Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Beatriz Martinez-Delgado and Version 2 by Lindsay Dong.
Non-alcoholic fatty liver disease (NAFLD) is a type of steatosis commonly associated with obesity, dyslipidemia, hypertension, and diabetes. Other diseases such as inherited alpha-1 antitrypsin deficiency (AATD) have also been related to the development of liver steatosis. The primary reasons leading to hepatic lipid deposits can be genetic and epigenetic, and the outcomes range from benign steatosis to liver failure, as well as to extrahepatic diseases. Progressive hepatocellular damage and dysregulated systemic immune responses can affect extrahepatic organs, specifically the heart and lungs.
- NAFLD
- AATD
- cardiovascular disease
- lipids
- meta-inflammation
1. Non-Alcoholic Fatty Liver Disease (NAFLD)
Non-alcoholic fatty liver disease (NAFLD) is a common liver condition characterized by an excess of lipid accumulation in hepatocytes (steatosis), which is present in about 25% of the adult population [1]. This term includes a range of liver diseases from benign steatosis to cirrhosis, passing through steatohepatitis (NASH) to hepatocellular carcinoma (HCC) [2]. There are different environmental or genetic risk factors that can lead to NAFLD [3], including insulin resistance and obesity. MAFLD (metabolic associated fatty liver disease) has been proposed as a new name that is expected to better mirror the heterogeneities and similarities between NAFLD and metabolic syndrome [4][5][4,5]; however, some controversies remain regarding this new name [6].
The pathology typically begins with an altered lipid homeostasis, the intracellular increment of fats followed by an uncontrolled inflammatory response, which can eventually lead to cirrhosis and/or to HCC [7]. Initially, most of the NAFLD patients are asymptomatic and blood markers typically do not reflect liver impairment [8]. The progression to NASH is associated with liver inflammation usually followed by fibrosis, whereas in some cases, the development of liver failure requires liver transplantation. However, cardiovascular diseases (CVD) are among the main causes of death among NAFLD patients [9].
It is widely accepted that free fatty acids act as primary triggers of NAFLD, although there are other factors implicated in disease progression such as dietary habits, obesity, insulin resistance, intestinal microbiota, or epigenetic factors [10]. Patients with NASH typically have high levels of blood endotoxins, suggesting that bacterial endotoxins play a role in NASH pathogenesis [11][12][11,12].
Steatosis is defined by the presence of lipid droplets (LDs) in the cytosol of more than 5% of hepatocytes, which is a consequence of altered lipid metabolism when fatty acid obtention exceeds fatty acid removal [13][14]. Lipid droplets are dynamic organelles composed of neutral lipids, mainly triglycerides and cholesterol esters [14][15], which act as energy storage but also as protectors against the deleterious effects of free fatty acids [15][16]. LDs are increasingly recognized as having important non-pathological roles in cell signalling and function. The properties of LDs are highly regulated by proteins coating the surface of LDs to control lipid trafficking and flux [16][17]. LDs also play roles in endoplasmic reticulum (ER) stress response, protein storage and degradation, and in infection and immunity [17][18].

