Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Luis Aristizabal.
Coffee berry borer (CBB) is the most serious insect pest of coffee worldwide, causing significant reductions in yield and quality. Here we are addressing tThe integrated pest management of CBB in Hawaii and Puerto Rico are addressed, the only two USA locations that produce commercial specialty coffee.
- biological control
- cultural practices
- monitoring
- natural enemies
1. Coffee BBerry Borer Biology
Adult female coffee berry borer (CBB) are attracted to developing berries and cherries that are 60–240 days old [1][2]. Coffee fruits are commonly called berries, and ripe fruits are called cherries, even though neither term is botanically correct; coffee fruits are drupes. Once an appropriate berry is located, the female bores a small entry hole, typically in the central disc area. The degree of penetration into the berry can be described by the AB and CD positions [3]. The AB position is the initial perforation of the exocarp and mesocarp, in which part of the CBB body is still visible (Figure 1A). CBB can persist in this AB position for 30–90 days until the endosperm (part of the coffee seed or “bean”) has reached <20% moisture content [1]. In the AB position, the CBB female is vulnerable to insecticides, pathogens, natural predators, and parasitoids and can be killed before damage to the endosperm occurs [3][4]. When the endosperm reaches > 20% dry weight (berries ~120–150 days old; [1]) the female will begin boring tunnels for reproduction. CBB entry into the endosperm and the building of galleries in which to initiate oviposition is known as the CD position (Figure 1B). In the CD position, damage to the coffee seed has occurred, and the CBB female and its progeny are protected from pesticides and the entomopathogenic fungus Beauveria bassiana (Figure 1C) [3][4]. Male and female siblings mate inside their natal berry, the males die, and the mated females fly in search of a new berry, thus starting a new cycle of infestation.
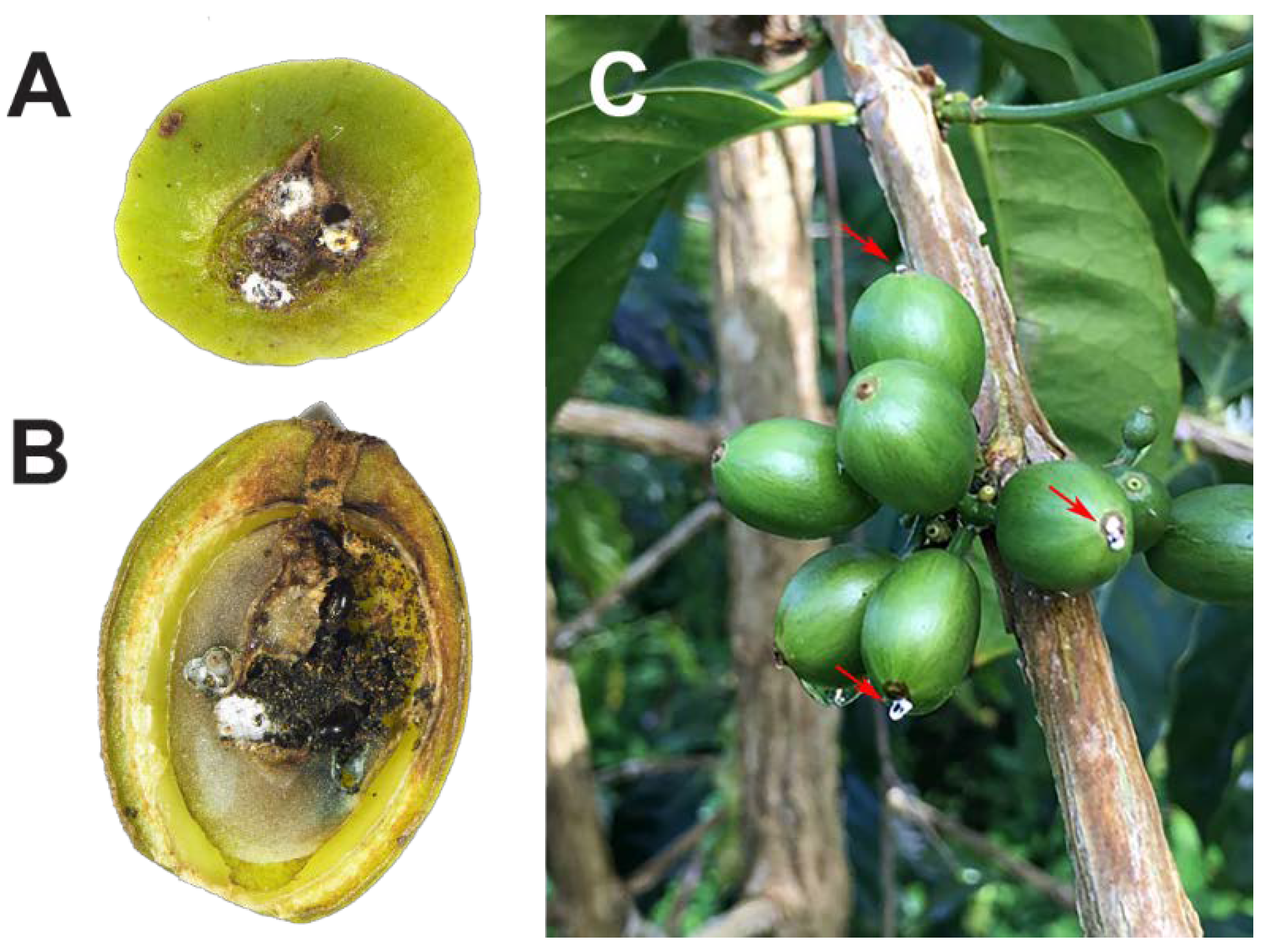
Figure 1. CBB females in the AB position killed by the entomopathogenic fungus Beauveria bassiana (A); CBB adult females in the CD position: note larval feeding on the seed tissue (B); red arrows show the white mycelia of B. bassiana growing from the bodies of the CBB in AB position (C). Photos: (A,B) M. A. Johnson; (C) L. F. Aristizábal.
2. Cultural Control
2.1. Harvesting and Sanitation Picks
Frequent and efficient harvesting, as well as post-harvest strip-picking (collection of all remaining green, ripe, and over-ripe berries at the end of the harvest season), are the most important cultural practices for regulating CBB as they remove population reservoirs from the fields [3][5][6][7]. While these practices are relatively simple in concept, their implementation is often limited by the cost of labor, field worker availability, and the quality of training they receive [1][3][8][9][10].
