Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yeimmy Peralta-Ruiz and Version 2 by Sirius Huang.
Fruits and vegetables are constantly affected by postharvest diseases, of which anthracnose is one of the most severe and is caused by diverse Colletotrichum species, mainly C. gloeosporioides. Colletotrichum gloeosporioides is a cosmopolitan pathogen widely disseminated as a common plant pathogen.
- anthracnose
- biocontrol
- climate change
- cultivar resistance
1. Introduction
Plant pathogens, such as Colletotrichum gloeosporioides, which cause the disease known as anthracnose, can attack vegetables and fruit crops both in the field and during the postharvest storage and commercialization phases, reducing their shelf life and generating the need to use pesticides, which are usually produced through chemical synthesis, as a disease mitigation strategy [1]. For several decades, this solution has been the most used solution for postharvest disease management; however, some studies have reported that chemical pesticides have adverse effects on the environment [2]. Additionally, consumer interest in high-quality, microbiologically safe, and sustainable products, together with initiatives approved by the United Nations (UN) known as the 2030 Agenda for Sustainable Development, has increased the interest in seeking new alternatives to plant and food protection [3]. Several technologies have been used to extend the shelf life of fruits during the postharvest stage, including freezing, sterilization, ozone, ethylene pretreatment, modified atmosphere, and heat treatments [4]. Although these technologies are effective, there are still some disadvantages to their application, including cost and changes in the physicochemical and sensory properties of the food products [5].
The use of natural substances has gained relevance, and countless studies have shown their advantages, including the postharvest conservation of fruits’ physicochemical and sensory qualities for a longer time. The most common materials used are biopolymers, including polysaccharides, proteins, and fats. In recent years, a wide variety of extracts and essential oils have been studied for their antimicrobial, antioxidant, insecticidal, and herbicidal activity, and the films and coatings prepared with the substances mentioned above are generally nontoxic and biodegradable [6]. Another up-and-coming green alternative is the so-called biological control, where residential or introduced biotic organisms are used to minimize the activities and population of plant pathogens [7]. These organisms include bacteria, fungi, viruses, protozoans, and insects, which can control plant diseases directly or indirectly [8]. Biocontrol also includes nonliving agents of biological origin and the compounds secreted by several organisms.
2. Colletotrichum gloeosporioides
Colletotrichum gloeosporioides is a cosmopolitan pathogen widely disseminated as a common plant pathogen globally [9]. It was first isolated from citrus in Italy and proposed by Penzig (1882) as Vermicularia gloeosporioides. The fungus includes Glomerella cingulata as a sexual teleomorph state (perfect) and C. gloeosporioides as an anamorph state (imperfect), and this concept was taxonomically formalized in 1957 [10].
The Colletotrichum gloeosporioides complex is one of the major clades of the genus Colletotrichum, comprising more than 22 species to date. The characterization of this fungus has mainly been based on its production of oblong to ovoid, hyaline, obtuse-ended conidia, as well as colony color, growth rates, appressoria shape [11], host type, presence of setae, whether or not the teleomorph develops, and the use of internal transcribed spacer (ITS) sequence [12]. However, some challenges and problems have been presented due to the variability of its morphological characteristics. This variability depends on the culture medium, growth conditions, difficulty in standardization, variable host range, pathogenicity, and erroneous sequence names of Colletotrichum strains in public databases generating deficient identification in delimiting species of Colletotrichum [13].
Molecular techniques have been used to resolve systematic problems in Colletotrichum species; in particular, the multilocus phylogenetic analyses with the uses of seven housekeeping genes [14], such as glutamine synthetase (GS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), calmodulin (CAL), actin (ACT), chitin synthase (CHS–1), β-tubulin (TUB2), DNA lyase (APN2), and mating-type protein (MAT1-2) genes that show high resolution to distinguish species in the Colletotrichum gloeosporioides complex [15]. Currently, the multilocus phylogenetic analyses are used together with the pathogenicity test, morphological description, and ecological and metabolite studies to accurately identify C. gloeosporioides [16].
