Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Catherine Yang and Version 2 by Catherine Yang.
Direct biocatalytic processes for CO2 capture and transformation in value-added chemicals may be considered a useful tool for reducing the concentration of this greenhouse gas in the atmosphere. Among the other enzymes, carbonic anhydrase (CA) and formate dehydrogenase (FDH) are two key biocatalysts suitable for this challenge, facilitating the uptake of carbon dioxide from the atmosphere in complementary ways.
- carbonic anhydrase
- formate dehydrogenase
- cofactor regeneration
1. Chemical Absorption
Chemical absorption has been the most used CCS method for decades [1]. This procedure involves the scrubbing of exhaust gas at low pressures and temperatures with alkaline solutions typically containing amines and/or carbonates or hydroxide solutions [1]. Amines are weak bases that can capture protons from Brönsted acid CO2. The reactions of primary/secondary or tertiary amines produce carbamates or bicarbonate anions, respectively, according to the reactions described below.
The amines typically used for these purposes are alkanolamines. An alcohol group increases water solubility and decreases vapor pressure compared to analogous amines. The main chemical solvent used as an absorber is monoethanolamine (MEA). The solutions typically consist of an aqueous solution of 20–30 wt% MEA. CO2 is captured at low pressure (ca. 1 bar) and in a mixed gas containing other gases, such as N2, SOx, and NOx. Secondary amine diethanolamine (DEA) and tertiary amine N-methyl diethanolamine (MDEA) are also amply used amines.
Several points should be considered when choosing a suitable solvent for CO2 sequestration. First, the enthalpy of the reaction: the higher the enthalpy of the reaction, the higher the cost of solvent regeneration. This is a crucial point because it is estimated that 60–80% of the costs of these processes arise from solvent regeneration [2]. The enthalpy of (exothermic) reactions 6 and 7 increases from tertiary to secondary and primary amines; thus, primary amines improve both energetic and economic costs. Moreover, the power of corrosion also follows the same order (primary amines are the most corrosive). Another decisive issue is their ability to load CO2. Tertiary amines have the highest capabilities in this regard [3]. According to these thermodynamic aspects, tertiary amines are the best ones to use. However, another crucial point is the kinetics of CO2 sequestration; indeed, tertiary amines have low reaction rates and are kinetically much more inert than primary amines [4][5]. Owing to the slow kinetics of tertiary amines, primary amines (or secondary amines) are currently preferred. However, the high costs of cooperation and maintenance due to the ease of amine degradation and the formation of highly corrosive salts are drawbacks when operating with amines.
Consequently, for kinetic reasons, primary amines, specifically MEA, are by far the most widely used alkanolamines in the industry. Numerous plants have been developed using MEA solutions. These systems are typically coupled with industrial processes and CO2 is captured with postcombustion gases. For instance, their use is extended to the iron and steel industries (responsible for approximately 31% of all industrial CO2 emissions). These plants can recover 85–95% of the CO2 in gas. As an example, a steel production plant recently established by Emirate Steel Industries has a yield plant using CCS based on amine absorption that captures 0.8 Mt CO2/year [6].
In the last decade, CA has been revealed as a tool for accelerating CO2 uptake in chemical absorption processes. Gundersen et al. studied CA stability and activity for a long time (150 days) as a function of pH, temperature, and the solvent, combining MEA and MDEA solutions, among others [7]. They concluded that CA was suitable for these purposes; the biocatalyst was stable and active between pH 7 and 11, with maximum activity at 40 °C. In addition, the enzyme preserved its activity between 12 and 91% of the original activity depending on the solvent employed. The absorption of CA was also accomplished in MOF ZIF-L-1 in the presence of MDEA [8]. In ZIF-L (a zeolitic imidazolate framework), the imidazolate groups enhance CA immobilization, and CO2 uptake is hence greatly increased. The authors highlighted that this new MOF obtained excellent CO2 absorption rates at 40 °C and a CO2 partial pressure of 15 kPa, while the activity was maintained for six reuse cycles [9]. Additionally, a pilot-scale plant was set up with CA in solution in the presence of MDEA. The authors observed an enhancement in CO2 capture in the presence of the enzyme and demonstrated the possibility of translating the laboratory results to higher scales [10]. However, because the free enzyme is damaged by amines, immobilization is necessary. Kim et al. also studied the effect of CA on CO2 absorption rates in the presence of MEA and MDEA, although they used a membrane contactor with hydrophobic and hydrophilic supports [11]. This system allows an expanded contact surface to enhance CO2 absorption.
