Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Fazheng Ren.
By regulating skin health and gut–skin axis interactions, probiotics can be used as potential management tools to suppress and improve skin diseases in multiple ways, including decreasing oxidative stress, suppressing inflammatory responses, and keeping immune effects.
- skin
- probiotics
- intestinal microbiota
- gut–skin axis
1. Introduction
Skin accounts for about 15 percent of the total body weight of adults, with an average surface area of 1.5–2 m2 [1]. One of the main functions of the skin is its use as a mechanical barrier to disease-causing microorganisms and harmful substances; in fact, it could be viewed as one of the host’s vital defenses against infections, as well as the innate and adaptive immune system [2]. Other important features include inhibition of transcutaneous water loss (TEWL), thermoregulation, structural support, and vitamin synthesis, all of which assist in maintaining a healthy host [2,3,4][2][3][4].
The quest for beauty never ends. It is often hard to know whether skin issues, including skin pigmentation, skin wrinkles, skin aging, and skin dehydration, occur due to external elements or internal changes. Skin issues have various causes, and investigators are continuously researching safe and efficient skin treatment products to address skin issues. Today, various cosmetic products contain chemicals, including titanium dioxide, which are more or less toxic and may be harmful to an individual’s health [5]. There are also various researchers who use raw materials extracted from herbal medicines as important components in skin treatment products, while they show certain results due to the complexity of herbal ingredients, their influences sometimes fail to meet expectations and their quality still needs to be improved [6,7][6][7]. Thus, there is an urgent need to explore safe and efficient ingredients for skin treatment products that can effectively address skin issues.