Colletotrichum gloeosporioides Infection Process
The Colletotrichum gloeosporioides initiates plant infection in a biotrophic manner (Figure 1), establishing itself in the plant after conidial adhesion to the leaf surface and subsequent spore germination. The fungus then penetrates the plant’s epidermal cells through an appressorium following the development of a small vesicle [17]. Primary hyphae grow and colonize only select cells, while the fungus secrets small proteins and absorbs secondary metabolites from the plant through its interaction with the apoplast. During this stage, genes critical to endocytosis, such as C1H1 glycoprotein and CgDN3, are expressed by the fungus [18]. The necrotic phase is characterized by the appearance of disease symptoms in the plant; the fungus produces secondary hyphae that ramify through host tissues and release carbohydrate-active enzymes (CAZymes), such as polygalacturonase and pectate lyase, which degrade plant cells and reduce host defense responses due to the high activity of oligogalacturonides that are released [17].
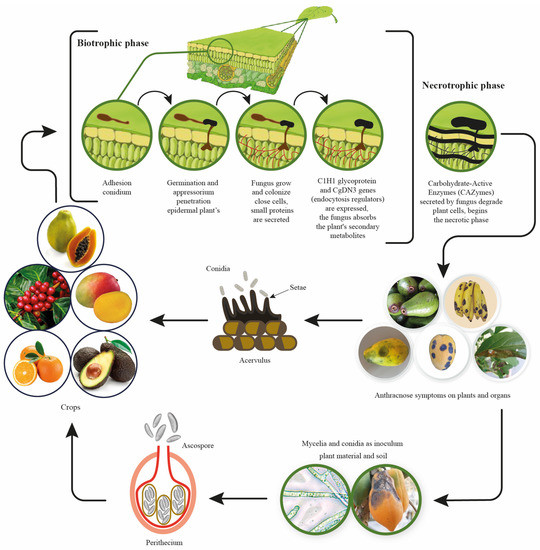
Figure 1.
Colletotrichum gloeosporioides
3. Anthracnose: The Big Problem with Colletotrichum gloeosporioides
Anthracnose disease is associated with significant economic losses of fruit and vegetable crops and evergreen trees and shrubs due to its incidence in preharvest and postharvest stages, affecting many crops globally, mainly in tropical and subtropical climates. It is caused by diverse species of Colletotrichum such as C. coccodes, C. fructicola, C. siamense, C. capsici, C. gloeosporioides, C. acutatum, C. dematium, C. panacicola, C. boninense, and C. godetiae. However, C. gloeosporioides has been identified as the prevalent cause of anthracnose [17].
Colletotrichum spp. infects fruits and other plant organs, including the leaves, flowers, twigs, and branches. It has been reported that the fungus can present an endophyte behavior and remain quiescent, infecting the plants without showing any symptoms [17]. Anthracnose causes the wilting and drying of tissues. Infected plants develop dark-colored spots on their leaves, as well as browning, curling, cupping (in the young leaves), and early drop. The above symptoms can also occur in flowers. In the preharvest stage, the fruit can present small, rounded spots on the surface; however, the fungus can remain dormant until the postharvest as appressorium, developing dark spots, decay, and rot in the fruit until the postharvest [18]. It is well known that some species of Colletotrichum infect specific hosts, while others infect multiple hosts. Anthracnose pathogens that infect multiple hosts may indicate the development of cross-infection ability. For example, isolates of C. gloeosporioides sensu lato from mango could infect and produce symptoms in guava, chili, and papaya [19].
The environmental conditions that favor anthracnose disease are relative humidity greater than 90% and temperatures between 22 and 32 °C. These conditions are where most symptoms develop [10]. However, during postharvest, tissue damage can occur in drier conditions due to the presence of the fungus and the aging of the fruits. The pathogen can survive in crop residues and grow saprotrophically after being dispersed by rain splashes and air transmission [20]. Anthracnose has been reported in different hosts, affecting plant productivity and products such as mango, papaya, olive, coffee, banana, eggplant, and guava, among others (Figure 2).