2. Chemical Carbonation
Chemical carbonation is probably the most efficient method for capturing CO2. This is performed when CO2 is bubbled through an alkaline solution, typically consisting of dissolved KOH or Ca(OH)2, where potassium or calcium carbonates precipitate. The limiting step for capturing CO2 in postcombustion processes. However, CO2 capture is much faster in alkaline media since carbonates are formed, and so hydrogen carbonate concentration decreases. Indeed, once bicarbonate anion (soluble) is formed, reactions such as Equations (1) and (2) (amine formation, Scheme 1) occur much faster in alkaline media. Even so, the limiting step, for kinetic reasons, continues to be the CO2 gas uptake, as commented previously. Thus, the main challenge in applying alkaline solutions, either amines or carbonates, to the CCS approach is speeding up CO2 conversion to bicarbonate [12]. Consequently, numerous studies on CA to increase the mass transfer of CO2 capture have been proven not only at the laboratory level, and its feasibility has been demonstrated on an industrial scale [3][13]. Novozymes NS81239 CA (NCA) at 2 μM increased the absorption rate of CO2 into potassium carbonate by ca. 30%, augmenting this uptake at temperatures in the range of 40–60 °C [12][14]. Power et al. demonstrated that bovine CA accelerated the carbonation rate of brucite Mg(OH)2 from CO2 gas by up to 240% [15]. In these studies, CA was supplied as a free enzyme; therefore, its regeneration was not studied. Biological tools have also been used to enhance carbonation. Jin et al. accelerated calcium carbonate precipitation by employing Bacillus mucilaginosus on steel slag powder [16], increasing the carbonation degree from 66.34 to 86.25%. Moreover, the mechanical properties and durability of the treated steel slag were enhanced. The CA immobilization, as described in the previous section, strongly improves the reusability of the enzyme as well as the chemical carbonation. For instance, Jo and coworkers proved the suitability of CA encapsulated in a biosilica matrix, obtaining good yields for carbonation compared to the free enzyme [8].
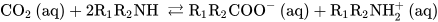
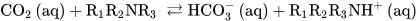
Scheme 1. Reactions of CO2 with primary, secondary (Equation (1)), and tertiary (Equation (2)) amines in water solutions.
3. Mineralization
Biomineralization is a very slow and exothermic process by which carbonate minerals are formed from silicates and CO2 under basic conditions [17]. The starting silicates usually contain divalent metals such as Ca(II) and Mg(II) or trivalent metals such as Fe(III). This event occurs in nature on a regular basis and is responsible for the formation of inorganic structures in living organisms such as exoskeletons in protozoa, algae or invertebrates, and shells or plant mineral structures. It is also responsible for the presence of large amounts of limestone on the Earth’s surface [18][19]. When trying to emulate biomineralization, which takes place over very large timescales, the main drawback is speeding up the process. Artificial mineralization mimics nature, although in short periods. It involves the injection of CO2 directly into geological formations to promote a carbonate-forming reaction with alkaline minerals [20]. This mineral sequestration would be a viable alternative for subsequent storage because the carbonate products formed would not require monitoring owing to their high stability and safety. On the other hand, in-ground or ex situ mineralization is based on the exposure of crushed rock material in a processing plant where CO2 is introduced, facilitating the formation of carbonate minerals. Natural minerals or alkaline solid waste can be used [20][21]. The use of natural silicates requires a large amount of material, which implies a very large operational size and an unfeasible economic mineral impact. Ex situ mineralization can also be carried out using alkaline wastes containing divalent metals such as ash originating from the coal or metallurgical industry, cement and concrete wastes, or iron and steel slag [22][23]. This method would reduce not only environmental CO2 but also the accumulation of waste from industrial activities, although a major disadvantage in that its capacity is much smaller than that of CO2 mineralization from silicates. At laboratory scale, this mineralization has been satisfactorily performed by directly extracting CO2 from the air, and its direct extraction by passing the air through cooling towers using NaOH solutions has also been proposed for larger scales [24]. However, the same authors pointed out the elevated costs of this approach on a large scale.