In Hawaii, strip-picking is recommended as a critical part of the IPM program for CBB control [11][12]. This practice is regularly conducted in the Kona district of Hawaii Island on coffee farms that are at low and middle elevations (200–600 m), where the harvest season is short (3–4 months). In contrast, farms in the Ka’u district and at high elevations (>600 m) in the Kona district typically conduct sanitation picks (pre- or post-harvest removal of all ripe and over-ripe berries) since the harvest season is longer (6–9 months) in these locations [13]. The longer harvesting season is due to high precipitation in these locations, which allows multiple flowerings throughout the year. Flowers, developing green berries, and ripe and over-ripe berries are observed simultaneously during most of the year, making strip-picking economically unfeasible for coffee farmers in these locations. Instead of strip-picking, more frequent and efficient harvesting practices and sanitation picking (Figure 2) are recommended in areas that experience a year-round harvest [14].
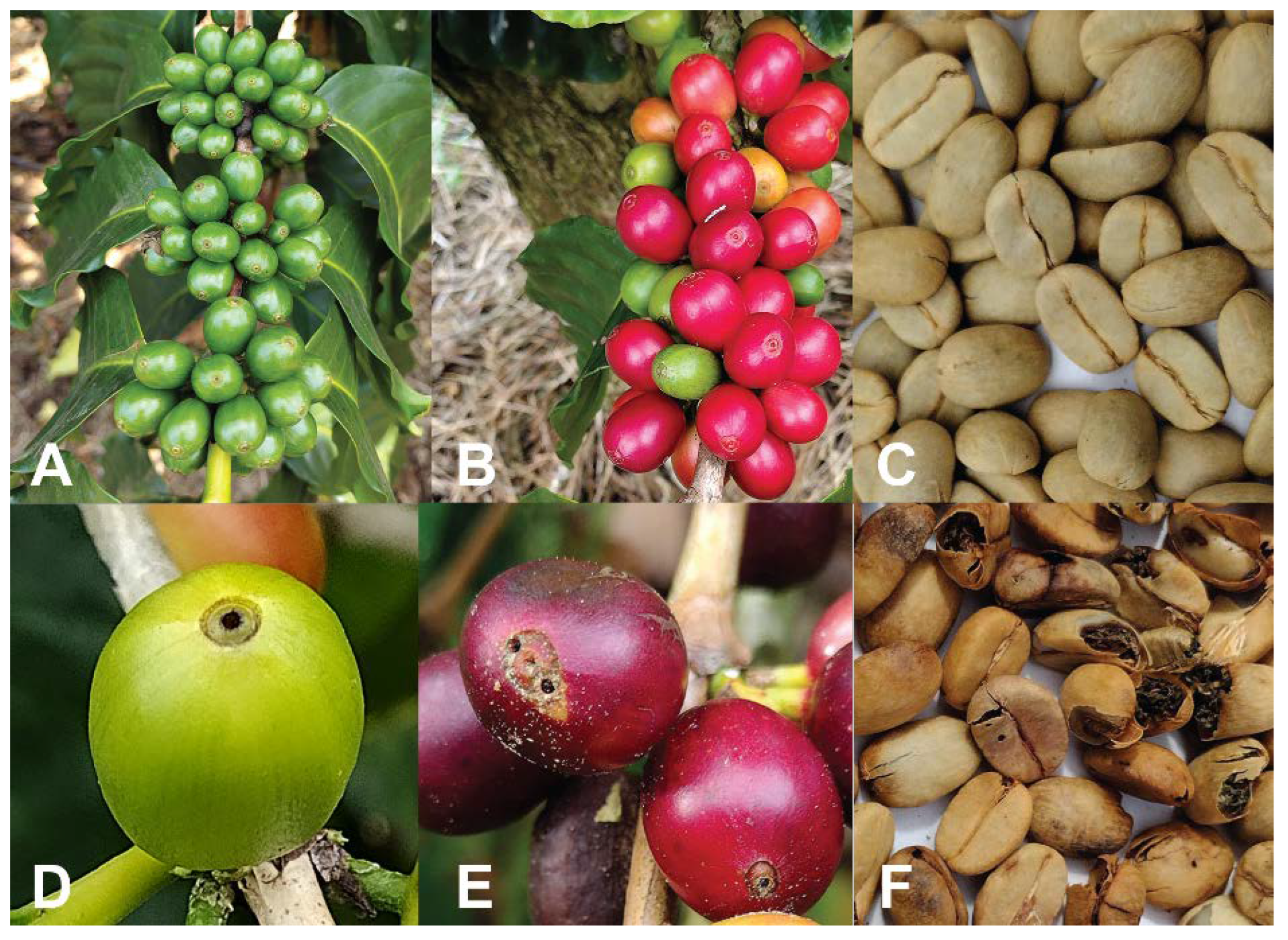
Figure 2. Standard harvesting and sanitation pick, in which only cherry and raisin berries are collected (A), strip-pick at the end of harvest season in which all berries are collected (green, cherry and raisin berries) (B), and a coffee picker working during the harvest (C). Photos: Luis F. Aristizábal.
The first evaluation of harvesting efficacy in Hawaii was conducted in 2016 on 11 coffee farms, with 36 harvesting rounds assessed [15]. The efficacy of each harvesting round was evaluated by randomly selecting 10 trees and counting the number of ripe and over-ripe berries left per tree (Excellent < 5 berries, Good = 5–10 berries, Bad > 10 berries [3]. Only 9.1% of rounds were scored as “Excellent”, while 21.2% were scored as “Good” and the remaining 69.7% were scored as “Bad” [15]. These results suggested that better training of coffee farmers and pickers was urgently needed to reduce CBB populations. In 2018, a second study evaluated 25 coffee farms in the Ka’u district. Prior to training coffee farmers and pickers in proper harvest techniques, there were an average of 15.6 cherries left per tree (“Bad”), and CBB infestation was 6.5% [16]. After farm visits and training were conducted, the average number of berries left per tree decreased to 6.3 (“Good”) and CBB infestation was reduced to 2.5% [16].
