IL-10 ↓ IL-17 ↓ IL-2 ↓ ALT ↓ AST ↓ TNF-α ↓ | None | [26][68] |
| - | Sprague Dawley rats (Cisplatin 10 mg/kg) | 8 mg/kg | caspase 3 ↓ caspase 9 ↓ tBilirubin↓ albumin ↑ caspase 8 ↓ MDA ↓ TAC ↑ IL-1 ↓ AST ↓ ALT ↓ caspase 1 ↓ p53 ↓ Bax ↓ Bcl-2 ↑ |
NLRP3 pathway | [93][65] |
N.B: ↑—upregulate/increase, ↓—downregulate/decrease.
6. Protective Effects of Betulin on Inflammation
Inflammation is a part of the host’s front line of defense against injury and infections. It is a biological process that results from the disruption of tissue homeostasis due to a variety of physical, chemical, or biological factors, including toxins, alcohol, pathogens, and so on [6] Inflammation is a complex, dynamic process regulated by multiple signaling pathways. It requires the interaction of different cells and modulates a wide range of cellular responses and homeostasis [7]. It is an adaptive response to noxious stimuli, such as tissue damage. Inflammation is divided into acute and chronic inflammation, where acute is beneficial to the host and is dominated by neutrophil infiltration characterized by classical signs of redness, heat, and swelling. However, when inflammation continues for a long time, mainly involving cellular infiltration of macrophages and lymphocytes, it becomes chronic, leading to the development of different chronic diseases [6]. The inflammatory pathway includes four parts: inducers, sensors, mediators, and effectors. Inflammatory inducers such as PAMPs, allergens, and AGEs trigger the production of multiple inflammatory mediators that change the functionality of the inflammatory effectors (organs and tissues) [6]. Several groups of inflammatory mediators can be produced during an inflammatory response, among which chemokines, lipid mediators (platelet-activating factors and eicosanoids), and cytokines have been extensively studied. As inflammation is a hallmark of many chronic diseases, there is a need for natural compounds that can effectively inhibit inflammation and target multiple disease-related signaling pathways. Traditionally, drugs that block inflammatory mediators and inhibit eicosanoid biosynthesis have been used to treat inflammation; however, targeting multiple targets rather than a single target is preferable in complex diseases such as inflammation. Furthermore, exploring the anti-inflammatory potential of natural products is a safer option in terms of therapeutic efficacy, side effects, and negative compensatory mechanisms [96][69].
References
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409.
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951.
- Šiman, P.; Filipová, A.; Tichá, A.; Niang, M.; Bezrouk, A.; Havelek, R. Effective method of purification of betulin from birch bark: The importance of its purity for scientific and medicinal use. PLoS ONE 2016, 11, e0154933.
- Laavola, M.; Haavikko, R.; Hämäläinen, M.; Leppänen, T.; Nieminen, R.; Alakurtti, S.; Moreira, V.M.; Yli-Kauhaluoma, J.; Moilanen, E. Betulin Derivatives Effectively Suppress Inflammation in vitro and in vivo. J. Nat. Prod. 2016, 79, 274–280.
- Zhao, H.; Liu, Z.; Liu, W.; Han, X.; Zhao, M. Betulin attenuates lung and liver injuries in sepsis. Int. Immunopharmacol. 2016, 30, 50–56.
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435.
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218.
- Wexler, R.; Elton, T.; Pleister, A.; Feldman, D. Cardiomyopathy: An Overview. Am. Fam. Physician 2009, 79, 778–784.
- Ayyappan, J.P.; Lizardo, K.; Wang, S.; Yurkow, E.; Nagajyothi, J.F. Inhibition of SREBP Improves Cardiac Lipidopathy, Improves Endoplasmic Reticulum Stress, and Modulates Chronic Chagas Cardiomyopathy. J. Am. Heart Assoc. 2020, 9, e014255.
- Yu, C.; Cai, X.; Liu, X.; Liu, J.; Zhu, N. Betulin Alleviates Myocardial Ischemia–Reperfusion Injury in Rats via Regulating the Siti1/NLRP3/NF-κB Signaling Pathway. Inflammation 2021, 44, 1096–1107.