Probiotics can act through a variety of mechanisms (Figure 1).
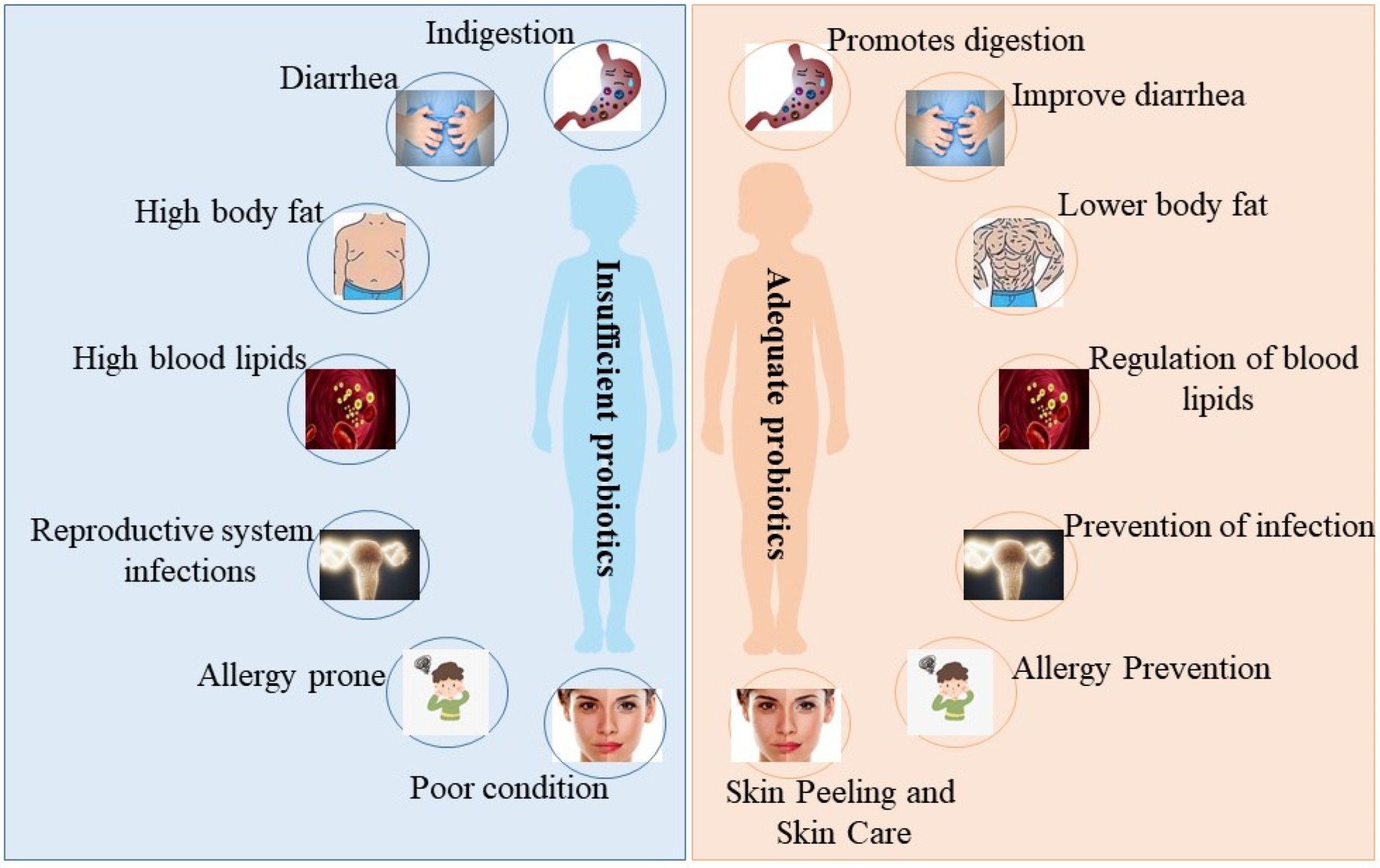

Figure 1. The beneficial effects of probiotics on the organism. When the abundance of probiotics in the organism is insufficient, the organism suffers from the following issues: indigestion, diarrhea, high body fat, high blood lipids, reproductive system infections, allergy prone, and poor skin condition (left picture). When the abundance of probiotics in the organism is sufficient, the organism behaves as follows: promotes digestion, improves diarrhea, lowers body fat, regulates blood lipids, prevents reproductive system infections, prevents allergies, and skin peeling and skin care (right picture).
2. The Different Effects of Probiotics on the Skin
2.1. Skin Whiting
Recently, there has been an increasing interest in skin lightening, and the focus of brightening products is to decrease melanin content and suppress overproduction pigmentation [13][8]. Melanin is photoprotective and protects the skin from ultraviolet (UV) radiation, but the overexpression of pigmentation can affect skin tone and even induce various skin disorders, including freckles and melasma [14,15,16][9][10][11]. The process of melanin production involves various enzymes and chemical catalytic reactions [17,18][12][13]. There are three main enzymes associated with melanogenesis, containing tyrosinase, tyrosinase-related protein 1 (TYRP-1), and tyrosinase-related protein 2 (TYRP-2), with tyrosinase being the indispensable primary enzyme [19][14]. A lot of whitening cosmetics can precisely suppress the tyrosinase activity, thus reducing melanin content and achieving a brightening effect. Recently, probiotics have been increasingly used in brightening products, which is tightly associated with their great antagonistic influence on tyrosinase (Figure 2).
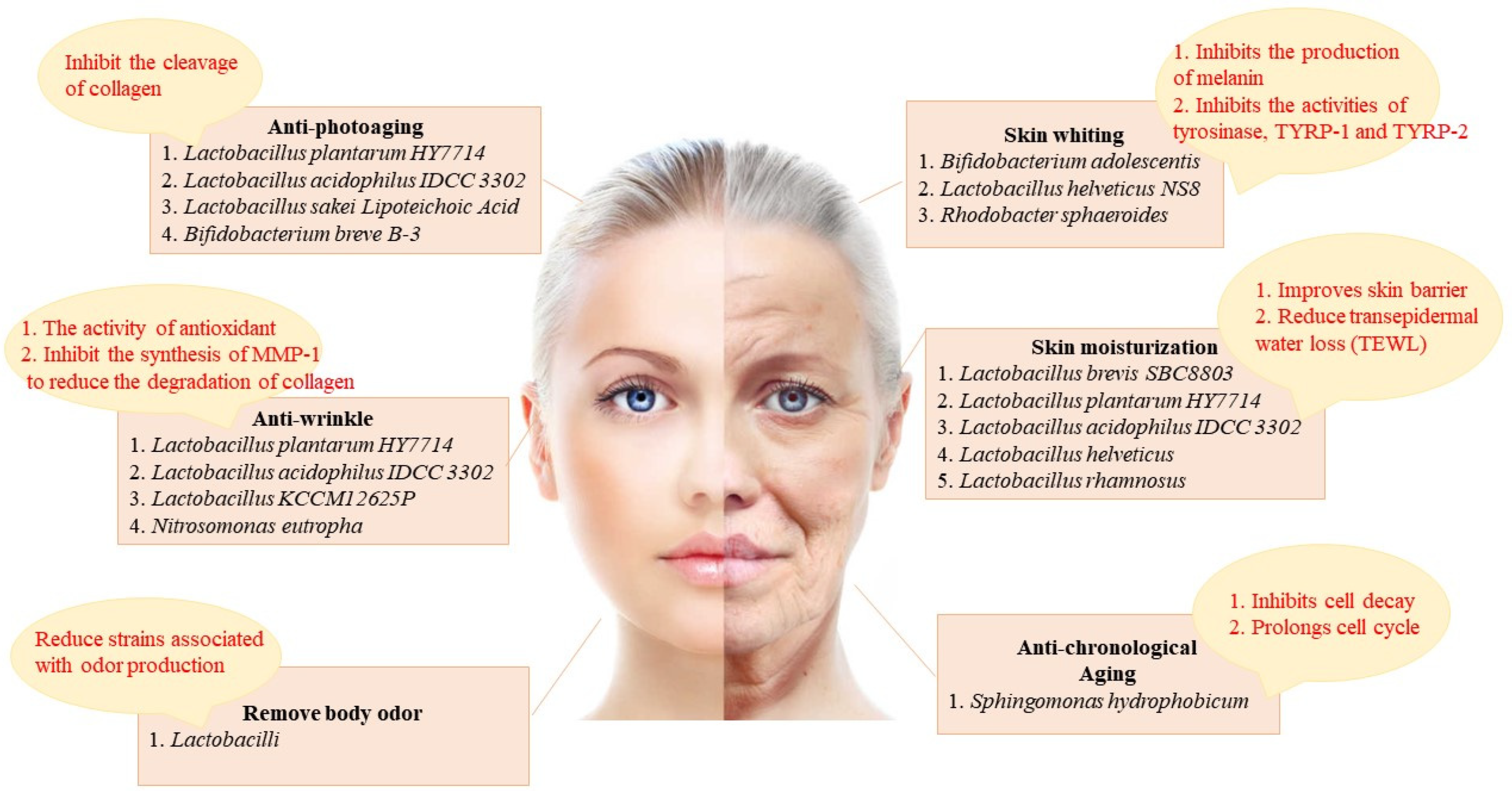

Figure 2. The skin improvement effect of probiotics and its related mechanism. The skin improving effects of probiotics include: anti-photoaging (inhibit the cleavage of collagen), skin whiting (inhibits the production of melanin and inhibits the activities of tyrosinase, TYRP-1 and TYRP-2), anti-wrinkle (the activity of antioxidant and inhibition of the synthesis of matrix metalloproteinase-1 (MMP-1) to reduce the degradation of collagen), skin moisturization (improves skin barrier and reduces TEWL), body odor removal (reduce strains associated with odor production), and anti-chronological aging (inhibits cell decay and prolongs cell cycle).
A study suggested that the antagonistic influence of Bifidobacterium adolescentis culture filtrate on mushroom tyrosinase and tyrosinase activity strengthened with increasing content, thereby reducing the melanin levels in B16F10 cells [20][15]. According to these studies, Bifidobacterium adolescentis culture filtrate could modulate tyrosinase activity via its antioxidant effect, thereby decreasing melanin content and achieving a whitening purpose. Moreover, they also uncovered that lactic acid in Lactobacillus can suppress melanin synthesis directly by down-regulating tyrosinase activity and also regulate melanin synthesis by affecting tyrosinase expression or tyrosinase, tyrosine 1 and tyrp-2 to exhibit a brightening effect [21][16].