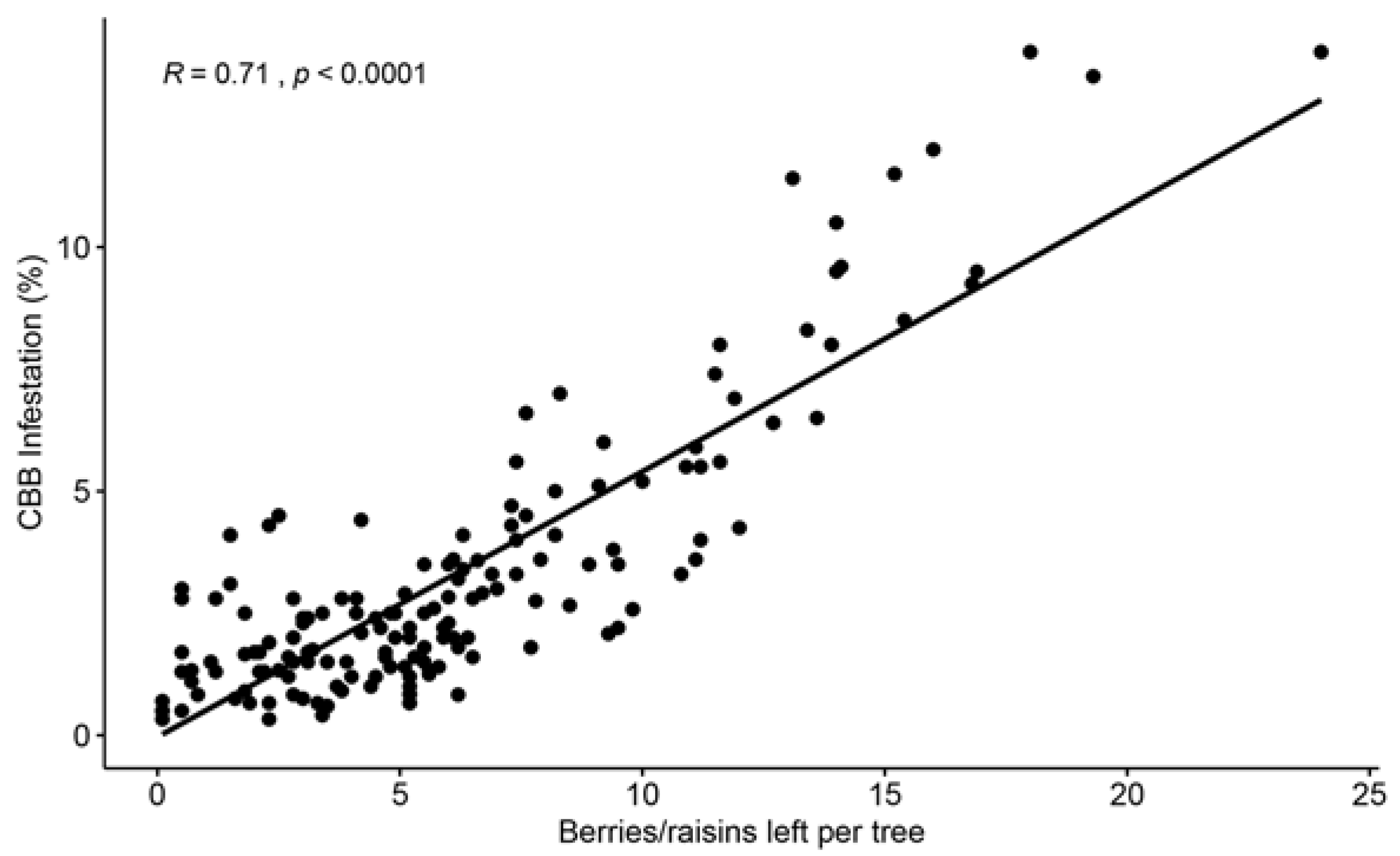
In a third study conducted on Hawaii Island, the efficacy of CBB control using conventional management (frequent sprays of insecticides and few harvesting rounds) was compared to cultural management (frequent and efficient harvesting and few insecticide sprays) over two years [14]. A positive relationship was found between CBB infestation, and the number of berries left per tree after a harvesting round (Figure 3). Frequent and efficient harvesting not only significantly reduced CBB populations and damage to processed coffee but was also found to be economically feasible for commercial coffee farms in Hawaii, which have some of the highest costs to produce coffee around the world. A cost–benefit analysis showed that a profit was obtained after sanitation, harvesting, and strip-picking were conducted, and the collected coffee was processed and sold [14].

Figure 3. Positive correlation between the number of cherries and raisin berries left on trees after a harvesting round was conducted and CBB infestation. Evaluations were conducted on 10 commercial coffee farms from the Kona and Ka’u districts of Hawaii Island during the 2019–2020 coffee season (n = 184 evaluations). Data from Aristizábal et al. [14].
2.2. Pruning
2.2. Pruning
Pruning renews trees while helping to regulate CBB populations by allowing better access to berries during and after the harvest and improving spray coverage [3][17]. In Hawaii, the traditional pruning system is known as “Kona style”, in which each tree has multiple verticals of different ages [18]. In this pruning system, old verticals (>4 years old) with low production are removed, and only productive younger verticals are maintained [18]. This traditional pruning style promotes the growth of new verticals to improve the productivity and health of trees. However, it does not significantly reduce the CBB population since there is no interruption in berry development, which deprives CBB of food and shelter for reproduction and survival.