Figure 2. Anthracnose lesion caused by Colletotrichum. gloeosporioides in ripened fruits. (a) Apple, (b) papaya, (c) mango, (d) orange, (e) avocado, (f) eggplant, (g) pepper, (h) pomegranate, (i) tomato, and (j) banana. The lesions appear on the fruit as dark spots, gangrene, and rots.
Colletotrichum gloeosporioides, a Globally Distributed Fungus
Anthracnose affects several fruits worldwide, mainly those produced in tropical and subtropical regions (Figure 3). High damage incidence caused by C. gloeosporioides has been reported in many countries including India, China [21], Indonesia, Mexico, Brazil, Ecuador, Guatemala [19], Belize, Costa Rica [22], Peru [23], Dominican Republic [24], Colombia [13], Australia [25], Italy [26], New Zeland [27], United States [28], and South Africa [19], among others.
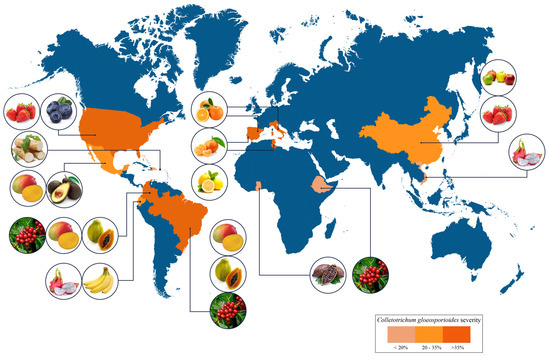
Figure 3.
Worldwide distribution, occurrence, and severity of anthracnose.
Tropical fruits are susceptible to anthracnose disease both preharvest and postharvest [19]. This disease is one of the causes of the papaya shelf-life limitations, resulting in significant losses in the postharvest stage of around 25% to 40% [29] of the 13 megatons produced globally [30]. In the last decade, anthracnose has severely affected avocado production in Mexico, reducing fruit quality and commercial yield [31]. Similarly, mango fruit, one of the most important tropical fruits globally with a production of 58 megatons in 2019 [32], experienced direct and indirect losses in pre- and postharvest stages caused by anthracnose up to 30% in 2019 [33][34][33,34].
Recently, it has been reported that C. gloeosporioides causes the presence of anthracnose in bananas (Musa AAA Cavendish), the most crucial crop in Ecuador [19], as well as in blueberry crops in the United States [35]. Regarding citrus, Italy and Portugal have reported anthracnose disease in orange fruits, mandarin leaves, and unripe and ripe lemon fruits, respectively [26].
In 2019, Ghana reported economic losses from an anthracnose outbreak in cacao crops, the main export product [36]. In addition, coffee anthracnose is widely distributed in coffee-growing countries globally, causing devastating damage and significant crop losses [37]. The most harmful yam pathogen is C. gloeosporioides, which has reduced the production yield in Africa, the Caribbean, Asia, and South Pacific regions; in countries such as Puerto Rico, it has reported losses of 250 tones due to anthracnose disease [38][39][38,39]. Emerging super crops such as dragon fruit, highly desired for their nutraceuticals and functional properties and cultivated in many countries, including China, Colombia, Ecuador, and Vietnam, are affected by anthracnose, reducing their quality and decreasing the opportunity for export [19]. Anthracnose also presents difficulties for dried fruit; China has reported walnut production problems caused by fruit gangrene, which is caused by C. gloeosporioides [21]. On the other hand, the disease also attacks popular and globally produced fruit such as strawberries. The United States and China are reported as the principal causal agent of the strawberry anthracnose caused by C. gloeosporioides [28].
4. Climate Change (CC) and Its Probable Effects on C. gloeosporioides and Anthracnose Disease
It is well established that environmental changes, such as increasing temperature, light and water availability, soil fertility, wind speeds, atmospheric ozone, methane, CO2 concentrations, and changing precipitation, are linked directly to changes in plant-pathogen incidence and severity [40]. Thus, climate change will probably alter plant diseases’ geographical and temporal distribution; therefore, new problems will emerge, while other problems will be reduced. However, it is essential to consider how plants and pathogens adapt to changing environmental conditions and how the interaction could be harmful in the new climatic scenario. In this context, there is very little information about host and pathogen adaptation to climatic change, and accurate predictive models still do not exist for many diseases [41].