Probiotics can decrease melanin content not only by regulating tyrosinase activity but also by other means to achieve a whitening effect. Jingjing Rong et al. indicated the brightening effect of Lactobacillus helveticus NS8 fermented milk supernatant (NS8-FS) [22][17]. Data demonstrated that NS8-FS decreased melanin levels in B16F10 cells by suppressing the activity of tyrosinase and proteins associated with tyrosinase expression. Furthermore, a UV radiation-induced pigmentation model was established in guinea pigs. Masson-Fontana staining and tyrosinase staining tests confirmed that NS8-FS improved skin pigmentation. The potential mechanism by which NS8-FS improved skin pigmentation is the modulation of the Nrf2 activity, which promotes melanogenesis in melanocytes under UV-mediated oxidative stress [23][18]. Liu et al. explored the inhibitory effect of Rhodobacter spheroides (Lysogen™) on melanin synthesis [24][19]. Research demonstrated that melanin content in B16F10 cells up-regulated after α-MSH supplementation and down-regulated in a dose-dependent manner after Lysogen™ treatment.
2.2. Skin Moisturization
There are various reasons for dry skin, such as seasonal alterations, skin barrier damage, and disorderly flaking [25][20]. Skin hydration is important for health and beauty, and it exerts vital effects on maintaining proper body activity and beauty. Probiotics can decrease TEWL and improve skin dryness, which can be used to modulate dry skin. Moreover, probiotics can also decrease skin water-loss by modulating skin–barrier function and are good skin moisturizers [26][21].
A study confirmed that the oral supplementation of Lactobacillus plantarum HY7714 increased ceramide levels by elevating serine palmitoyltransferase (SPT) mRNA expression and decreasing ceramidase mRNA expression [27][22]. Ceramides exert an important effect on keeping the structural support of the epidermal barrier and the epidermal hydration [28,29][23][24]. Elevated ceramide contents lead to lower TEWL values and up-regulated hydration. Studies used ELISA to detect hyaluronic acid (HA) content and found that the use of acidophilic lactic acid IDCC 3302 exerts a beneficial effect on skin hydration [30,31][25][26]. In conclusion, the treatment of Lactobacillus acidophilus IDCC 3302 resulted in improved skin dryness and decreased TWEL, thereby up-regulating skin hydration. Hidoko BABA et al. indicated that the administration of Lactobacillus helveticus-fermented milk whey (LHMW) led to an obvious reduction in TWEL of intact skin and an increase in skin water content, proving that LHMW milk has a moisturizing effect and is used in cosmetics [32][27].
2.3. Skin Barrier Integrity
Damage to the skin barrier function can adversely affect the skin by disrupting the moisture balance on the skin surface [33][28]. Ye-On Jung et al. demonstrated experimentally that Lactobacillus rhamnosus (LR) can effectively improve the skin barrier and can be regarded as a moisturizing skin care product [34][29]. They used immunofluorescence staining to identify up-regulated expression levels of Claudin-1 and Occludin, two tightly bound molecules and indicated that the stratum corneum of tissues treated with LR lysate was tighter and more organized. Moreover, qPCR results suggested elevated expression levels of loricrin and filaggrin which exert a vital effect on the restoration of skin barrier function [35][30]. Furthermore, the strengthened skin barrier function was further suggested by reducing sodium dodecyl sulfate (SLS)-induced cytotoxicity and decreasing skin permeability.
2.4. Anti-Aging
Chronological aging and photoaging are two primary forms of skin aging [36][31]. Chronological aging is mainly influenced by internal elements, whereas photoaging is mainly influenced by external elements [37][32]. These influences are different but have similar regulatory mechanisms, but probiotics have a beneficial effect on both forms of skin aging.
2.4.1. Anti-Chronological Aging
Chronological aging is mainly associated with genetic elements and is a regular physiological process in the human body. As we age, the body ages, and so does the skin, which is characterized by thinning and dryness [38][33]. Probiotics achieve anti-aging mainly by suppressing cell decay and prolonging the cell cycle. Sandie Gervason et al. indicated that the exclusion of Sphingomonas hydrophobicum (SH) could suppress the production of proteins associated with aging, such as P16 and P21, using an immunohistological experiment. P16 and P21 are cell cycle antagonists, which suppress the cell cycle and result in cell aging [39,40][34][35]. The production level of P16 and P21 in the experimental group were obviously down-regulated versus the control group without SH extraction. SH extraction was also demonstrated to suppress the SA-β-galactosidase level, which is linked to aging, to improve cell senescence. Moreover, the level of fibrillin-1 and Versican was up-regulated after SH extraction supplementation. Previous research indicated that fibrillin-1 participates in the production of elastic skin fibers [41][36] and that an up-regulated Versican level can inhibit the apoptosis response of fibroblasts [42][37], both of which can slow down cell senescence. In the case of SH extraction, it can be used as an anti-aging skin-care product.