- Wen, Y.; Geng, L.; Zhou, L.; Pei, X.; Yang, Z.; Ding, Z. Betulin alleviates on myocardial inflammation in diabetes mice via regulating Siti1/NLRP3/NF-κ-B pathway. Int. Immunopharmacol. 2020, 85, 106653.
- Tang, J.J.; Li, J.G.; Qi, W.; Qiu, W.W.; Li, P.S.; Li, B.L.; Song, B.L. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 2011, 13, 44–56.
- Gui, Y.; Yan, H.; Gao, F.; Xi, C.; Li, H.; Wang, Y. Betulin attenuates atherosclerosis in apoE−/− mice by up-regulating ABCA1 and ABCG1. Acta Pharmacol. Sin. 2016, 37, 1337–1348.
- Muceniece, R.; Namniece, J.; Nakurte, I.; Jekabsons, K.; Riekstina, U.; Jansone, B. Pharmacological research on natural substances in Latvia: Focus on lunasin, betulin, polyprenol and phlorizin. Pharmacol. Res. 2016, 113, 760–770.
- Ma, C.; Long, H. Protective effect of betulin on cognitive decline in streptozotocin (STZ)-induced diabetic rats. Neurotoxicology 2016, 57, 104–111.
- Ko, B.S.; Kang, S.; Moon, B.R.; Ryuk, J.A.; Park, S. A 70% Ethanol Extract of Mistletoe Rich in Betulin, Betulinic Acid, and Oleanolic Acid Potentiated β-Cell Function and Mass and Enhanced Hepatic Insulin Sensitivity. Evid.-Based Complement. Altern. Med. 2016, 2016, 7836823.
- Alsaadi, J.H.H. Isolation, Purification and Identification of Active Chemical Compound Lup-20(29)-ene-3, 28-diol (Betulin) from Tetradium daniellii Leaves and Study the hypoglycemic Effect on Rabbits. Univ. Thi-Qar J. Sci. 2016, 6, 54–61.
- Ratna Wulan, D.; Priyo Utomo, E.; Mahdi, C. Antidiabetic Activity of Ruellia tuberosa L., Role of α -Amylase Inhibitor: In Silico, In Vitro, and In Vivo Approaches. Biochem. Res. Int. 2015, 2015, 349261.
- Wulan, D.R.; Utomo, E.P.; Mahdi, C. Molecular modeling of Ruellia tuberosa L compounds as a-amylase inhibitor: An in silico comparation between human and rat enzyme model. Bioinformation 2014, 10, 209–215.
- Qaisar, M.N.; Uzair, M.; Imran, M.; Chaudhary, B.A.; Hussain, S.N. New α-glucosidase inhibitors from Croton bonplandianum Croton bonplandianum Baill (Euphorbiaceae). Trop. J. Pharm. Res. 2016, 15, 319–326.
- Thengyai, S.; Thiantongin, P.; Sontimuang, C.; Ovatlarnporn, C.; Puttarak, P. α-Glucosidase and α-amylase inhibitory activities of medicinal plants in Thai antidiabetic recipes and bioactive compounds from Vitex glabrata R. Br. stem bark. J. Herb. Med. 2020, 19, 100302.
- Gurupriya, S.; Cathrine, L. Molecular docking studies of isolated compounds andrographolide and betulin from methanolic leaves extract of Andrographis echioides as alpha-amylase and alpha-glucosidase activators. Int. J. Appl. Pharm. 2021, 13, 121–129.
- Yuca, H.; Özbek, H.; Demirezer, L.; Güvenalp, Z. Assessment of α-glucosidase and α-amylase inhibitory potential of Paliurus spina-christi Mill. and its terpenic compounds. Med. Chem. Res. 2022, 31, 1393–1399.
- Ilyina, A.; Arredondo-Valdés, R.; Farkhutdinov, S.; Segura-Ceniceros, E.P.; Martínez-Hernández, J.L.; Zaynullin, R.; Kunakova, R. Effect of Betulin-containing Extract from Birch Tree Bark on α-Amylase Activity In vitro and on Weight Gain of Broiler Chickens In vivo. Plant Foods Hum. Nutr. 2014, 69, 65–70.