2. Alpha-1 Antitrypsin Deficiency (AATD)
Inherited alpha-1 antitrypsin deficiency (AATD) is a rare monogenic disorder (ORPHA 60) mainly related to lung and/or liver diseases, but also to neutrophilic panniculitis or systemic vasculitis [18][20]. AATD is characterized by low levels of circulating alpha-1 antitrypsin (AAT), an acute phase glycoprotein encoded by the SERPINA1 gene, in which more than 120 allelic variants have been described [19][21]. Some mutations in the SERPINA1 gene have no clinical relevance and are considered as normal variants or M alleles; however, deficient alleles, typically resulting from point mutations or small deletions, are related to low levels or functional activity of AAT, and mild to severe clinical manifestations. Among the deficient alleles, the most clinically relevant and best recognized is the Z allele (Glu342Lys), originating from a point mutation in exon 5 [20][22]. According to current data, the homozygosity in the Z allele is present in about 96% of AATD patients, whereas the remaining 4% are heterozygous carriers or contain other rare alleles [21][23]. AAT is primarily synthetized by hepatocytes (about 80%) and acts not only as a main inhibitor of neutrophil elastase and proteinase-3 [22][23][24,25], but also as a modulator of caspase activity and apoptosis, as an antioxidant, and/or as a broad immunomodulatory protein [24][25][26,27]. The complex tertiary structure of AAT makes it extremely vulnerable to conformational changes, as it happens in the Z allele where a change in just one amino acid triggers AAT polymerization. As mentioned above, AATD mainly affects the liver and lungs; hepatic manifestations are due to AAT intrahepatic polymer accumulation and cytotoxicity [26][28], whereas lung pathologies are due to low circulating levels, mostly polymeric forms of AAT resulting in an insufficient inhibition of neutrophil proteases [27][29].3. Meta-Inflammation in NAFLD and AATD
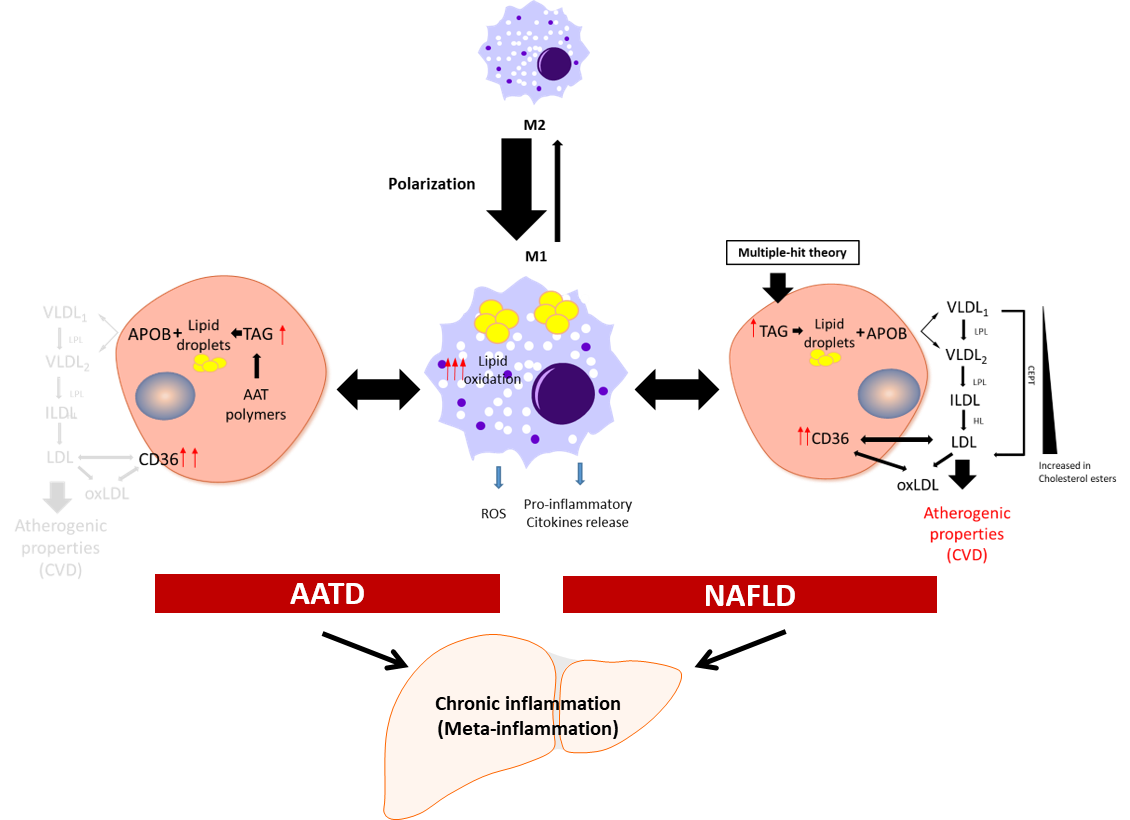
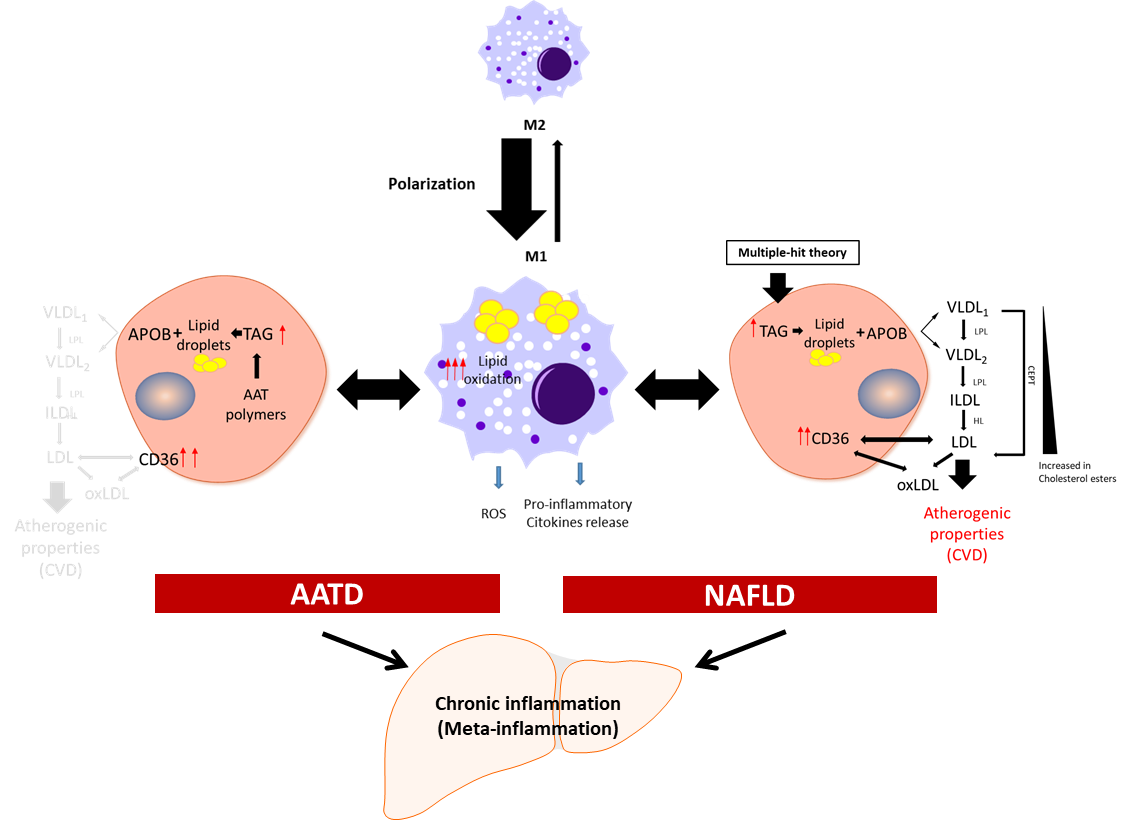