2.4.2. Anti-Photoaging
Photoaging is primarily influenced by external environmental elements, such as UV radiation and toxins. These external elements will induce injury to the skin, causing it to lose elasticity, lose moisture, thicken, and become rough and sluggish [43][38]. Probiotics have a significant influence on the treatment of photoaging, which is primarily achieved by suppressing collagen division.
Research indicated that patients taking Lactobacillus plantarum HY7714 had decreased epidermal moisture loss, decreased wrinkle depth, and ameliorated skin gloss and elasticity [44][39]. Research demonstrated that tyndallized Lactobacillus acidophilus IDCC 3302 could restore the reduction in collagen expression after UV irradiation via Western blot analysis [45][40]. Meanwhile, it was shown that tyndallized Lactobacillus acidophilus IDCC 3302 could obviously decrease the contents of MMP-1, MMP-2, and MMP-9 in HaCaT, which were up-regulated due to exposure to UV rays, primarily by suppressing the MAPK signaling pathway. Moreover, tyndallized Lactobacillus acidophilus IDCC 3302 can improve the inflammation response by reducing the levels of proinflammatory cytokines, including IL-1β, IL-8, and TNF-α. The above data showed that tyndallized Lactobacillus acidophilus IDCC 3302 can inhibit photoaging and ameliorate inflammation responses induced by UV irradiation. You et al. suggested that Lactobacillus sakei Lipoteichoic Acid (sLTA) could suppress the phosphorylation of MAPK and further block the MMP-1 synthesis when hosts are exposed to UV rays [46][41].
2.5. Anti-Wrinkle
Wrinkles are induced by atrophy of the skin and repeated contractions of facial muscles underneath [47][42]. The use of probiotics has been proven to regulate facial wrinkles. The antioxidant activity of probiotics is closely associated with their anti-wrinkle properties. Moreover, MMP-1 synthesis activates the degradation of collagen produced by fibroblasts, resulting in wrinkles on the surface of human skin [48][43]. Probiotics could suppress MMP-1 synthesis and decrease collagen degradation, resulting in anti-wrinkle properties.
Researchers found that tyndallized Lactobacillus KCCM12625P (AL) can suppress the MMP-1 synthesis, thus preventing wrinkle formation. AL can effectively inhibit the formation of facial wrinkles and act as an anti-wrinkle mainly through the above two aspects.
Hyun Mee Kim et al. found that Lactobacillus plantarum HY7714 had a powerful blocking function on UV-induced MMP-1 according to Western blot [49][44]. Moreover, Lactobacillus plantarum HY7714 inhibited the MMP-1 expression and the MMP-2 and MMP-9 activity, which effectively improved the area and depth of wrinkles and exerted a vital effect on wrinkle elimination. A study indicated that tyndallized Lactobacillus acidophilus IDCC 3302 could effectively decrease MMP-1, MMP-2, and MMP-9 contents in UV-exposed HaCaT cells measured by ELISA. Therefore, tyndallized Lactobacillus acidophilus IDCC 3302 could decrease wrinkles by suppressing MMPs.
References
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533.
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253.
- Oh, J.; Byrd, A.L.; Park, M.; Kong, H.H.; Segre, J.A. Temporal stability of the human skin microbiome. Cell 2016, 165, 854–866.
- Schommer, N.N.; Gallo, R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013, 21, 660–668.
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46.
- Hu, Y.; Zeng, H.; Huang, J.; Jiang, L.; Chen, J.; Zeng, Q. Traditional Asian aerbs in skin whitening: The current development and limitations. Front. Pharmacol. 2020, 11, 982.
- Yu, J.; Ma, X.; Wang, X.; Cui, X.; Ding, K.; Wang, S.; Han, C. Application and mechanism of probiotics in skin care: A review. J. Cosmet. Dermatol. 2022, 21, 886–894.
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115.
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549.
