Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Francesco Buonfiglio and Version 4 by Francesco Buonfiglio.
Glaucoma is the most prevalent optic nerve disease worldwide. Oxidative stress plays a pivotal role in the pathophysiology of optic nerve diseases such as glaucoma, Leber’s hereditary optic neuropathy (LHON), and anterior ischemic optic neuropathy (AION)AION. Imbalances between reactive oxygen species (ROS) as well as reactive nitrogen species (RNS) generation and antioxidant systems lead to reactive species overproduction, adenosine triphosphate (ATP) insufficiency, irreversible cellular injuries, and ultimately retinal ganglion cell (RGC)RGC loss.
- oxidative stress
- optic nerve
- retinal ganglion cell
- glaucoma
1. Introduction
Optic nerve diseases encompass a wide range of disorders characterized by optic nerve atrophy, resulting from the loss of retinal ganglion cells (RGCs) and leading to sight-threatening conditions [1][2][3][1,2,3]. These pathologies include:
-
Anterior ischemic optic neuropathies (AION): This category includes arteritic forms, like giant cell arteritis (GCA), which has a pooled prevalence of approximately 51.74 in 100,000 for individuals over the age of 50 [15]. Nonarteritic forms have a reported prevalence of approximately 102.87 in 100,000 in the general population over the age of 40 in the Republic of Korea [16];
-
Infiltrative optic neuropathies, such as leukemic optic neuropathy, which presents in approximately 16% and 18% of all chronic and acute leukemia cases, respectively [28];
Glaucoma is the most prevalent optic nerve disease worldwide [6][7][6,7]. LHON has a low estimated prevalence for complete penetrance cases [8][9][10][11][12][8,9,10,11,12], while the prevalence is much higher for carriers of mutation variants in the general population [13][14][13,14]. The common underlying feature in all optic nerve diseases is the damage and loss of RGCs and their axons, which gradually leads to optic nerve degeneration [3][33][34][3,33,34]. RGCs have high energy requirements and are particularly susceptible to alterations in their energy supply, mainly generated in the mitochondria through the electron transport chain (ETC) [35]. Oxidative stress plays a pivotal role in the pathophysiology of optic nerve diseases such as glaucoma, LHON, and AION. Imbalances between reactive oxygen species (ROS) as well as reactive nitrogen species (RNS) generation and antioxidant systems lead to reactive species overproduction, adenosine triphosphate (ATP) insufficiency, irreversible cellular injuries, and ultimately RGC loss [3][36][37][38][39][40][41][42][43][44][45][3,36,37,38,39,40,41,42,43,44,45].
2. Anatomy and Perfusion of the Visual Pathway
2. Anatomy and Perfusion of the Visual Pathway
The optic nerve, also known as the second cranial nerve [46], is composed of thin (0.1 µm) and lengthy (~50 mm) RGC axons that extend from the retina to the lateral geniculate nucleus, resulting in a soma/axon ratio of approximatively 1:10,000 [35]. Within the retinal layers, these axons merge to form the retinal nerve fiber layer [47], which runs parallel to the superficial blood vessels. The inner retina, including the outer plexiform layer through the nerve fiber layer, is supplied by the central retinal artery. On the other hand, the avascular outer retina, consisting of the outer nuclear layer and photoreceptors, receives its blood supply through diffusion from the choriocapillaris. The choriocapillaris is nourished by short posterior ciliary arteries, which branch from the ophthalmic artery [48][49][48,49]. Notably, retinal oxygenation exhibits variability depending on light or dark conditions due to the different oxygen demand of rods and cones. In humans, rods, which are responsible for vision in low light, are present in approximately 120 million, while cones number around 6 million [50]. Consequently, retinal oxygen consumption is reduced by half in the presence of light, attributed to the decreased activity of rods compared to cones [50].
Optic nerve axons account for approximately 38% of all axons within the central nervous system [51]. Around 1.2 million RGC axons converge to form the optic nerve head (ONH), also referred to as the optic papilla or optic disc. The ONH exhibits a brighter central depression known as the optic cup [51][52][53][54][51,52,53,54]. Blood supply to the ONH in humans is provided by the arterial circle of Zinn–Haller [48].