Meta-inflammation is defined as a low-grade chronic inflammation associated with metabolic syndrome [28][39]. Most scientists agree that meta-inflammation, as a component of immune system, links chronic inflammatory diseases and obesity [29][40]. In this scenario, adipose tissue macrophages can react to high concentrations of fatty acids and initiate signalling pathways promoting monocyte mobilization and differentiation into macrophages, which further contribute to the inflammatory response [30][31][41,42]. Macrophages derived from hematopoietic progenitors are involved in homeostatic and pathogenic processes. In adult tissues, the functions of macrophages are dependent on the microenvironment, and thus macrophages can acquire a proinflammatory (M1) or an anti-inflammatory (anti-fibrotic) (M2) phenotype [32][33][43,44]. Bone-marrow monocyte-derived macrophages can also acquire a pro-inflammatory phenotype and contribute to inflammation [34][45]. Because of lipid accumulation in NAFLD, not only is macrophage polarization altered in favour of the M1 phenotype, but macrophages also undergo metabolic reprogramming leading to increased fatty acid intake and worsen steatosis [35][46]. Activated liver Kupffer cells release pro-inflammatory cytokines, which in turn activate hepatic stellate cells, hepatocytes, or endothelial cells [36][37][47,48], promoting monocyte infiltration and boosting macrophage population. Furthermore, fat accumulation in Kupffer cells leads to oxidative stress and structural changes in the plasmatic and mitochondrial membranes, while in the context of AATD, due to AAT protein accumulation in the ER, this also leads to activation of the unfolded protein response [13][14]. An increase in free fatty acids intensifies lipid oxidation, mainly in the mitochondria and peroxisomes, as well as free-radical production, which can lead to mitochondrial damage and fragmentation [38][39][49,50]. On the other hand, ER stress induced by misfolded proteins triggering the unfolded protein response elicits p53 expression, mitochondrial cytochrome c release, and apoptosis [40][51]. Hence, liver Kupffer cells can contribute not only to the sustained meta-inflammation, but also to the progression of NAFLD (Figure 1).


Figure 1. Schematic presentation of the development of chronic inflammation in NAFLD and AATD. Activated liver macrophages promote inflammation characterized by cytokine and free radical (ROS) production and increased lipid oxidation. In this scenario, to diminish the net increment of lipids, hepatocytes fuse triglycerides (TAG) stored in lipid droplets into