- Iozumi, K.; Hoganson, G.E.; Pennella, R.; Everett, M.A.; Fuller, B.B. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J. Investig. Dermatol. 1993, 100, 806–811.
- Li, G.; Ju, H.K.; Chang, H.W.; Jahng, Y.; Lee, S.H.; Son, J.K. Melanin biosynthesis inhibitors from the bark of Machilus thunbergii. Biol. Pharm. Bull. 2003, 26, 1039–1041.
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Inhibitors of melanogenesis: A patent review (2009–2014). Expert. Opin. Ther. Pat. 2015, 25, 775–788.
- Schiaffino, M.V. Signaling pathways in melanosome biogenesis and pathology. Int. J. Biochem. Cell Biol. 2010, 42, 1094–1104.
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425.
- Huang, H.C.; Chang, T.M. Antioxidative properties and inhibitory effect of Bifidobacterium adolescentis on melanogenesis. World J. Microbiol. Biotechnol. 2012, 28, 2903–2912.
- Huang, H.C.; Lee, I.J.; Huang, C.; Chang, T.M. Lactic acid bacteria and lactic acid for skin health and melanogenesis inhibition. Curr. Pharm. Biotechnol. 2020, 21, 566–577.
- Lim, H.Y.; Jeong, D.; Park, S.H.; Shin, K.K.; Hong, Y.H.; Kim, E.; Yu, Y.G.; Kim, T.R.; Kim, H.; Lee, J.; et al. Antiwrinkle and antimelanogenesis effects of tyndallized Lactobacillus acidophilus KCCM12625P. Int. J. Mol. Sci. 2020, 21, 1620.
- Chaiprasongsuk, A.; Onkoksoong, T.; Pluemsamran, T.; Limsaengurai, S.; Panich, U. Photoprotection by dietary phenolics against melanogenesis induced by UVA through Nrf2-dependent antioxidant responses. Redox Biol. 2016, 8, 79–90.
- Liu, W.S.; Kuan, Y.D.; Chiu, K.H.; Wang, W.K.; Chang, F.H.; Liu, C.H.; Lee, C.H. The extract of Rhodobacter sphaeroides inhibits melanogenesis through the MEK/ERK signaling pathway. Mar. Drugs 2013, 11, 1899–1908.
- Kim, H.; Kim, J.T.; Barua, S.; Yoo, S.Y.; Hong, S.C.; Lee, K.B.; Lee, J. Seeking better topical delivery technologies of moisturizing agents for enhanced skin moisturization. Expert. Opin. Drug Deliv. 2018, 15, 17–31.
- Harding, C.R.; Watkinson, A.; Rawlings, A.V.; Scott, I.R. Dry skin, moisturization and corneodesmolysis. Int. J. Cosmet. Sci. 2000, 22, 21–52.
- Ra, J.; Lee, D.E.; Kim, S.H.; Jeong, J.W.; Ku, H.K.; Kim, T.Y.; Choi, I.D.; Jeung, W.; Sim, J.H.; Ahn, Y.T. Effect of oral administration of Lactobacillus plantarum HY7714 on epidermal hydration in ultraviolet B-irradiated hairless mice. J. Microbiol. Biotechnol. 2014, 24, 1736–1743.
- Elias, P.M.; Menon, G.K. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv. Lipid Res. 1991, 24, 1–26.
- Holleran, W.M.; Uchida, Y.; Halkier-Sorensen, L.; Haratake, A.; Hara, M.; Epstein, J.H.; Elias, P.M. Structural and biochemical basis for the UVB-induced alterations in epidermal barrier function. Photodermatol. Photoimmunol. Photomed. 1997, 13, 117–128.
- Im, A.R.; Lee, B.; Kang, D.J.; Chae, S. Skin moisturizing and antiphotodamage effects of tyndallized Lactobacillus acidophilus IDCC 3302. J. Med. Food 2018, 21, 1016–1023.
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm.-Endocrinol. 2012, 4, 253–258.
- Baba, H.; Masuyama, A.; Yoshimura, C.; Aoyama, Y.; Takano, T.; Ohki, K. Oral intake of Lactobacillus helveticus-fermented milk whey decreased transepidermal water loss and prevented the onset of sodium dodecylsulfate-induced dermatitis in mice. Biosci. Biotechnol. Biochem. 2010, 74, 18–23.