The optic nerve can be divided into four compartments: the intraocular segment (1–2 mm), which includes the retinal nerve fiber layer (RNFL) and extends from the ONH to the lamina cribrosa; the intraorbital segment (25–30 mm), which spans from the retrobulbar tract to the optic canal; the intracanalicular segment (5–9 mm); and the intracranial segment (9–10 mm), which extends from the optic canal to the optic chiasm [51]. Four distinct regions can be identified within the optic nerve head: the nerve fiber layer, the prelaminar region, the lamina cribrosa, and the retrolaminar region [47]. The lamina cribrosa serves as a supportive structure for the RGC axons within the ONH [55][56][55,56] and consists of approximately 200–300 porous apertures through which the optic nerve passes from the sclera into the retrobulbar cavity [51]. Deformations of the lamina cribrosa may indicate RGC loss and can signify the initial stages of glaucomatous optic neuropathy [57]. Once beyond the lamina cribrosa, the optic nerve becomes myelinated by oligodendrocytes, increasing its diameter from 1–2 mm to 3–4 mm [51]. The intraorbital, intracanalicular, and intracranial segments receive their blood supply from the posterior ciliary arteries as well as the circle of Willis [58].
The two optic nerves converge at the optic chiasm, where nerve fibers originating from the nasal retina of each eye cross over to join the temporal fibers of the contralateral eye [47][59][60][47,59,60]. The blood supply to the chiasm is provided by the circle of Willis [47][52][47,52]. From the chiasm, the RGC axons continue their course into the optic tract, which receives perfusion from the posterior communicating and internal carotid artery [52]. Within the optic tract, the nerve fibers undergo rearrangement to align with their corresponding positions in the lateral geniculate nucleus [59]. Fibers carrying visual information from the right visual field project to the left cerebral hemisphere and vice versa [59]. In the lateral geniculate nucleus, the RGC axons synapse with the second-order neurons of the visual pathway, organized in six layers consisting mainly of large and small neurons [47]. Some fibers from the optic tract also synapse with the olivary pretectal nucleus, regulating the pupillary light reflex [52][61][52,61]. Additionally, RGC axons containing melanopsin terminate in the suprachiasmatic nucleus, a crucial center for controlling the circadian rhythms [47][61][47,61].
The large and small axons of the lateral geniculate nucleus form optic radiation, which initially projects anteriorly and then turns posteriorly, terminating in the occipital lobe where the visual cortex (Brodmann area 17) is located [47][59][47,59]. These regions receive blood supply from branches of the internal carotid artery (lateral geniculate nucleus and optic radiation) and the posterior cerebral artery (visual cortex) [52]. In Figure 12, the perfusion and the anatomy of the visual pathway are illustrated.
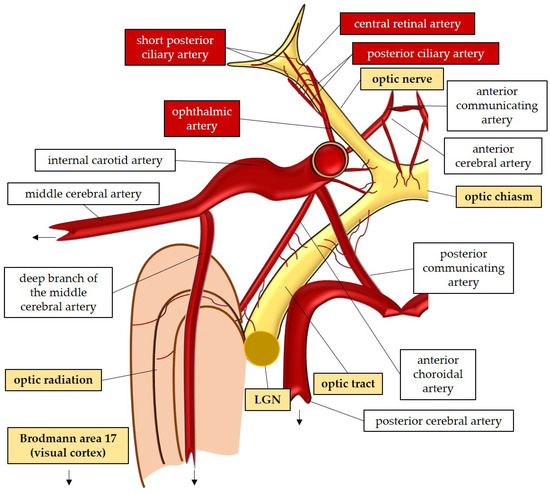
Figure 12.
Anatomy and perfusion of the visual pathway. LGN: lateral geniculate nucleus.
An interesting aspect of the optic nerve anatomy is the presence of an unmyelinated portion in the RGCs [35]. In the unmyelinated compartment, specifically in the intraocular segment of the optic nerve, potential signals cannot be transmitted through saltatory conduction due to the absence of myelin [62]. To compensate for this limitation and enable rapid transmission, RGCs generate higher quantities of ATP in their axons to repolarize the plasma membrane [63]. Mitochondrial bidirectional transport (antero- and retrograde) along the axons plays a crucial role in this process. These organelles move toward regions with high energy demands, such as the unmyelinated portions, and ATP gradients are believed to guide this transport [35]. This mechanism may explain the specific vulnerability of RGCs to mitochondrial dysfunction, leading to the triggering of ROS production in a vicious cycle [44][63][44,63].