APOB-containing lipoproteins and increase the expression of the CD36 receptor to export the lipids out of the cells. In NAFLD patients, this increases the plasma lipoprotein levels with the concomitant risk of cardiovascular disease (CVD). In AATD patients, despite the increased expression of CD36, the accumulation of Z-AAT protein impairs lipids secretion and lowers the risk of CVD. LPL: lipoprotein lipase; HL: hepatic lipase; CETP: cholesteryl ester transfer protein; oxLDL: oxidized LDL.
4. Features of Lipid Metabolism in NAFLD and AATD
Lipids are key cellular components involved in maintaining the integrity of cellular membranes and energy homeostasis, although they also contribute to pathologies [41][63]. Lipid homeostasis in the liver depends on the equilibrated balance between lipid acquisition (de novo formation and uptake), storage, and removal [42][64]. Neutral lipids (sterol esters and triglycerides) are stored in LDs, and in a healthy liver, these lipids do not exceed 5% [43][65]. Fatty acids stored as sterol esters and triglycerides are used during liver homeostasis to generate energy via fatty acid oxidation or are transported to other organs in very-low-density lipoprotein (VLDL) [44][45][66,67] (Figure 1).
A composite route required for VLDL assembly is the lipidation of APOB100, a main and highly hydrophobic apolipoprotein. Initially, VLDLs are pre-assembled in ER lumen by the microsomal triglyceride transfer protein [46][68] and are subsequently moved to the secretory pathway as VLDL2 particles (TAG poor). These particles are secreted out of the hepatocytes or undergo additional lipidation through LD fusion and become VLDL1 particles (TAG enriched) [47][69].
NAFLD is a multifactorial disorder, in which genetic alterations play a role [48][72]. For example, genes such as PNPLA3 [49][73] and TM6SF2 [50][74] are linked to a high risk of NAFLD. The patatin-like phospholipase domain-containing 3 gene (PNPLA3) encodes a membrane-associated lipase that mediates triacylglycerol hydrolysis to manage the increasing amount of lipids after a meal intake. The nonsynonymous transversion from cytosine to guanine (rs738409) renders an amino acid change at codon 148 (isoleucine to methionine) that results in an imbalance between the liver triglyceride content and VLDL secretion [51][75].