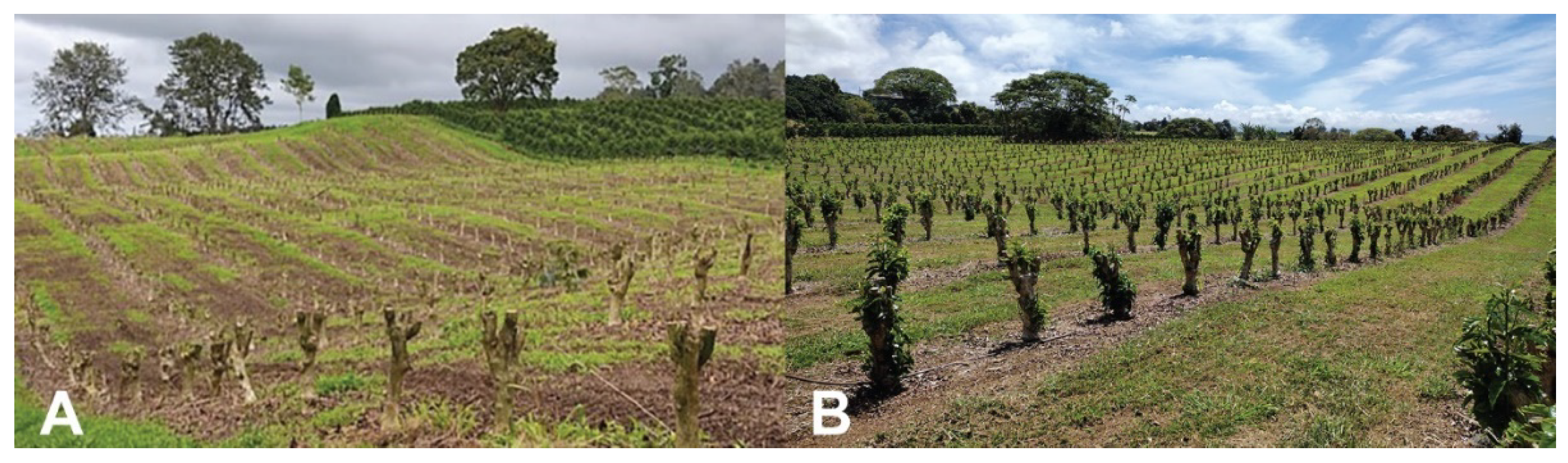
In contrast, stump pruning in blocks (Figure 4), initially developed in Colombia [3], guarantees a significant reduction in CBB populations in coffee lots since all verticals are removed and berry production is interrupted for 12–15 months. On stumped coffee lots, many CBB females emerge from berries fallen on the ground or left on stumped trees. CBB continues to emerge from these berries over a 3-month period [1][3] and can infest neighboring coffee lots. Keeping 1–2 rows of coffee trees along the border of stumped lots helps to capture flying females and limit the number that escape into neighboring fields [3][19]. These trap trees may be sprayed with B. bassiana and the berries collected every three weeks over the 3-month emergence period for the regulation of CBB [3][19]. Trap trees are then stumped to break the cycle. In Hawaii, stump pruning resulted in low CBB infestation (1–4%) in comparison to lots that used the Beaumont-Fukunaga pruning system (multiple verticals of the same age on each tree, with rows stumped in 3–5-year cycles), in which CBB infestation was 2–12% [15]. Additional studies are needed to fully evaluate the impacts of the various pruning systems on CBB populations as well as the cost and benefits of this cultural practice.

Figure 4. A coffee field block-stumped for the renewal of trees and control of CBB and CLR (A). Coffee field three months after stumping was conducted; note new leaf growth (B). Photos: Luis F. Aristizábal.

3. Biological Control
3.1. Beauveria Bassiana
The entomopathogenic fungus Beauveria bassiana (Basl. Criv.) Vuill. (Hypocreales: Cordycipitaceae) is a natural enemy of CBB in coffee-producing regions worldwide [3][5][6][20][21][22]. However, its efficacy as an inundative biological control agent is highly variable, with mortality ranging from 10–75% [1][3][23][24][25][26]. Weather conditions, virulence, pathogenicity, concentration, specificity of the B. bassiana strain, and formulation are some factors that determine the effectiveness of B. bassiana [27][28][29].
In 2011, the Hawaii Department of Agriculture (HDOA) authorized the distribution and application of commercial formulations of B. bassiana for the control of CBB. BotaniGard® ES and Mycotrol® ESO are two commercial formulations of the B. bassiana GHA strain (short for Grass Hopper Active, as it was originally formulated to control grasshoppers and locusts) that are widely used in Hawaii. The authorization of commercial formulations of B. bassiana marked the first step towards the establishment of an environmentally responsible IPM. Initially, coffee farmers conducted monthly calendar sprays of B. bassiana each season, which was costly in terms of products and labor and did not necessarily provide the level of control expected [15].
Later, the timing of applications was optimized based on trapping studies that elucidated peak CBB flight times [30][31], and susceptibility based on high percentages of CBB in the AB position [15][30][32]. Applications of B. bassiana became more effective and less costly since fewer sprays (4–5) were needed to obtain the same quality of coffee in comparison with calendar spray strategies (7–11 sprays per season) [29]. Cumulative mortality by the GHA strain on Hawaii coffee farms was estimated to be 20–60%, with sprays being most effective when conducted early in the season and under favorable weather conditions (overcast and humid, but not raining); half the recommended rate (16 Fl oz per acre) of BotaniGard® ES was also reported to be as effective as full rates (32 Fl oz per acre) [33].
Several naturally occurring or “wild” strains of B. bassiana have been observed to infect CBB on Hawaii Island [32][34][35][36]. CBB infection and mortality induced by wild and commercial strains of B. bassiana were observed to increase with increasing elevation, emphasizing how microclimate influences the efficacy of this control [32][35]. Despite the initial optimism that wild strains of B. bassiana might offer the potential for improved CBB control in Hawaii, field [32] and laboratory studies [37] showed that the commercially formulated GHA strain was more virulent than wild strains, despite wild strains being more persistent in CBB populations [33].
In contrast, a recent study in Puerto Rico supported the potential benefits of wild B. bassiana strains. As in Hawaii, B. bassiana is often seen sporulating on CBB that have begun boring into coffee fruits in Puerto Rico and is often observed on farms where commercial strains have never been applied. Based on microsatellite DNA typing of numerous strains, the only commercially available strain in Puerto Rico (Mycotrol®) is genetically distinct from wild strains [38]. In lab experiments, the Mycotrol® strain was very effective at killing CBB, and some wild strains were equally effective (though many were less virulent). When two wild strains (chosen for high virulence) were applied in the field, they were more effective than the Mycotrol® strain in reducing the proportion of fruits with CBB damage and the number of CBB per fruit. Furthermore, the wild strains were recovered at a much higher rate than the Mycotrol® strains, implying that they were more successful at surviving and reproducing under local field conditions after application [38]. This is not surprising considering that the Mycotrol® strain was originally isolated in Oregon, USA, a very different climate than that of the coffee farms in Puerto Rico. However, US EPA regulations prohibit the use of strains and formulations not specified in product registrations. In other words, the registration of Mycotrol® and Botanigard® does not apply to other strains of the same species; the use of other strains would mean that the crop they are applied to cannot be sold legally in the US. The registration process would have to be repeated for each strain, a very expensive and lengthy process.
3.2. Flat-Bark Beetles
In Hawaii, two flat-bark beetles that persist as generalist predators and are commonly found in macadamia nut crops, Cathartus quadricollis (Coleoptera: Silvanidae) and Leptophloeus spp. (Coleoptera: Laemophloeidae), were found to feed on immature stages of CBB [39][40]. The flat bark beetle Cathartus quadricollis has also been found inside infested coffee fruits in Puerto Rico [41] and may play a small role in controlling the CBB population, especially in infested raisin berries left on trees [40][42][43]. To promote the increase in flat bark beetle predation on CBB, USDA-ARS researchers created predator breeding stations to distribute to coffee growers in Hawaii and augment existing populations. Studies to assess the contribution of these beetles to CBB mortality and the reduction in berry damage are ongoing (P. Follett, pers. comm.).