It is important to highlight the trophic role of myelin in the optic nerve sheath. Myelin has been shown to play a vital role in supplying nutrients to the axon, as the entire mitochondrial respiratory chain has been detected in the myelin sheath of the optic nerve [64]. This finding provides possible explanations for the link between myelin loss and axonal degeneration observed in neuropathies or demyelinating disorders [64]. A decrease in myelin-related mitochondrial respiration may be one of the main triggers responsible for neurodegenerative events [65]. With aging, there is a loss of structural integrity in the myelin sheath, which can subsequently lead to axonal deterioration [66]. This process may underlie various neurodegenerative pathologies, including Alzheimer’s disease, as recent studies have suggested [67]. Therefore, an intriguing future therapeutic approach for neurodegenerative disorders involves improving the integrity of the myelin sheath, which could potentially slow disease progression [67]. In this regard, the comanipulation of microglia and a specific signaling pathway, such as the G protein-coupled receptor 17 pathway in oligodendrocyte precursor cells, has been shown to induce robust myelination and promote axonal regeneration following injury [68]. Exploring these avenues may also offer new therapeutic perspectives for other neurodegenerative diseases, including optic neuropathies [69].
3. General Mechanisms of Nitro-Oxidative Stress in the Optic Nerve
3.1. Generation of Reactive Oxygen and Nitrogen Species
Mitochondria are vital intracellular organelles responsible for essential chemical reactions that produce energy substrates [70][71][70,71]. In addition to their various cellular functions, such as modulating intracellular calcium levels, synthesizing nucleotides, lipids, and amino acids, and regulating apoptosis, mitochondria also generate ROS [70][72][73][74][70,72,73,74]. ROS, at basal levels, serve as critical mediators of signaling pathways, including hypoxic and inflammatory pathways [44][70][75][76][44,70,75,76]. The fundamental function of mitochondria is to regulate oxygen metabolism and produce energy in the form of ATP [70][71][77][70,71,77]. The electron transport chain (ETC) within the inner mitochondrial membrane plays a central role in this process [78]. Despite the efficiency of oxidative phosphorylation, electron leaks can occur, leading to the direct interaction of electron carriers with molecular oxygen (O2) in the mitochondrial matrix. This interaction results in the donation of electrons and the generation of superoxide (O2•−) [71][77][79][80][71,77,79,80]. While mitochondria are recognized as the main source of ROS in the cell, other significant sources include the enzymatic activities of nitric oxide synthase (NOS) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) [3][79][81][82][3,79,81,82]. NOS generates nitric oxide (NO), while NOX (comprising seven isoforms: NOX1, -2, -3, -4, -5, DUOX1, -2) transfers electrons from cytosolic NADPH to molecular O2, generating O2•− [83][84][83,84]. NO is a free radical that plays a pivotal role in various physiological functions [85]. It serves as a regulator of vascular tone [86][87][88][86,87,88]. Additionally, NO acts as a signaling molecule in neurotransmission and as a regulator of gene transcription [89][90][91][92][93][94][89,90,91,92,93,94]. The production of NO is facilitated by the activity of NOS, an enzyme that has three isoforms: neuronal NOS (nNOS or NOS I); inducible NOS (iNOS or NOS II); and endothelial NOS (eNOS or NOS III) [85][95][85,95]. NO rapidly and spontaneously reacts with O2•− through a “diffusion-limited reaction” [96][97][96,97]. As a result, a highly damaging RNS termed peroxynitrite (ONOO−) is generated [85][96][97][85,96,97]. Peroxynitrite contributes to the pathogenesis of diverse retinal disorders, being also newly proposed as a critical factor in the pathogenesis of glaucoma [98][99][100][98,99,100].3.2. Oxidative Damage and Antioxidant Defense Systems