The efficiency of the biomineralization process can also be accelerated by modifying certain parameters such as increasing the temperature, pressure, or retention time. The biomineralization process is also favored by the presence of purines, NaCl, or CA. The presence of CA accelerates the rate of hydration of CO2 dissolved in water; therefore, possible modifications to CA to support high pH and temperature conditions without losing its advantageous functionality have been studied [25][26]. On the other hand, some varieties of carbonic anhydrase are inhibited in the presence of high concentrations of hydrogen carbonate, which becomes a problem for its use in industry. However, this can be circumvented, at least partially, by increasing the pH to values equal to or higher than 9.0, conditions under which some CAs are still stable and functional. Immobilization improves the stability of CA at high temperatures or alkaline conditions, as confirmed by Arias et al. when forming calcite in vitro by mineralization using CA immobilized in eggshell membranes [26]. Recombinant CAs have also been used to accelerate mineralization under extreme conditions. For instance, CA from the alkalistable Aeribacillus pallidus was genetically modified, achieving acceptable yields in the presence of pollutants such as NOx and SOx [27]. Similarly, CA from the thermophilic bacterium Sulfurihydrogenibium azorense was modified, and its half-life was found to be 8 days when the biomineralization process was carried out at 70 °C and 53 days at a reaction temperature of 50 °C [28]. Di Lorenzo et al. studied the effect of CA and a Zr-based MOF in the carbonation process of wollastonite (CaSiO3) to produce calcite (CaCO3) [29]. Although CA accelerated CO2 uptake by the silicate, the total gas absorber quantity was lower than that of the MOF. Jin et al. also took advantage of CA to accelerate the carbonation of γ-dicalcium silicate, which is also present in steel slag. They used a powder containing alkali-resistant CA bacteria, increasing the yield by 19.0% [30].
References
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-Combustion CO2 Capture with Chemical Absorption: A State-of-the-Art Review. Chem. Eng. Res. Des. 2011, 89, 1609–1624.
- Heldebrant, D.J.; Koech, P.K.; Glezakou, V.-A.; Rousseau, R.; Malhotra, D.; Cantu, D.C. Water-Lean Solvents for Post-Combustion CO2 Capture: Fundamentals, Uncertainties, Opportunities, and Outlook. Chem. Rev. 2017, 117, 9594–9624.
- Sharif, M.; Zhang, T.; Wu, X.; Yu, Y.; Zhang, Z. Evaluation of CO2 Absorption Performance by Molecular Dynamic Simulation for Mixed Secondary and Tertiary Amines. Int. J. Greenh. Gas. Control 2020, 97, 103059.
- Bernhardsen, I.M.; Krokvik, I.R.T.; Jens, K.-J.; Knuutila, H.K. Performance of MAPA Promoted Tertiary Amine Systems for CO2 Absorption: Influence of Alkyl Chain Length and Hydroxyl Groups. Energy Procedia 2017, 114, 1682–1688.
- Liu, B.; Cui, Z.; Tian, W. The Kinetics Investigation of CO2 Absorption into TEA and DEEA Amine Solutions Containing Carbonic Anhydrase. Processes 2021, 9, 2140.
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon Capture and Storage (CCS): The Way Forward. Energy Environ. Sci. 2018, 11, 1062–1176.
- Gundersen, M.T.; Von Solms, N.; Woodley, J.M. Enzymatically Assisted CO2 Removal from Flue-Gas. In Energy Procedia; Department of Chemical and Biochemical Engineering, Technical University of Denmark: Lyngby, Denmark, 2014; Volume 63, pp. 624–632.
- Jo, B.H.; Seo, J.H.; Yang, Y.J.; Baek, K.; Choi, Y.S.; Pack, S.P.; Oh, S.H.; Cha, H.J. Bioinspired Silica Nanocomposite with Autoencapsulated Carbonic Anhydrase as a Robust Biocatalyst for CO2 Sequestration. ACS Catal. 2014, 4, 4332–4340.
- Zhang, S.; Du, M.; Shao, P.; Wang, L.; Ye, J.; Chen, J.; Chen, J. Carbonic Anhydrase Enzyme-MOFs Composite with a Superior Catalytic Performance to Promote CO2 Absorption into Tertiary Amine Solution. Environ. Sci. Technol. 2018, 52, 12708–12716.
- Gladis, A.; Lomholdt, N.F.; Fosbøl, P.L.; Woodley, J.M.; von Solms, N. Pilot Scale Absorption Experiments with Carbonic Anhydrase-Enhanced MDEA-Benchmarking with 30 wt% MEA. Int. J. Greenh. Gas Control. 2019, 82, 69–85.
- Kim, T.-J.; Lang, A.; Chikukwa, A.; Sheridan, E.; Dahl, P.I.; Leimbrink, M.; Skiborowski, M.; Roubroeks, J. Enzyme Carbonic Anhydrase Accelerated CO2 Absorption in Membrane Contactor. In Energy Procedia; SINTEF Materials and Chemistry: Trondheim, Norway, 2017; Volume 114, pp. 17–24.
- Thee, H.; Smith, K.H.; Da Silva, G.; Kentish, S.E.; Stevens, G.W. Carbonic Anhydrase Promoted Absorption of CO2 into Potassium Carbonate Solutions. Greenh. Gases Sci. Technol. 2015, 5, 108–114.
- Knoche, W. Chemical Reactions of CO2 in Water. In Biophysics and Physiology of Carbon Dioxide, Proceedings in Life Sciences; Bauer, C., Gros, G., Bartels, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1980.