Meteorological parameters that predict crop disease occurrence include air temperature, leaf wetness, precipitation, and relative humidity. As mentioned above, the Colletotrichum growth is favored at temperatures and relative humidity above 20 °C and 95%, respectively. Guyader [42] reported the importance of preserving the genetic diversity of C. gloeosporioides strains since some strains/races have better resilience than others. Colletotrichum gloeosporioides spores can survive in leaf wetness absence for up to 48 h, and infectious capacity is recovered after high-temperature stress of up to 40 °C, depending on the strain. Misra [43], suggested that the decreasing rainfall resulting in lower humidity will also be unfavorable for the anthracnose disease of mango.
The last decades have seen a dramatic increase in atmospheric CO2 concentrations, which is one of the major concerns for crop production in the face of CC. Some studies have demonstrated that increasing CO2 concentrations could increase crop yield; however, it might modulate host–pathogen interaction and related disease epidemics differently [44]. For the fungal pathogen C. gloeosporioides, it has been reported that an elevated CO2 concentration resulted in a modulation of the development and disease severity, which is highly dependent on the variety of the plant host and CO2 concentration. For example, Chakraborty et al. [45] reported that 700 ppm of CO2 delayed and reduced germination, germ tube growth, and appressoria production of Colletotrichum, thus decreasing the anthracnose severity. Changes in CO2 concentration from 700 ppm to ambient exposure influenced the susceptibility of the host (Stylosanthes scabra) under field conditions. In addition, aggressiveness has increased towards the resistant one but not the susceptible one.
Further studies have shown that the genetic fingerprint and karyotype of C. gloeosporioides isolates changed for some CO2 cultivar combinations but these changes were not related to increased aggressiveness [45]. Additionally, Cogliati et al. [46] demonstrated that a combination of 800 ppm CO2 and temperature between 22 and 26 °C resulted in a significant increase in anthracnose disease in terms of the percentage of affected leaves of plants, among other environmental conditions. However, increased levels of CO2 can decrease the susceptibility of coffee to C. gloeosporioides due to the high concentration of endogenous caffeine, which is reduced under high CO2 concentrations. Although the studies did not consider the combined effect with the temperature changes, these results are relevant not only to the genetics of C. gloeosporioides–host interaction but also to the epidemiology of the disease because it is the demonstration that the pathogen can adapt to a new environment; thus, it may make the expansion of the geographic area of C. gloeosporioides more concerning. Instead, three years of study by Koo et al. [47] showed that the aggressiveness of the anthracnose pathogen in hot pepper was significantly reduced in environmental conditions of 700 ppm CO2 and response to a temperature increase of 5 °C after 100 inoculation cycles. Studies have shown that increasing CO2 concentrations could boost crop yield; however, it might modulate host–pathogen interaction and the related disease epidemics differently. Li et al. [48] elevated the concentration of CO2-reduced caffeine (an alkaloid in tea that has long been known for its role in plant defense) and jasmonic acid (a crucial signaling molecule in plant defense) concentration, sharply increasing the susceptibility of tea to C. gloeosporioides. In addition, it should be noted that enlarged plant-canopy architecture under elevated CO2 can trap more spores, leading to more severe anthracnose under favorable weather.
Finally, CC could reduce the effectiveness of biological control agents for C. gloeosporioides, which could be a significant problem in future pest-management programs. CC causes a considerable reduction in the yield of major crops worldwide and food security. However, how the combined effects of multiple environmental conditions impact disease outcomes remains one of the most outstanding and challenging questions for the future study of plant–pathogen interactions [40]. The studies mentioned above did not consider the coevolutionary processes of Colletotrichum spp. with superior plant varieties, fungicides, and agricultural practices.