- Wardecki, T.; Werner, P.; Thomas, M.; Templin, M.F.; Schmidt, G.; Brandner, J.M.; Merfort, I. Influence of Birch Bark Triterpenes on Keratinocytes and Fibroblasts from Diabetic and Nondiabetic Donors. J. Nat. Prod. 2016, 79, 1112–1123.
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 55.
- Esposito, S.; Bianco, A.; Russo, R.; Di Maro, A.; Isernia, C.; Pedone, P.V. Therapeutic Perspectives of Molecules from Urtica dioica Extracts for Cancer Treatment. Molecules 2019, 24, 2753.
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277.
- Tuli, H.S.; Sak, K.; Gupta, D.S.; Kaur, G.; Aggarwal, D.; Parashar, N.C.; Choudhary, R.; Yerer, M.B.; Kaur, J.; Kumar, M.; et al. Anti-inflammatory and anticancer properties of birch bark-derived betulin: Recent developments. Plants 2021, 10, 2663.
- Novío, S.; Cartea, M.E.; Soengas, P.; Freire-Garabal, M.; Núñez-Iglesias, M.J. Effects of Brassicaceae isothiocyanates on prostate cancer. Molecules 2016, 21, 626.
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525.
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262.
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321.
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714.
- Pyo, J.S.; Si, H.R.; Dae, K.K.; Jin, G.L.; Yong, Y.L.; Soon, S.H.; Sung, W.K.; Jeong, H.P. Anti-cancer effect of betulin on a human lung cancer cell line: A pharmacoproteomic approach using 2 D SDS PAGE coupled with nano-HPLC tandem mass spectrometry. Planta Med. 2009, 75, 127–131.
- Gauthier, C.; Legault, J.; Lavoie, S.; Rondeau, S.; Tremblay, S.; Pichette, A. Synthesis and cytotoxicity of bidesmosidic betulin and betulinic acid saponins. J. Nat. Prod. 2009, 72, 72–81.
- Şoica, C.; Dehelean, C.; Peev, C.; Aluas, M.; Zupkó, I.; Kása, P., Jr.; Alexa, E. Physico-chemical comparison of betulinic acid, betulin and birch bark extract and in vitro investigation of their cytotoxic effects towards skin epidermoid carcinoma (A431), breast carcinoma (MCF7) and cervix adenocarcinoma (HeLa) cell lines. Nat. Prod. Res. 2012, 26, 968–974.
- Dehelean, C.A.; Feflea, S.; Molnár, J.; Zupko, I.; Soica, C. Betulin as an antitumor agent tested in vitro on A431, HeLa and MCF7, and as an angiogenic inhibitor in vivo in the CAM assay. Nat. Prod. Commun. 2012, 7, 981–985.
- Li, Y.; Liu, X.; Jiang, D.; Lin, Y.; Wang, Y.; Li, Q.; Liu, L.; Jin, Y.H. Betulin induces reactive oxygen species-dependent apoptosis in human gastric cancer SGC7901 cells. Arch. Pharm. Res. 2016, 39, 1257–1265.
- Li, Y.; He, K.; Huang, Y.; Zheng, D.; Gao, C.; Cui, L.; Jin, Y.H. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010, 49, 630–640.
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulin is a potent anti-tumor agent that is enhanced by cholesterol. PLoS ONE 2009, 4, e1.
- Rzeski, W.; Stepulak, A.; Szymański, M.; Juszczak, M.; Grabarska, A.; Sifringer, M.; Kaczor, J.; Kandefer-Szerszeń, M. Betulin Elicits Anti-Cancer Effects in Tumour Primary Cultures and Cell Lines in vitro. Basic Clin. Pharmacol. Toxicol. 2009, 105, 425–432.
- Han, Y.H.; Mun, J.G.; Jeon, H.D.; Kee, J.Y. Betulin inhibits lung metastasis by inducing cell cycle arrest, autophagy, and apoptosis of metastatic colorectal cancer cells. Nutrients 2020, 12, 66.
