Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Béla Völgyi.
Gap junctions (GJs) are not static bridges; instead, GJs as well as the molecular building block connexin (Cx) proteins undergo major expression changes in the degenerating retinal tissue. Various progressive diseases, including retinitis pigmentosa, glaucoma, age-related retinal degeneration, etc., affect neurons of the retina and thus their neuronal connections endure irreversible changes as well.
- retina
- gap junction
- connexin
- circadian rhythm
1. Introduction
The mammalian retina is a sheet of nervous tissue that covers the posterior aspect of the eye. Its neurons are organized into three cellular layers incorporating the light-sensitive photoreceptors (PRs), three interneuron populations including bipolar cells (BCs) horizontal cells (HCs) and amacrine cells (ACs), as well as the retinal ganglion cells (RGCs) that provide the sole output of the retina towards the brain. Neurons in the retina like in other brain areas communicate via chemical and/or electrical synapses. Following the initial descriptions [1[1][2][3][4],2,3,4], electrical synapses (also called gap junctions—GJ) were considered scarce and were thought to play insignificant roles, but their essential interplay in neuronal signaling became evident just recently. Experimental work over the past 3 decades also showed that GJs are just as important in the retina as in any other brain area [5,6][5][6]. Although the retinal distribution pattern of the retinal connexins (Cx; molecular building blocks of GJs) gained firm support, it is well documented that their expression levels are subject to changes in the external and internal milieu.
2. GJs and Connexin Building Blocks
GJs serve as conduits allowing for the free transcellular diffusion of ions and small molecules up to 1 kD [1[1][2],2], which paves the way for a molecular exchange between cells. In addition to small molecules, charged ions pass through GJs as well, thereby altering the membrane potential of both cells, which is a process that is utilized by neurons to perform fast and two-directional signaling. Due to this latter phenomenon, GJs are also called electrical synapses. At GJ sites, the membranes of neighboring cells form close appositions leaving only a very thin (1–2 nm) synaptic space in between. These intimate physical contacts harbor a piece of molecular machinery whose integral parts are the so-called connexons/hemichannels on each side. A functional channel is formed by two opposing