3.3. Ants
Several species of ants are predators of CBB larvae and pupae and can remove them from coffee berries [43][44][45]. In many cases, the ants nest inside the berries [45], while other species can predate or remove adult females before they begin boring into the endosperm [46]. Most of these reports are from observations in the field; relatively few experimental studies have tested the capacity of ants as predators of CBB. In Puerto Rico, the six most common species of ants on coffee farms are Wasmannia auropunctata (Roger), Tapinoma melanocephalum (Fabricius), Monomorium floricola (Jerdon), Brachymyrmex heeri (Forel), Solenopsis invicta (Buren), and Paratrechina longicornis (Latreille) [46][47]. The first four species are small ants, which could penetrate CBB entry holes in coffee berries and remove immature and adult CBBs. The other two species, S. invicta and P. longicornis, are larger species that could predate adult females before they reach and penetrate the berries. Of these six species, only S. invicta, W. auropunctata, and Tapinoma sp. significantly reduced the damage caused by CBB [46][48]. Wasmannia auropunctata (LFA, little fire ant) also significantly reduced CBB survival; this species has a great capacity to predate adult CBB inside coffee berries [46][49]. Its small size, high activity, and abundance in coffee plantations in Puerto Rico and Hawaii make LFA a candidate for biological control of the CBB. However, it is considered an agricultural pest due to its interference with harvesting and other field work; farm workers avoid areas with high abundance of this species due to its painful sting. Solenopsis invicta (RIFA, red imported fire ant) is larger than LFA but also potentially useful for the biological control of CBB in Puerto Rico. RIFA was observed to reduce CBB damage by predating adults before they reached the coffee berries [46]. Like LFA, its painful sting makes it extremely unpopular among orchard workers. Additional studies are needed in both Puerto Rico and Hawaii to fully characterize ant diversity on coffee farms and determine the level of CBB predation and removal from berries.
3.4. Parasitoids
Three species of parasitoid wasps have been identified as natural enemies of CBB in Africa: Cephalonomia stephanoderis, Prorops nasuta (Hymenoptera: Bethylidae), and Phymastichus coffea (Hymenoptera: Eulophidae) [50]. Of these, C. stephanoderis and P. nasuta can parasitize immature stages of CBB (larvae and pupae) and predate mainly small larvae and eggs [51][52][53], while P. coffea parasitizes adult CBB females [54][55] (Figure 5). Both P. nasuta and C. stephanoderis are also able to complete their life cycle inside the CBB-infested coffee berries [53][56]. Once inside the berry, female wasps paralyze or kill the CBB female and use its body as a barrier to limit the entry of other organisms [53]. Host feeding and oviposition take place after CBB females or bigger larvae and pupae are paralyzed [51][52][53][57]. Usually, the parasitoid attacks the CBB female through the dorsal part of the abdomen, laying two eggs [55].

Figure 5. Coffee berry borer (A) and the parasitoid wasp Phymastichus coffea (B), a natural enemy of CBB that is slated to be released as a biocontrol in Hawaii. Photos: (A) M. A. Johnson, (B) David Honsberger.
Parasitism by C. stephanoderis, P. nasuta, and P. coffea tends to vary greatly from study to study, as summarized in Table 1. This variation is a disincentive to attempts to implement classical biocontrol programs. Competition and displacement between species of parasitoids have been observed. For example, competition for the host has been reported between C. stephanoderis and P. nasuta. Cephalonomia stephanoderis is more often successful, sometimes paralyzing and/or killing females of P. nasuta [58][59]. However, parasitoids can be used in tandem, with the release of P. coffea early in the season when coffee berries are developing, and most CBB females are in the AB position. This can be followed by the release of C. stephanoderis, which can parasitize and predate immature stages of the CBB [60].
Table 1. Summary of studies using the parasitoid wasps Cephalonomia stephanoderis, Prorops nasuta, and Phymastichus coffea for biological control of the coffee berry borer (CBB).
| Parasitism Type | Parasitoid | Country | CBB Parasitized (%) | Result | References |
|---|---|---|---|---|---|
| Natural | C. stephanoderis | Brazil | 0.5–83 | Not reported | [61] |
| Natural | C. stephanoderis | Brazil | 2–24 | Not reported | [62] |
| Previous Release |
C. stephanoderis | Mexico | 0.3–26 | Not reported | [63][64] |
| Natural | P. nasuta | Brazil | 2–33 | Not reported | [65] |
| Previous Release |
P. nasuta | Colombia | 0.25–50 | Not reported | [66][67] |
| Release (1–3) | C. stephanoderis | Colombia | 2–8 25–65 |
CBB mortality 95% CBB adult predation 94% |
[68] *, [69][70] |
| Release (1) | C. stephanoderis | Guatemala | 5–91 | Reduction in infestation (1–15%). Reduction in CBB population (2–43.1%) |
[71] * |
| Release (1) | C. stephanoderis | Ecuador | 3–52 | Not reported | [72] |
| Release (1) | C. stephanoderis | Colombia | Not reported | Reduction in CBB population (43–73%) | [57] * |
| Release | C. stephanoderis | Colombia | Not reported | Reduction in infestation (11%) | [73] * |
| Release (3) | P. nasuta | Colombia | 1.5–44 | Reduction in infestation (46% on average) | [70] *, [74] * |
| Release (1) | P. nasuta | Ecuador | 0.3–22 | Not reported | [72] |
| Release | P. coffea | Colombia | 2–95 | Reduction in infestation (47%) | [73] *, [74][75][76][77][78] |
| Release | P. coffea | Mexico | 10–97 | Reduction in CBB infestation (2–81%) Reduction in CBB population (90–96%) |
[55] *, [79] * |
| Release | P. coffea | Colombia | Not reported | Reduction in CBB infestation (47%) | [73] * |
| Release | P. coffea | Brazil | Not reported | Reduction in CBB infestation (18%) | [73] * |
* In these studies, the effect of parasitoid releases on number of CBB and/or CBB infestation was reported. Numbers in parentheses indicate number of releases of parasitoids in field.