ROS and RNS play a physiological role in cellular responses to hypoxia, cell proliferation, cell death, inflammation, or infection [44][76][44,76]. Immune cells, such as phagocytes, produce ROS, which provide reactions necessary for an appropriate killing of pathogens [101][102][101,102]. Due to endogenous or exogenous trigger factors, the balance between pro- and antioxidant systems can be critically undermined, resulting in nitro-oxidative stress. In this context, radicals begin to compete for paired electrons with intracellular substrates [103], creating oxidative damage. Oxidative injuries are recognized to be a crucial player in the pathogenesis of a variety of pathologies, including ocular diseases [36][104][105][106][107][36,104,105,106,107]. At the biomolecular level, three general forms of injuries caused by reactive species can be distinguished: DNA lesions [108][109][108,109], protein alterations [110][111][110,111], and lipid peroxidation [96][112][96,112]. The consequences of DNA damage are modifications in the expression of proteins and the altered regulation of fundamental activities, like oxidative phosphorylation, according to the vicious cycle theory [113][114][115][113,114,115]. In this context, mitochondrial ROS also induce activation of the nod-like receptor family pyrin domain-containing 3 (Nlrp3) inflammasome, a key factor in pyroptotic cell death during inflammation [116]. Antioxidant systems are responsible for defending cells and tissues from the damaging impact of reactive species, which are constantly produced as a “by-product” of oxidative phosphorylation but also serve, at basal levels, physiological functions [76]. Enzymatic antioxidants comprise SOD, catalase (CAT), glutathione peroxidase (GPX), glutathione-S-transferase (GST), heme oxygenase (HO), peroxiredoxin, and thioredoxin [103][117][118][119][120][121][122][103,117,118,119,120,121,122]. Nonenzymatic antioxidants can be classified into direct and indirect agents. Direct antioxidants react with ROS or RNS, “being sacrificed in the process of their antioxidant actions” [123][124][123,124]. Free radical scavengers are, for example, glutathione (GSH) [125], carotenoids [126], vitamin C (ascorbic acid) [127], and vitamin E (α-tocopherol) [128]. Alternatively, indirect antioxidants are molecules, such as vitamin C, that upregulate antioxidant proteins, for example, via the nuclear factor erythroid-2-related factor 2 (Nrf2) [123][127][123,127] or molecules, like α-lipoic acid [129]. Examples of antioxidant compounds adsorbed with the food are resveratrol and betulinic acid [98][130][131][98,130,131].3.3. Oxidative Stress in Retinal Ganglion Cells
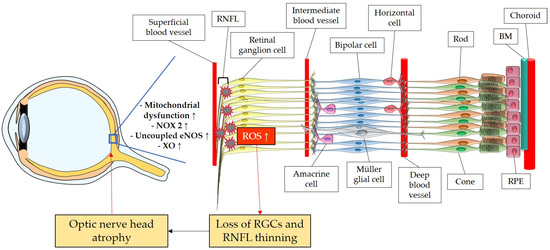
The retina belongs to the metabolically most active organs in the human body [132] and requires a relatively large amount of energy substrates [133], which makes it particularly vulnerable to energy insufficiency [134]. Oxygen supply is essential for retinal function [135], and its consumption occurs very rapidly, like in the brain [136][137][138][136,137,138]. Hence, conditions that can modify the supply of molecules, such as O2, necessary for the production of energy substrates, like ATP, may rapidly generate significant damage in RGCs due to their susceptibility to oxygen deficiency (Figure 23). Thus, an appropriate blood supply via retrobulbar and retinal vessels is crucial for the proper function of RGCs. Studies in retrobulbar blood vessels reported that ROS blunted endothelial function partially by reducing the contribution of the NOS pathway to endothelium-dependent vasodilation [139]. Likewise, moderately elevated IOP induced endothelial dysfunction in retinal arterioles together with RGC loss [140][141][140,141]. Zadeh et al. found in apolipoprotein E (ApoE)-deficient mice that hypercholesterolemia caused oxidative stress and endothelial dysfunction in retinal arterioles but did neither lead to increased ROS levels in the RGC layer nor to loss of RGCs, indicative of compensatory effects [142]. In contrast, a study in pigs reported that after only 12 min of ocular ischemia and 20 h of reperfusion, endothelial dysfunction, retinal edema, and RGC loss occurred [143]. ROS generation due to ischemia/reperfusion (I/R) injury is reported to be caused by diverse enzymes involved in the regulation of oxidative metabolism, such as NOX2, xanthine oxidase (XO), uncoupled eNOS, and by ETC dysfunction [143][144][145][146][143,144,145,146]. Hyperglycemia was also described to be a cause of endothelial dysfunction and oxidative stress in the retina [147][148][149][147,148,149] via the involvement of NOX2 due to the activation of the receptor of an advanced glycation end product (RAGE)-, mitogen-activated protein kinase (MAPK)-, polyol-, protein kinase C (PKC)-, renin–angiotensin system (RAS) signaling pathways [150][151][152][153][154][155][150,151,152,153,154,155].
Figure 23. Model representing the ROS impact on the retina and on the optic nerve. ROS: reactive oxidative species; NOX2: NADPH oxidase type 2; XO: xanthine oxidase; eNOS: endothelial nitric oxide synthase; RGC: retinal ganglion cell; RNFL: retinal nerve fiber layer; RPE: retinal pigment epithelium; BM: Brunch’s membrane. Up arrows mean increase or upregulation.