hemichannels that are located in plasma membranes of neighboring cells. Each connexon is formed by a hexameric conglomerate of six membrane-spanning connexin (Cx) protein subunits. Cx-s are formed by four transmembrane domains: two extracellular and one intracellular loops and cytoplasmic C- and N-terminal endings. The amino acid sequence and the Cx makeup determine the molecular permeability and unitary conductance of each pore. The functional properties of the pore can also be modified by the intracellular molecular milieu, and thus the on- and offset of various signal-transduction pathways [7]. In the human and mouse genomes that have been studied most extensively, over 20 Cx genes were identified and sequenced [8]. Cx proteins are named simply after their molecular weights expressed in kilodaltons (kDa; ranging between 21–70 kDa, including Cx43, Cx40, Cx26, etc.). The Cx phylogenetic tree has three main branches comprising α-connexins (e.g., Cx38 and Cx40, Cx43, Cx45, Cx46, Cx50) with long intracellular C- and N-terminal chains, small β-connexins (e.g., Cx26, Cx30.2, Cx31 and Cx32) with short intracellular N-terminal [7] and medium-sized γ-connexins (e.g., mammalian Cx36, skate, perch and zebrafish Cx35, perch Cx34.7). In contrast to α- and/or β-Cx-s, pores formed by γ-Cx-s have unique features as they have low (or no) voltage- and pH-sensitivity and are also unable to form heterologous channels with α- and β-Cx subunits. It has now been firmly established that Cx-s are important in development, differentiation and growth control in all organs, and every vertebrate class uses a unique set of Cx-s to build GJs [9].3. GJs in the Retina
GJs are abundant in all brain areas including the mammalian retina (Figure 1), where all major neuron classes have been shown to form GJs to couple neighbor cells [6,10,11,12,13,14][6][10][11][12][13][14]. In addition to the great variety of connecting cell types, the diverse Cx makeup also suggests that retinal GJs play a number of roles in signal processing. The best-studied GJ sites in the vertebrate retina are those highly conductive GJs that couple HCs into extensive laterally oriented syncytia. GJs of this circuit are used to average signals of the ambient background light across the coupled HC array, the signal of which can be then utilized by BCs to detect contrasts prevailing against this uniform background. Homologous GJ synapses (connecting neurons of the same subtype) are commonly used to average noise, thereby increasing the signal-to-noise