- Dehelean, C.A.; Feflea, S.; Gheorgheosu, D.; Ganta, S.; Cimpean, A.M.; Muntean, D.; Amiji, M.M. Anti-angiogenic and anti-cancer evaluation of betulin nanoemulsion in chicken chorioallantoic membrane and skin carcinoma in Balb/c mice. J. Biomed. Nanotechnol. 2013, 9, 577–589.
- Feng, J.H.; Duan, X.Z.; Pan, J.Y.; Li, W.M.; Zhang, X.D.; Zhang, Y.S. Involvement of protein kinase C-δ activation in betulin-induced apoptosis of neuroblastoma. Trop. J. Pharm. Res. 2017, 16, 2097–2105.
- Oh, S.-H.; Choi, J.-E.; Lim, S.-C. Protection of betulin against cadmium-induced apoptosis in hepatoma cells. Toxicology 2006, 220, 1–12.
- Yim, N.H.; Jung, Y.P.; Kim, A.; Kim, T.; Ma, J.Y. Induction of apoptotic cell death by betulin in multidrug-resistant human renal carcinoma cells. Oncol. Rep. 2015, 34, 1058–1064.
- Zehra, B.; Ahmed, A.; Sarwar, R.; Khan, A.; Farooq, U.; Ali, S.A.; Al-Harrasi, A. Apoptotic and antimetastatic activities of betulin isolated from Quercus incana against non-small cell lung cancer cells. Cancer Manag. Res. 2019, 11, 1667–1683.
- Yin, F.; Feng, F.; Wang, L.; Wang, X.; Li, Z.; Cao, Y. SREBP-1 inhibitor Betulin enhances the antitumor effect of Sorafenib on hepatocellular carcinoma via restricting cellular glycolytic activity. Cell Death Dis. 2019, 10, 672.
- Kvasnica, M.; Sarek, J.; Klinotova, E.; Dzubak, P.; Hajduch, M. Synthesis of phthalates of betulinic acid and betulin with cytotoxic activity. Bioorganic Med. Chem. 2005, 13, 3447–3454.
- Gao, H.; Wu, L.; Kuroyanagi, M.; Harada, K.; Kawahara, N.; Nakane, T.; Umehara, K.; Hirasawa, A.; Nakamura, Y. Antitumor-promoting constituents from Chaenomeles sinensis Koehne and their activities in JB6 mouse epidermal cells. Chem. Pharm. Bull. 2003, 51, 1318–1321.
- Taiyi, S.J. Caspase-9 activation-critical for betulin-induced apoptosis of human hepatoma cells. Chem. Res. Chin. Univ. 2010, 26, 792–797.
- Zhang, J.; Zhou, B.; Sun, J.; Chen, H.; Yang, Z. Betulin ameliorates 7,12-dimethylbenz(a)anthracene-induced rat mammary cancer by modulating MAPK and AhR/Nrf-2 signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22779.
- Zhou, Z.; Zhu, C.; Cai, Z.; Zhao, F.; He, L.; Lou, X.; Qi, X. Betulin induces cytochrome c release and apoptosis in colon cancer cells via NOXA. Oncol. Lett. 2018, 15, 7319–7327.
- Cheng, W.; Ji, S.; Zhang, H.; Han, Z.; Liu, Q.; Wang, J.; Ping, H. mTOR activation is critical for betulin treatment in renal cell carcinoma cells. Biochem. Biophys. Res. Commun. 2017, 482, 1030–1036.
- Lin, Y.C.; Chen, H.Y.; Hsieh, C.P.; Huang, Y.F.; Chang, I.L. Betulin inhibits mTOR and induces autophagy to promote apoptosis in human osteosarcoma cell lines. Environ. Toxicol. 2020, 35, 879–887.
- Yu, J.; Li, M.; Zhan, D.; Shi, C.; Fang, L.; Ban, C.; Zheng, W.; Veeraraghavan, V.; Mohan, S.; Tang, X. Inhibitory effects of triterpenoid betulin on inflammatory mediators inducible nitric oxide synthase, cyclooxygenase-2, tumor necrosis factor-alpha, interleukin-6, and proliferating cell nuclear antigen in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Pharmacogn. Mag. 2020, 16, 836.