In Puerto Rico, C. stephanoderis was imported in 2011 from CENICAFE in Colombia to quarantine facilities at the University of Puerto Rico in Mayagüez. However, delays in shipment meant that viable colonies could not be established. Cephalonomia stephanoderis has been observed to occur naturally on coffee farms in Puerto Rico; it was first reported in 2009, only two years after the first report of CBB on the island [41][80][81]. A viable colony established from individuals collected from coffee fruits in the field was established at the Agricultural Experimental Station in Adjuntas. In 2014, natural parasitism of CBB with C. stephanoderis was observed to reach 8%, and this increased to 20% following the release of lab-raised parasitoids [82]. Reductions in CBB-inflicted losses attributable to parasitoid impacts on beetle populations were estimated at 12–20% (USD 2.6–4.4M) at the farm level [82]. The parasitoid P. coffea was also imported to Puerto Rico from CENICAFE in 2010 and 2011. However, this species could not be released in the field because of requirements for host specificity tests and federal restrictions (F. Gallardo, pers. comm.). There are no reports of natural occurrences of P. coffea or P. nasuta in Puerto Rico. More detailed and extensive studies on the presence of these parasitoids in Puerto Rico are needed.
Phymastichus coffea is being considered for introduction into Hawaii as recent work has shown the high host specificity and capacity of these wasps to kill CBB females before they penetrate and damage the coffee endosperm [83]. Host specificity tests included 43 different species of Coleoptera, including non-target native Hawaiian species, exotic species, and beneficial species [83]. Results showed that only H. hampei and four other Hypothenemus species (H. obscurus, H. seriatus, H. birmanus, and H. crudiae) were parasitized [83]. Among the Hypothenemus species tested, those most distantly related to H. hampei were least parasitized, or not parasitized at all (H. eruditis) [83]. These results are promising, as high host specificity is among the most relevant aspects to consider for the introduction of biological control agents into Hawaii to ensure minimal risk for non-target native species. No native species of Hypothenemus occur in Hawaii; those that do occur are all invasive, and some are significant pests of other important crops such as Macadamia nut (H. obscurus) [83].
Yousuf et al. [83] suggest that the introduction of P. coffea as a biological control agent is highly likely to be environmentally safe. Now that permits have been obtained (May 2023) for importation and release, efforts will be made to establish P. coffea in Hawaii. Should establishment fail, P. coffea may be mass reared for inundative releases and incorporated into the current IPM program for CBB in Hawaii. Studies on rearing techniques, establishment, dispersal, impact on CBB populations, and compatibility with other CBB control strategies need to be addressed to facilitate the successful incorporation of this parasitoid into CBB management plans.
3.5. Entomopathogenic Nematodes
The potential use of entomopathogenic nematodes (EPNs), Heterorhabditis sp. and Steinernema sp., to control CBB has been reported in other coffee-producing countries [84][85][86][87]. In Hawaii, preliminary results showed the potential of Steinernema carpocapsae (Weiser) on infested green and raisin berries on the ground, in which CBB larvae mortality was 17.1% and 4.7% for adults [88]. In addition, two endemic EPNs from Hawaii (S. feltiae strain MG-14 and Heterorhabditis indica strain OM-160) tested on infested berries on the ground showed low mortality of CBB but high abandonment of CBB from infested berries [30]. Results suggest that there is potential for the use of EPNs against CBB, but additional field studies are needed to fully understand the role, effectiveness, and incorporation of those EPNs into an IPM for CBB.
3.6. Wolbachia Bacteria
One intriguing aspect of the CBB is its skewed sex ratio of approximately 10:1 females to males. A less skewed sex ratio would be advantageous because fewer female CBB would be available to attack coffee fruits. In many insects, skewed sex ratios are caused by infections of the endosymbiotic bacterium Wolbachia. The detection of Wolbachia in CBB Vega et al. [89] led Mariño et al. [90] to investigate its potential role in sex determination and reproduction in Puerto Rico. CBB colonies were fed artificial diets with the antibiotic tetracycline added to reduce populations of Wolbachia. After ten generations, Wolbachia was substantially reduced but not eliminated. The sex ratio was significantly less skewed than in controls, but not to the extent predicted. Thus, other factors appear to control the sex ratio in CBB. However, females on diets with tetracycline produced significantly fewer progeny, suggesting that reduction of Wolbachia (or other groups of bacteria; see Mariño et al. [91]) might affect CBB reproduction [90]. More detailed studies are needed to manipulate the Wolbachia infection of CBB for biological control. It is important to determine what mechanism causes the skewed sex ratio. Vega et al. [89] suggested that Wolbachia could induce cytoplasmic incompatibility in CBB, in which case the incompatible insect technique (IIT) [92] could be used. This technique involves mating populations of Wolbachia-free CBB females with infected males, and the resulting incompatible crosses would cause a decrease in CBB populations.
4. Chemical Control
The use of insecticides is intended to target CBB females when they are first colonizing and infesting new berries (AB position), before damage to the endosperm occurs. However, their effectiveness depends on timing sprays with CBB emergence, making good contact with the berries, applying them during favorable weather conditions, and proper calibration of sprayers. In many coffee-producing countries, synthetic insecticides containing highly toxic active ingredients (e.g., endosulfan, DDT, lindane, fenitrothion, fenthion, phenthoate, chlorpyrifos, and pirimiphos methyl-methyl) are the tools of choice used by farmers to control CBB [3][93][94]. In many cases, the use of insecticides is the first control strategy used by farmers since they are looking for a fast and effective solution. However, relying only on insecticides for control of CBB is not the best strategy since most of the CBB population is protected inside the berries and insecticides cannot reach them. In addition, their negative impact on human and environmental health, as well as the potential for CBB to develop resistance, has led to these chemicals being banned or phased out in several regions [93][95]. In Hawaii, relatively few products are authorized to be used in coffee to control CBB. A pyrethrin-based contact insecticide (Pyronil) has shown effective control of CBB [96], along with protectants such as Kaolin clay (Surround WP; Steiman and Burbano, unpub. data) and repellents (Verbenone; Wright et al., unpub. data). These products are applied alone or in a tank mixture with B. bassiana [12][29][96]. A recent field study testing the efficacy of spinetoram, whose active ingredient is derived from the fermentation of Saccharopolyspora spinosa, a naturally occurring soil organism, reported up to 73% control when CBB were in the AB position [97].
On Hawaii Island, several applications (4–5) of Pyronil or B. bassiana alone or in combination during the early coffee season (May, June, and July) were found to be as effective as monthly calendar sprays in controlling CBB but less costly [29]. However, sprays alone are often ineffective and must be combined with cultural control practices to achieve year-round control of this pest [29][33]. A viable economic strategy for controlling CBB in Hawaii includes a combination of monitoring, a few sprays of insecticides early in the season, frequent harvesting, and post-harvest sanitation [14]. Reducing the number of chemical sprays, and the use of less toxic insecticides, should be considered by farmers to preserve beneficial insects such as pollinators, predators, and parasitoids.