ratio. Such noise-reducing GJs exist in both plexiform layers, including the cone–cone GJs in the outer retina as well as those that couple AII ACs in extended homologous arrays in the inner retina [15]. On the other hand, heterologous GJs connecting rods to cone PRs provide an alternative route for rod-mediated signals [6,16,17,18,19,20,21][6][16][17][18][19][20][21]. Finally, a cohort of studies showed that GJs formed between RGC neighbors or connecting RGCs to nearby ACs partake in the correlation of the RGC spike output sent towards the brain [22,23,24,25,26][22][23][24][25][26]. Overall, these data indicate that electrical synaptic circuitry in the retina is as complex and dynamic as the well-described circuits formed by chemical neurotransmitters.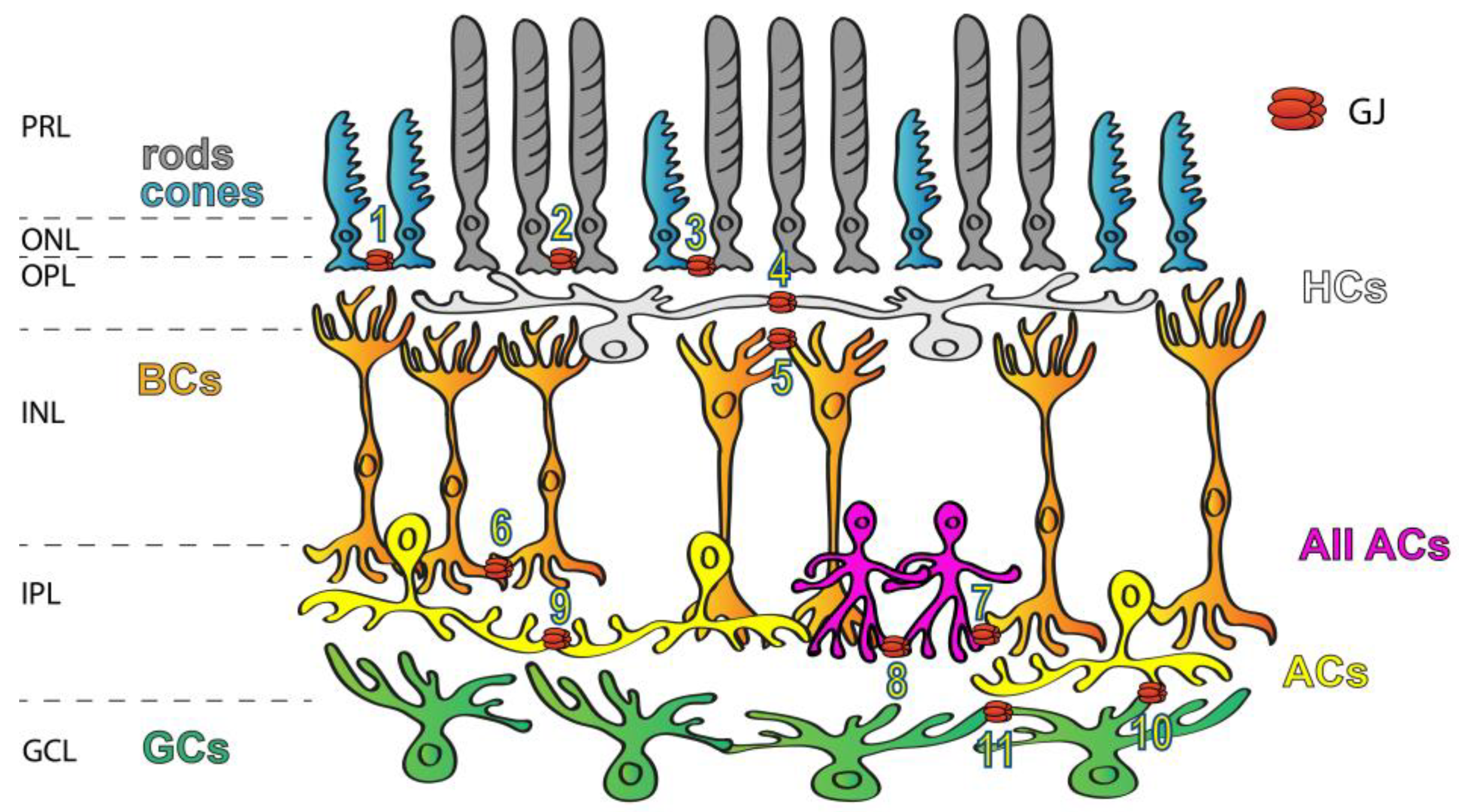
Figure 1. Expression of GJs in the mammalian retina. GJs connecting retinal neurons are ubiquitous and they form a variety of connections, including rod/rod (1), cone/cone (2), rod/cone (3), HC/HC (4), dendritic BC/BC (5), axonal BC/BC (6), AII AC/ON BC (7), AII AC/AII AC (8), AC/AC (9), AC/RGC (10) and RGC/RGC (11) GJs. Labels to the left mark the layers of the retinal tissue: PRL—photoreceptors layer, ONL—outer nuclear layer, OPL—outer plexiform layer, INL—inner nuclear layer, IPL—inner plexiform layer and GCL—ganglion cells layer.
4. Connexin Composition of Retinal GJs
Cx36 has been found in both plexiform layers [27] of the retina of various mammalian species, whereas other subunits are restricted to either plexiform layer (Figure 2). Cx45 for example can be found mostly in the inner retina [28], whereas both Cx50 and Cx57 are expressed by the outer retinal HCs and thus their distribution is confined to the OPL [29,30][29][30]. Cx36 GJs have been reported in multiple sites of the rod pathways, including AII AC junctions and those formed between rod and cone PRs [27,31,32,33,34,35,36][27][31][32][33][34][35][36]. In the proximal retina, Cx45 has been localized to some of the axon terminals of ON cone BCs at sites where they form GJs with the Cx36-expressing AII ACs. While these latter connections are clearly heterotypic, the rest of the AII AC/ON cone BC connections are homotypic and show the presence of Cx36 in both neuron populations [37,38][37][38]. Therefore, both Cx36 and Cx45 are essential subunits for transmitting rod-mediated signals for night vision pathways. Apart from mediating signals of the night vision pathways, both Cx36 and Cx45 participate in RGC circuits to serve visual feature