- Segre, J.A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Investig. 2006, 116, 1150–1158.
- Jung, Y.O.; Jeong, H.; Cho, Y.; Lee, E.O.; Jang, H.W.; Kim, J.; Nam, K.; Lim, K.M. Lysates of a probiotic, Lactobacillus rhamnosus, can improve skin barrier function in a reconstructed human epidermis model. Int. J. Mol. Sci. 2019, 20, 4289.
- Draelos, Z.D. New treatments for restoring impaired epidermal barrier permeability: Skin barrier repair creams. Clin. Dermatol. 2012, 30, 345–348.
- Cho, S. The role of functional foods in cutaneous anti-aging. J. Lifestyle Med. 2014, 4, 8–16.
- Trojahn, C.; Dobos, G.; Lichterfeld, A.; Blume-Peytavi, U.; Kottner, J. Characterizing facial skin ageing in humans: Disentangling extrinsic from intrinsic biological phenomena. Biomed Res. Int. 2015, 2015, 318586.
- Mora Huertas, A.C.; Schmelzer, C.E.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular-level insights into aging processes of skin elastin. Biochimie 2016, 128–129, 163–173.
- Gervason, S.; Napoli, M.; Dreux-Zhiga, A.; Lazzarelli, C.; Garcier, S.; Briand, A.; Albouy, M.; Thepot, A.; Berthon, J.Y.; Filaire, E. Attenuation of negative effects of senescence in human skin using an extract from Sphingomonas hydrophobicum: Development of new skin care solution. Int. J. Cosmet. Sci. 2019, 41, 391–397.
- Dolan, D.W.; Zupanic, A.; Nelson, G.; Hall, P.; Miwa, S.; Kirkwood, T.B.; Shanley, D.P. Integrated stochastic model of DNA damage repair by non-homologous end joining and p53/p21-mediated early senescence signalling. PLoS Comput. Biol. 2015, 11, e1004246.
- Penner, A.S.; Rock, M.J.; Kielty, C.M.; Shipley, J.M. Microfibril-associated glycoprotein-2 interacts with fibrillin-1 and fibrillin-2 suggesting a role for MAGP-2 in elastic fiber assembly. J. Biol. Chem. 2002, 277, 35044–35049.
- Sheng, W.; Wang, G.; Wang, Y.; Liang, J.; Wen, J.; Zheng, P.S.; Wu, Y.; Lee, V.; Slingerland, J.; Dumont, D.; et al. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol. Biol. Cell 2005, 16, 1330–1340.
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470.
- Lee, D.E.; Huh, C.S.; Ra, J.; Choi, I.D.; Jeong, J.W.; Kim, S.H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.H.; et al. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: A randomized, double blind, placebo-controlled study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168.
- Im, A.R.; Lee, B.; Kang, D.J.; Chae, S. Protective effects of tyndallized Lactobacillus acidophilus IDCC 3302 against UVB-induced photodamage to epidermal keratinocytes cells. Int. J. Mol. Med. 2019, 43, 2499–2506.
- You, G.E.; Jung, B.J.; Kim, H.R.; Kim, H.G.; Kim, T.R.; Chung, D.K. Lactobacillus sakeilipoteichoic acid inhibits MMP-1 induced by UVA in normal dermal fibroblasts of human. J. Microbiol. Biotechnol. 2013, 23, 1357–1364.
- Small, R. Botulinum toxin injection for facial wrinkles. Am. Fam. Physician 2014, 90, 168–175.
- Park, S.H.; Lee, K.H.; Han, C.S.; Kim, K.H.; Kim, Y.H. Inhibitory effects of carex humilis extract on elastase activity and matrix metalloproteinase-1 expression. J. Soc. Cosmet. Sci. Korea 2010, 36, 129–136.
- Kim, H.M.; Lee, D.E.; Park, S.D.; Kim, Y.T.; Kim, Y.J.; Jeong, J.W.; Jang, S.S.; Ahn, Y.T.; Sim, J.H.; Huh, C.S.; et al. Oral administration of Lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B induced photoaging. J. Microbiol. Biotechnol. 2014, 24, 1583–1591.
More