References
- Baker, P. The Coffee Berry Borer in Colombia. DIFI-Cenicafe-CABI-Bioscience IMP for Coffee Project (CNTR93/1536A); Cenicafé: Chinchiná, Colombia, 1999; p. 154.
- Ruiz-Cárdenas, R.; Baker, P. Life table of Hypothenemus hampei (Ferrari) in relation to coffee berry phenology under Colombian field conditions. Sci. Agric. 2010, 67, 658–668.
- Bustillo, A.; Cárdenas, R.; Villalba, D.; Benavides, P.; Orozco, J.; Posada, F. Manejo Integrado de la Broca del café Hypothenemus Hampei (Ferrari) en Colombia; Cenicafé: Chinchiná, Colombia, 1998; p. 134. (In Spanish)
- Aristizábal, L.F.; Lara, O.; Arthurs, S.P. Implementing an integrated pest management program for coffee berry borer in a specialty coffee plantation in Colombia. J. Integr. Pest Manag. 2012, 3, G1–G5.
- Damon, A. A review of the biology and control of the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae). Bull. Entomol. Res. 2000, 90, 453–465.
- Jaramillo, J.; Borgemeister, C.; Baker, P. Coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae): Searching for sustainable control strategies. Bull. Entomol. Res. 2006, 96, 223–233.
- Saldarriaga, G. Evaluación de Prácticas Culturales en el Control de la Broca del Café Hypothenemus hampei (Ferrari 1867) (Coleoptera: Scolytidae). Bachelor’s Thesis, Universidad Nacional de Colombia, Facultad de Ciencias Agropecuarias, Medellín, Colombia, 1994. (In Spanish).
- Aristizábal, L.F. Challenges faced by coffee growers establishing an integrated pest management for coffee berry borer in Hawaii. J. Agric. Sci. Technol. 2018, 14, 555919.
- Aristizábal, L.F.; Jiménez, M.; Bustillo, A.E.; Arthurs, S.P. Monitoring cultural practices for coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae) management in a small coffee farm in Colombia. Fla. Entomol. 2011, 94, 685–687.
- Duque, H.; Baker, P.S. Devouring Profit the Socio-Economics of Coffee Berry Borer IPM; 9589721842; The Commodities Press, CABI-Biosciense, Cenicafé: Chinchiná, Colombia, 2003.
- Johnson, M.A.; Fortna, S.; Hollingsworth, R.G.; Manoukis, N.C. Postharvest population reservoirs of coffee berry borer (Coleoptera: Curculionidae) on Hawai’i Island. J. Econ. Entomol. 2019, 112, 2833–2841.
- Kawabata, A.; Nakamoto, S.T.; Miyahira, M.; Curtiss, R.T. Recommendations for Coffee Berry Borer Integrated Pest Management in Hawai’i, Insects Pests-47; College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa: Honolulu, HI, USA, 2020.
- Aristizábal, L.F. Controlling the Coffee Berry Borer through Integrated Pest Management: A Practical Manual for Coffee Growers & Field Workers in Hawaii; Steuben Press: Kailua-Kona, Hawaii, 2018.
- Aristizábal, L.F.; Johnson, M.A.; Shriner, S.; Wall, M. Frequent and efficient harvesting as an economically viable strategy to regulate coffee berry borer on commercial farms in Hawaii. J. Econ. Entomol. 2023, 116, 513–519.
- Aristizábal, L.F.; Johnson, M.; Shriner, S.; Hollingsworth, R.; Manoukis, N.C.; Myers, R.; Bayman, P.; Arthurs, S.P. Integrated pest management of coffee berry borer in Hawaii and Puerto Rico: Current status and prospects. Insects 2017, 8, 123.
- Aristizábal, L.F. Insect pests affecting coffee: Understanding agroecosystems and alternative methods of control. In Climate-Smart Production of Coffee: Improving Social and Environmental Sustainability; Muschler, R., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2022; pp. 205–255.
- Aristizábal, L.F.; Bustillo, A.E.; Arthurs, S.P. Integrated pest management of coffee berry borer: Strategies from Latin America that could be useful for coffee farmers in Hawaii. Insects 2016, 7, 6.
- Smith, V.E.; Bittenbender, H. Growing Coffee in Hawaii; College of Tropical Agriculture and Human Resources, University of Hawai’i: Honolulu, HI, USA, 2008; pp. 1–40.
- Aristizábal, L.F.; Salazar, E.H.M.; Mejía, M.C.G. Evaluacion de dos componentes del manejo de la broca en la renovacion de cafetales mediante investigacion participativa. Av. Técnicos Cenicafé 2002, 295, 8.
- Vega, F.E.; Infante, F.; Castillo, A.; Jaramillo, J. The coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae): A short review, with recent findings and future research directions. Terr. Arthropod Rev. 2009, 2, 129–147.
- Haraprasad, N.; Niranjana, S.R.; Prakash, H.S.; Shetty, H.S.; Wahab, S. Beauveria bassiana a potential mycopesticide for the efficient control of coffee berry borer, Hypothenemus hampei (Ferrari) in India. Biocontrol Sci. Technol. 2001, 11, 251–260.
- Infante, F. Pest management strategies against the coffee berry borer (Coleoptera: Curculionidae: Scolytinae). J. Agric. Food. Chem. 2018, 66, 5275–5280.
- Benavides, P.; Bustillo, A.; Góngora, C. IPM program to control coffee berry borer Hypothenemus hampei, with emphasis on highly pathogenic mixed strains of Beauveria bassiana, to overcome insecticide resistance in Colombia. In Insecticides Advances in Integrated Pest Management; Perveen, F., Ed.; INTECH Open Access Publisher: Rijeka, Croatia, 2012; pp. 512–540.
- Posada, F.; Salazar, E.; Aristizábal, L.F.; Mejía, C.G.; Jiménez, M. Taller con caficultores en el control de Hypothenemus hampei (Ferrari) (Coleoptera: Scolytidae). Rev. Colomb. Entomol. 2003, 29, 63–70. (In Spanish)
- Cruz, L.P.; Gaitan, A.L.; Gongora, C.E. Exploiting the genetic diversity of Beauveria bassiana for improving the biological control of the coffee berry borer through the use of strain mixtures. Appl. Microbiol. Biotechnol. 2006, 71, 918–926.