encoding. It has been shown for instance that Cx36 is expressed by α-GCs and that Cx45 comprises GJs of ON–OFF direction-selective RGCs, serving spike synchronization and encoding of the direction of movement, respectively [39,40,41,42][39][40][41][42]. In addition to Cx36 and Cx45, only one other subunit, Cx30.2, has been shown to be expressed by A1 type of RGCs [43]. Besides the retinal distribution pattern of the Cx protein subunits, the expression levels of corresponding Cx36 mRNA transcripts have been studied as well, and their distribution patterns correlated with those of the subunit proteins [44,45,46][44][45][46]. However, GJs are not static bridges but, similar to chemical synapses, their prevalence and gating properties are highly regulated by changes throughout the postnatal development by external factors like adaptation to various light conditions and/or by the circadian master clock [38,47][38][47]. Many of the rapid changes in GJ coupling appear to be due to changes in post-translational modification (i.e., phosphorylation) rather than changes in protein expression, but longer lasting intrinsic and/or environmental alterations also induce expression changes as well.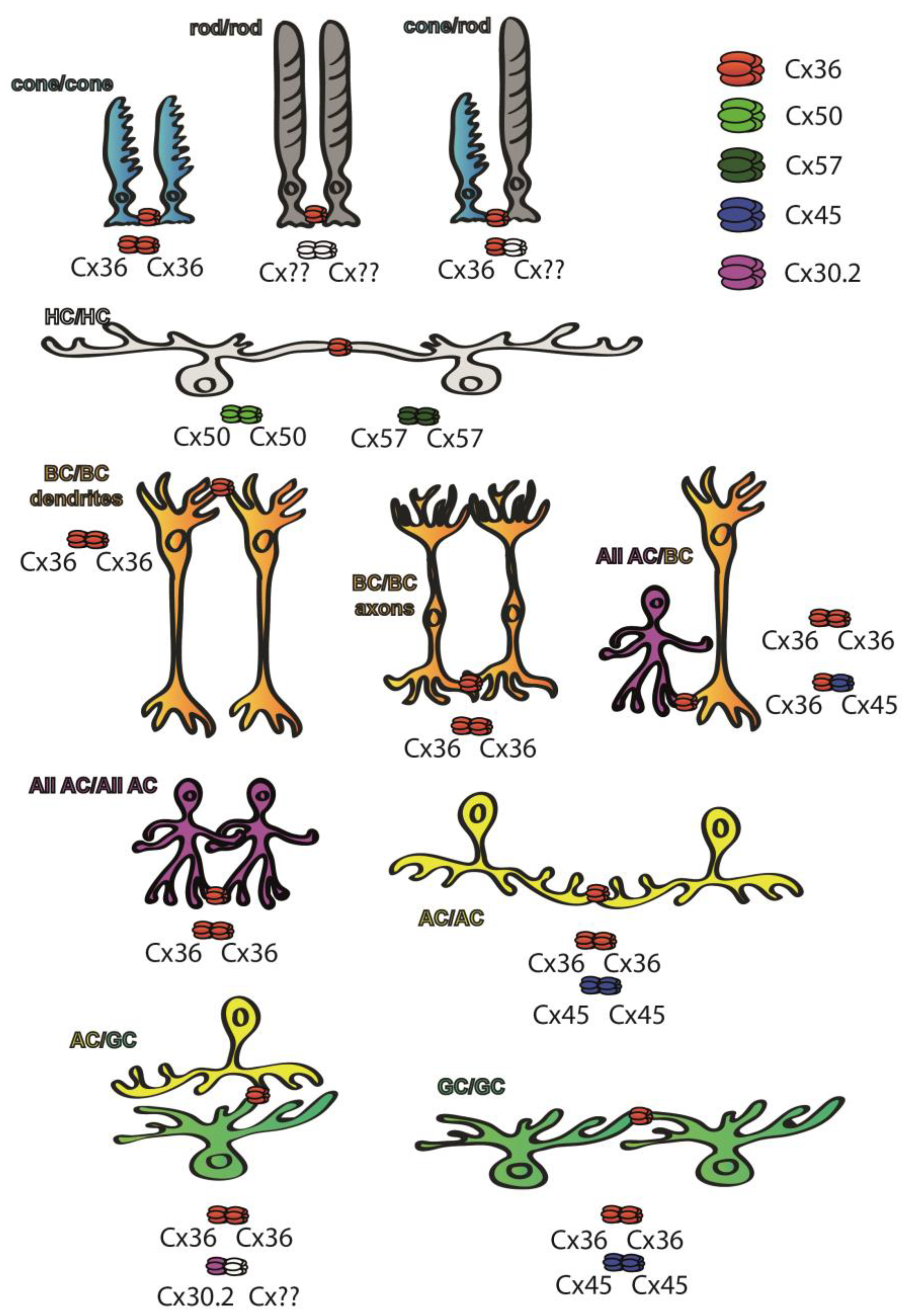
Figure 2. Cx subunit composition of GJs connecting neurons of the mouse retina. While Cx36 subunits are ubiquitous throughout the retina [27], Cx50 and Cx57 subunits are only found in outer retinal HC GJ connections [29[29][30],30], whereas Cx45 is majorly expressed in the inner retina [28]. Some of the retinal GJs are homotypic thus connecting neurons that express the same Cx subunits in their hemichannels, including cone/cone GJs [27,31,32,33[27][31][32][33][34][35][36],34,35,36], AII GJs [31,32,33][31][32][33] that comprise Cx36 or ON–OFF DS RGCs that express Cx45 [41]. Other connections are clearly heterotypic, thus connecting neurons expressing at least two different Cx subunits. The best known such connection is formed between BC axon terminals and AII ACs, in which AII cells express Cx36 while BCs express either Cx36 or Cx45 depending on their cellular subtype [37,38][37][38]. A third cohort of connections comprise a connexon whose identity has been confirmed, while the connecting hemichannel has not been determined yet (marked by ??). These latter connections include rod hemichannels [27[27][31][32][33][34][35][36],31,32,33,34,35,36], GJs that connect α-GCs into an array [39,40,42][39][40][42] or the ACs that form GJs with Cx30.2-expressing A1 type RGCs [43].