- Hu, G.; Nicholas, N.J.; Smith, K.H.; Mumford, K.A.; Kentish, S.E.; Stevens, G.W. Carbon Dioxide Absorption into Promoted Potassium Carbonate Solutions: A Review. Int. J. Greenh. Gas Control 2016, 53, 28–40.
- Power, I.M.; Harrison, A.L.; Dipple, G.M. Accelerating Mineral Carbonation Using Carbonic Anhydrase. Environ. Sci. Technol. 2016, 50, 2610–2618.
- Jin, P.; Zhang, S.; Liu, Y.; Zhang, W.; Wang, R. Application of Bacillus mucilaginosus in the Carbonation of Steel Slag. Appl. Microbiol. Biotechnol. 2021, 105, 8663–8674.
- Ehrlich, H.; Bailey, E.; Wysokowski, M.; Jesionowski, T. Forced Biomineralization: A Review. Biomimetics 2021, 6, 46.
- Bose, H.; Satyanarayana, T. Microbial Carbonic Anhydrases in Biomimetic Carbon Sequestration for Mitigating Global Warming: Prospects and Perspectives. Front. Microbiol. 2017, 8, 1615.
- Wang, F.; Dreisinger, D.B.; Jarvis, M.; Hitchins, T. The Technology of CO2 Sequestration by Mineral Carbonation: Current Status and Future Prospects. Can. Metall. Q. 2018, 57, 46–58.
- Hills, C.D.; Tripathi, N.; Carey, P.J. Mineralization Technology for Carbon Capture, Utilization, and Storage. Front. Energy Res. 2020, 8, 142.
- Bhatia, S.K.; Bhatia, R.K.; Jeon, J.-M.; Kumar, G.; Yang, Y.-H. Carbon Dioxide Capture and Bioenergy Production Using Biological System–A Review. Renew. Sust. Energy Rev. 2019, 110, 143–158.
- Zajac, M.; Krol, M.; Bullerjahn, F.; Deja, J. Effect of Temperature on Carbon Dioxide Mineralisation in Recycled Cement Paste. Adv. Cem. Res. 2023, 35, 1–12.
- Yin, B.; Xu, H.; Fan, F.; Qi, D.; Hua, X.; Xu, T.; Liu, C.; Hou, D. Superhydrophobic Coatings Based on Bionic Mineralization for Improving the Durability of Marine Concrete. Constr. Build. Mater. 2023, 362, 129705.
- Heldebrant, D.J.; Kothandaraman, J.; Mac Dowell, N.; Brickett, L. Next Steps for Solvent-Based CO2 Capture; Integration of Capture, Conversion, and Mineralisation. Chem. Sci. 2022, 13, 6445–6456.
- Rodriguez-Navarro, C.; Cizer, O.; Kudlacz, K.; Ibanez-Velasco, A.; Ruiz-Agudo, C.; Elert, K.; Burgos-Cara, A.; Ruiz-Agudo, E. The Multiple Roles of Carbonic Anhydrase in Calcium Carbonate Mineralization. CrystEngComm 2019, 21, 7407–7423.
- Fernández, M.S.; Montt, B.; Ortiz, L.; Neira-Carrillo, A.; Arias, J.L. Effect of Carbonic Anhydrase Immobilized on Eggshell Membranes on Calcium Carbonate Crystallization In Vitro. In Biomineralization; Endo, K., Kogure, T., Nagasawa, H., Eds.; Springer: Singapore, 2018; pp. 31–37.
- Bose, H.; Satyanarayana, T. Suitability of the Alkalistable Carbonic Anhydrase from a Polyextremophilic Bacterium Aeribacillus pallidus TSHB1 in Biomimetic Carbon Sequestration. Bioprocess Biosyst. Eng. 2016, 39, 1515–1525.
- De Luca, V.; Vullo, D.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. An Alpha-Carbonic Anhydrase from the Thermophilic Bacterium Sulphurihydrogenibium azorense Is the Fastest Enzyme Known for the CO2 Hydration Reaction. Bioorg. Med. Chem. 2013, 21, 1465–1469.
- Di Lorenzo, F.; Ruiz-Agudo, C.; Ibañez-Velasco, A.; Gil-San Millán, R.; Navarro, J.A.R.; Ruiz-Agudo, E.; Rodriguez-Navarro, C. The Carbonation of Wollastonite: A Model Reaction to Test Natural and Biomimetic Catalysts for Enhanced CO2 Sequestration. Minerals 2018, 8, 209.
- Jin, P.; Wang, R.; Zhang, S.; Chen, Y. Effect of Carbonic Anhydrase Bacteria on the Carbonation Process of γ-C2S. Adv. Cem. Res. 2021, 34, 15–27.
More