- Yang, Q.; Fei, Z.; Huang, C. Betulin terpenoid targets OVCAR-3 human ovarian carcinoma cells by inducing mitochondrial mediated apoptosis, G2/M phase cell cycle arrest, inhibition of cell migration and invasion and modulating mTOR/PI3K/AKT signalling pathway. Cell. Mol. Biol. 2021, 67, 14–19.
- Xu, G.M.; Zan, T.; Li, H.Y.; Han, J.F.; Liu, Z.M.; Huang, J.; Dong, L.H.; Zhang, H.N. Betulin inhibits lipopolysaccharide/D-galactosamine-induced acute liver injury in mice through activating PPAR-γ. Biomed. Pharmacother. 2018, 106, 941–945.
- Buko, V.; Kuzmitskaya, I.; Kirko, S.; Belonovskaya, E.; Naruta, E.; Lukivskaya, O.; Shlyahtun, A.; Ilyich, T.; Zakreska, A.; Zavodnik, I. Betulin attenuated liver damage by prevention of hepatic mitochondrial dysfunction in rats with alcoholic steatohepatitis. Physiol. Int. 2019, 106, 323–334.
- Bai, T.; Yang, Y.; Yao, Y.-L.; Sun, P.; Lian, L.-H.; Wu, Y.-L.; Nan, J.-X. Betulin alleviated ethanol-induced alcoholic liver injury via SIRT1/AMPK signaling pathway. Pharmacol. Res. 2016, 105, 1–12.
- Szuster-Ciesielska, A.; Plewka, K.; Kandefer-Szerszeń, M. Betulin, betulinic acid and butein are inhibitors of acetaldehyde-induced activation of liver stellate cells. Pharmacol. Rep. 2011, 63, 1109–1123.
- Wan, Y.; Jiang, S.; Lian, L.H.; Bai, T.; Cui, P.H.; Sun, X.T.; Jin, X.J.; Wu, Y.L.; Nan, J.X. Betulinic acid and betulin ameliorate acute ethanol-induced fatty liver via TLR4 and STAT3 in vivo and in vitro. Int. Immunopharmacol. 2013, 17, 184–190.
- Szuster-Ciesielska, A.; Kandefer-Szerszeń, M. Protective effects of betulin and betulinic acid against ethanol-induced cytotoxicity in HepG2 cells. Pharmacol. Rep. 2005, 57, 588–595.
- Eisa, N.H.; El-Sherbiny, M.; Abo El-Magd, N.F. Betulin alleviates cisplatin-induced hepatic injury in rats: Targeting apoptosis and Nek7-independent NLRP3 inflammasome pathways. Int. Immunopharmacol. 2021, 99, 107925.
- Dou, J.Y.; Jiang, Y.C.; Hu, Z.H.; Yao, K.C.; Yuan, M.H.; Bao, X.X.; Zhou, M.J.; Liu, Y.; Li, Z.X.; Lian, L.H.; et al. Betulin Targets Lipin1/2-Meidated P2X7 Receptor as a Therapeutic Approach to Attenuate Lipid Accumulation and Metaflammation. Biomol. Ther. 2022, 30, 246–256.
- Szuster-Ciesielska, A.; Plewka, K.; Daniluk, J.; Kandefer-Szerszeń, M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-α, TGF-β) production and by influencing intracellular signaling. Toxicology 2011, 280, 152–163.
- Zhou, Y.; Weng, X.; Dou, R.; Tan, X.; Zhang, T.; Fang, J.; Wu, X. Betulin from Hedyotis hedyotidea ameliorates concanavalin A-induced and T cell-mediated autoimmune hepatitis in mice. Acta Pharmacol. Sin. 2017, 38, 201–210.
- Koeberle, A.; Werz, O. Multi-target approach for natural products in inflammation. Drug Discov. Today 2014, 19, 1871–1882.
More