References
- Farquhar, M.G.; Palade, G.E. Cell junctions in amphibian skin. J. Cell Biol. 1965, 26, 263–291.
- Furshpan, E.J.; Potter, D.D. Mechanism of Nerve-Impulse Transmission at a Crayfish Synapse. Nature 1957, 180, 342–343.
- Rosenbluth, J. Smooth Muscle: An Ultrastructural Basis for the Dynamics of Its Contraction. Science 1965, 148, 1337–1339.
- Watanabe, A. The interaction of electrical activity among neurons of lobster cardiac ganglion. Jpn. J. Physiol. 1958, 8, 305–318.
- Brightman, M.W.; Reese, T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969, 40, 648–677.
- Raviola, E.; Gilula, N.B. Gap Junctions between Photoreceptor Cells in the Vertebrate Retina. Proc. Natl. Acad. Sci. USA 1973, 70, 1677–1681.
- Cruciani, V.; Mikalsen, S.-O. The Vertebrate Connexin Family. Cell. Mol. Life Sci. 2006, 63, 1125–1140.
- Söhl, G.; Willecke, K. An Update on Connexin Genes and Their Nomenclature in Mouse and Man. Cell Commun. Adhes. 2003, 10, 173–180.
- De Boer, T.P.; Van Der Heyden, M.A.G. Xenopus Connexins: How Frogs Bridge the Gap. Differentiation 2005, 73, 330–340.
- Marc, R.E.; Liu, W.L.; Muller, J.F. Gap Junctions in the Inner Plexiform Layer of the Goldfish Retina. Vis. Res. 1988, 28, 9–24.
- Mills, S.L.; Massey, S.C. Differential Properties of Two Gap Junctional Pathways Made by AII Amacrine Cells. Nature 1995, 377, 734–737.
- Raviola, E.; Gilula, N.B. Intramembrane Organization of Specialized Contacts in the Outer Plexiform Layer of the Retina. A Freeze-Fracture Study in Monkeys and Rabbits. J. Cell Biol. 1975, 65, 192–222.
- Vaney, D.I. Many Diverse Types of Retinal Neurons Show Tracer Coupling When Injected with Biocytin or Neurobiotin. Neurosci. Lett. 1991, 125, 187–190.
- Xin, D.; Bloomfield, S.A. Tracer Coupling Pattern of Amacrine and Ganglion Cells in the Rabbit Retina. J. Comp. Neurol. 1997, 383, 512–528.
- Bloomfield, S.A.; Völgyi, B. The Diverse Functional Roles and Regulation of Neuronal Gap Junctions in the Retina. Nat. Rev. Neurosci. 2009, 10, 495–506.
- Bloomfield, S.A.; Völgyi, B. Function and Plasticity of Homologous Coupling between AII Amacrine Cells. Vis. Res. 2004, 44, 3297–3306.
- Dacheux, R.; Raviola, E. Horizontal Cells in the Retina of the Rabbit. J. Neurosci. 1982, 2, 1486–1493.
- Nelson, R. Cat Cones Have Rod Input: A Comparison of the Response Properties of Cones and Horizontal Cell Bodies in the Retina of the Cat. J. Comp. Neurol. 1977, 172, 109–135.
- Schneeweis, D.; Schnapf, J. Photovoltage of Rods and Cones in the Macaque Retina. Science 1995, 268, 1053–1056.
- Smith, R.G.; Vardi, N. Simulation of the Aii Amacrine Cell of Mammalian Retina: Functional Consequences of Electrical Coupling and Regenerative Membrane Properties. Vis. Neurosci. 1995, 12, 851–860.
- Völgyi, B.; Deans, M.R.; Paul, D.L.; Bloomfield, S.A. Convergence and Segregation of the Multiple Rod Pathways in Mammalian Retina. J. Neurosci. 2004, 24, 11182–11192.
- Brivanlou, I.H.; Warland, D.K.; Meister, M. Mechanisms of Concerted Firing among Retinal Ganglion Cells. Neuron 1998, 20, 527–539.
- DeVries, S.H. Correlated Firing in Rabbit Retinal Ganglion Cells. J. Neurophysiol. 1999, 81, 908–920.
- Hu, E.H.; Bloomfield, S.A. Gap Junctional Coupling Underlies the Short-Latency Spike Synchrony of Retinal α Ganglion Cells. J. Neurosci. 2003, 23, 6768–6777.
- Mastronarde, D.N. Correlated Firing of Cat Retinal Ganglion Cells. I. Spontaneously Active Inputs to X- and Y-Cells. J. Neurophysiol. 1983, 49, 303–324.
- Völgyi, B.; Pan, F.; Paul, D.L.; Wang, J.T.; Huberman, A.D.; Bloomfield, S.A. Gap Junctions Are Essential for Generating the Correlated Spike Activity of Neighboring Retinal Ganglion Cells. PLoS ONE 2013, 8, e69426.
- Güldenagel, M.; Söhl, G.; Plum, A.; Traub, O.; Teubner, B.; Weiler, R.; Willecke, K. Expression Patterns of Connexin Genes in Mouse Retina. J. Comp. Neurol. 2000, 425, 193–201.
- Petrasch-Parwez, E.; Habbes, H.-W.; Weickert, S.; Löbbecke-Schumacher, M.; Striedinger, K.; Wieczorek, S.; Dermietzel, R.; Epplen, J.T. Fine-Structural Analysis and Connexin Expression in the Retina of a Transgenic Model of Huntington’s Disease: Retinal Degeneration in R6/2 Transgenic Mice. J. Comp. Neurol. 2004, 479, 181–197.
- Hombach, S.; Janssen-Bienhold, U.; Sohl, G.; Schubert, T.; Bussow, H.; Ott, T.; Weiler, R.; Willecke, K. Functional Expression of Connexin57 in Horizontal Cells of the Mouse Retina. Eur. J. Neurosci. 2004, 19, 2633–2640.
- Massey, S.C.; O’Brien, J.J.; Trexler, E.B.; Li, W.; Keung, J.W.; Mills, S.L.; O’Brien, J. Multiple Neuronal Connexins in the Mammalian Retina. Cell Commun. Adhes. 2003, 10, 425–430.
- Deans, M.R.; Volgyi, B.; Goodenough, D.A.; Bloomfield, S.A.; Paul, D.L. Connexin36 Is Essential for Transmission of Rod-Mediated Visual Signals in the Mammalian Retina. Neuron 2002, 36, 703–712.
- Feigenspan, A.; Teubner, B.; Willecke, K.; Weiler, R. Expression of Neuronal Connexin36 in AII Amacrine Cells of the Mammalian Retina. J. Neurosci. 2001, 21, 230–239.
- Feigenspan, A.; Janssen-Bienhold, U.; Hormuzdi, S.; Monyer, H.; Degen, J.; Söhl, G.; Willecke, K.; Ammermüller, J.; Weiler, R. Expression of Connexin36 in Cone Pedicles and OFF-Cone Bipolar Cells of the Mouse Retina. J. Neurosci. 2004, 24, 3325–3334.
- Han, Y.; Massey, S.C. Electrical Synapses in Retinal ON Cone Bipolar Cells: Subtype-Specific Expression of Connexins. Proc. Natl. Acad. Sci. USA 2005, 102, 13313–13318.
- Lee, E.-J.; Han, J.-W.; Kim, H.-J.; Kim, I.-B.; Lee, M.-Y.; Oh, S.-J.; Chung, J.-W.; Chun, M.-H. The Immunocytochemical Localization of Connexin 36 at Rod and Cone Gap Junctions in the Guinea Pig Retina. Eur. J. Neurosci. 2003, 18, 2925–2934.
- Mills, S.L.; O’Brien, J.J.; Li, W.; O’Brien, J.; Massey, S.C. Rod Pathways in the Mammalian Retina Use Connexin 36. J. Comp. Neurol. 2001, 436, 336–350.
- Lin, B.; Jakobs, T.C.; Masland, R.H. Different Functional Types of Bipolar Cells Use Different Gap-Junctional Proteins. J. Neurosci. 2005, 25, 6696–6701.
- Maxeiner, S.; Dedek, K.; Janssen-Bienhold, U.; Ammermüller, J.; Brune, H.; Kirsch, T.; Pieper, M.; Degen, J.; Krüger, O.; Willecke, K.; et al. Deletion of Connexin45 in Mouse Retinal Neurons Disrupts the Rod/Cone Signaling Pathway between AII Amacrine and ON Cone Bipolar Cells and Leads to Impaired Visual Transmission. J. Neurosci. 2005, 25, 566–576.
- Hidaka, S.; Akahori, Y.; Kurosawa, Y. Dendrodendritic Electrical Synapses between Mammalian Retinal Ganglion Cells. J. Neurosci. 2004, 24, 10553–10567.
- Schubert, T.; Degen, J.; Willecke, K.; Hormuzdi, S.G.; Monyer, H.; Weiler, R. Connexin36 Mediates Gap Junctional Coupling of Alpha-Ganglion Cells in Mouse Retina. J. Comp. Neurol. 2005, 485, 191–201.
- Schubert, T.; Maxeiner, S.; Krüger, O.; Willecke, K.; Weiler, R. Connexin45 Mediates Gap Junctional Coupling of Bistratified Ganglion Cells in the Mouse Retina. J. Comp. Neurol. 2005, 490, 29–39.
- Völgyi, B.; Abrams, J.; Paul, D.L.; Bloomfield, S.A. Morphology and Tracer Coupling Pattern of Alpha Ganglion Cells in the Mouse Retina. J. Comp. Neurol. 2005, 492, 66–77.
- Pérez De Sevilla Müller, L.; Dedek, K.; Janssen-Bienhold, U.; Meyer, A.; Kreuzberg, M.M.; Lorenz, S.; Willecke, K.; Weiler, R. Expression and Modulation of Connexin30.2, a Novel Gap Junction Protein in the Mouse Retina. Vis. Neurosci. 2010, 27, 91–101.
- Kihara, A.H.; Santos, T.O.; Osuna-Melo, E.J.; Paschon, V.; Vidal, K.S.M.; Akamine, P.S.; Castro, L.M.; Resende, R.R.; Hamassaki, D.E.; Britto, L.R.G. Connexin-mediated Communication Controls Cell Proliferation and Is Essential in Retinal Histogenesis. Int. J. Dev. Neurosci. 2010, 28, 39–52.
- Kihara, A.H.; Mantovani De Castro, L.; Belmonte, M.A.; Yan, C.Y.I.; Moriscot, A.S.; Hamassaki, D.E. Expression of Connexins 36, 43, and 45 during Postnatal Development of the Mouse Retina. J. Neurobiol. 2006, 66, 1397–1410.
- Kovács-Öller, T.; Raics, K.; Orbán, J.; Nyitrai, M.; Völgyi, B. Developmental Changes in the Expression Level of Connexin36 in the Rat Retina. Cell Tissue Res. 2014, 358, 289–302.
- Güldenagel, M.; Ammermüller, J.; Feigenspan, A.; Teubner, B.; Degen, J.; Söhl, G.; Willecke, K.; Weiler, R. Visual Transmission Deficits in Mice with Targeted Disruption of the Gap Junction Gene Connexin36. J. Neurosci. 2001, 21, 6036–6044.
More