- Vera, J.T.; Montoya, E.; Benavides, P.; Góngora, C. Evaluation of Beauveria bassiana (Ascomycota: Hypocreales) as a control of the coffee berry borer Hypothenemus hampei(Coleoptera: Curculionidae: Scolytinae) emerging from fallen infested coffee berries on the ground. Biocontrol Sci. Technol. 2011, 21, 1–14.
- Vega, F.E.; Infante, F.; Johnson, A.J. The genus Hypothenemus, with emphasis on H. hampei, the coffee berry borer. In Bark Beetles: Biology and Ecology of Native and Invasive Species, 1st ed.; Vega, F., Hofstetter, R., Eds.; Elsevier: London, UK, 2015; pp. 427–494.
- Mascarin, G.M.; Jaronski, S.T. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 2016, 32, 1–26.
- Hollingsworth, R.G.; Aristizábal, L.F.; Shriner, S.; Mascarin, G.M.; Moral, R.d.A.; Arthurs, S.P. Incorporating Beauveria bassiana into an integrated pest management plan for coffee berry borer in Hawaii. Front. Sustain. Food Syst. 2020, 4, 22.
- Aristizábal, L.F.; Shriner, S.; Hollingsworth, R.; Arthurs, S. Flight activity and field infestation relationships for coffee berry borer in commercial coffee plantations in Kona and Kau districts, Hawaii. J. Econ. Entomol. 2017, 110, 2421–2427.
- Johnson, M.A.; Manoukis, N.C. Influence of seasonal and climatic variables on coffee berry borer (Hypothenemus hampei Ferrari) flight activity in Hawaii. PLoS ONE 2021, 16, e0257861.
- Greco, E.B.; Wright, M.G.; Burgueño, J.; Jaronski, S.T. Efficacy of Beauveria bassiana applications on coffee berry borer across an elevation gradient in Hawaii. Biocontrol Sci. Technol. 2018, 28, 995–1013.
- Wraight, S.; Galaini-Wraight, S.; Howes, R.; Castrillo, L.; Griggs, M.; Carruthers, R.; Smith, R.; Matsumoto, T.; Keith, L. Efficacy of Beauveria bassiana strain GHA spray applications against coffee berry borer Hypothenemus hampei on Hawai’i Island. Biol. Control 2021, 161, 104587.
- Hollingsworth, R.G.; Lysy, A.M.; Matsumoto, T.K. Preliminary study of genetic variation in Hawaiian isolates of Beauveria bassiana (Hypocreales, Cordycipitaceae). J. Invertebr. Pathol. 2011, 106, 422–425.
- Wraight, S.P.; Galaini-Wraight, S.; Howes, R.L.; Castrillo, L.A.; Carruthers, R.I.; Smith, R.H.; Matsumoto, T.K.; Keith, L.M. Prevalence of naturally-occurring strains of Beauveria bassiana in populations of coffee berry borer Hypothenemus hampei on Hawaii Island, with observations on coffee plant-H. hampei-B. bassiana interactions. J. Invertebr. Pathol. 2018, 156, 54–72.
- Castrillo, L.A.; Wraight, S.P.; Galaini-Wraight, S.; Matsumoto, T.K.; Howes, R.L.; Keith, L.M. Genetic diversity among naturally-occurring strains of Beauveria bassiana associated with the introduced coffee berry borer, Hypothenemus hampei,(Coleoptera: Curculionidae) on Hawai ‘i Island. J. Invertebr. Pathol. 2020, 175, 107456.
- Wraight, S.P.; Howes, R.L.; Castrillo, L.A.; Griggs, S.; Galaini-Wraight, S.; Carruthers, R.I.; Matsumoto, T.K.; Keith, L.M. Laboratory studies assessing the microbial biocontrol potential of diverse strains of Beauveria bassiana isolated from coffee berry borer, with emphasis on strains from Hawaii Island and comparison to commercial strain GHA. J. Invertebr. Pathol. 2022, 194, 107819.
- Bayman, P.; Mariño, Y.A.; García-Rodríguez, N.M.; Oduardo-Sierra, O.F.; Rehner, S.A. Local isolates of Beauveria bassiana for control of the coffee berry borer Hypothenemus hampei in Puerto Rico: Virulence, efficacy and persistence. Biol. Control 2021, 155, 104533.
- Sim, S.; Yoneshi, N.M.; Brill, E.; Gleib, S.M.; Follet, P.A. Molecular markers detect cryptic predation on coffee berry borer (Coleoptera: Curculionidae) by silvanid and laemophloeid flat bark beetles (Coleoptera Silvanidae, Laemophloeidae) in coffee beans. J. Econ. Entomol. 2016, 109, 100–115.
- Follett, P.A.; Kawabata, A.; Nelson, R.; Asmus, G.; Burt, J.; Goschke, K.; Ewing, C.; Gaertner, J.; Brill, E.; Geib, S. Predation by flat bark beetles (Coleoptera: Silvanidae and Laemophloeidae) on coffee berry borer (Coleoptera: Curculionidae) in Hawaii coffee. Biol. Control 2016, 101, 152–158.
- González, O.P. Dinámica Poblacional de la Broca del Café Hypothenemus hampei (Ferrari) y sus Enemigos Naturales en el Cultivo de Café Coffea arabica L. en Puerto Rico. Master’s Thesis, Universidad de Puerto Rico, Facultad de Ciencias Agricolas, Mayagüez, Puerto Rico, 2013. (In Spanish).
- Kawabata, A.; Follet, P.A.; Wright, M.G.; Brill, E.; Curtiss, R.T. An Introduction to the Square-Necked Grain Beetle as a Predator of Coffee Berry Borer in Hawaii; Insect Pests-40; College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa: Honolulu, HI, USA, 2016.
- Bustillo, A.E.; Cardenas, R.; Posada, F.J. Inimigos Naturais e Competidores da Broca-do-Café Hypothenemus hampei (Ferrari) (Coleoptera: Scolytidae) na Colômbia. Neotrop. Entomol. 2002, 31, 635–639. (In Portuguese)
- Varón, E.H.; Hanson, P.; Borbón, O.; Carballo, M.; Hilje Quirós, L. Potencial de hormigas como depredadoras de la broca del café (Hypothenemus hampei) en Costa Rica. Manejo Integr. Plagas Agroecol. 2004, 73, 42–50. (In Spanish)
- Vázquez, L.; Jiménez, E.B.; Claro, O.E.; Brito, Y.M.; Simonetti, J.A. Observaciones sobre enemigos naturales de la broca del café (Hypothenemus hampei Ferrari) en Cuba. Fitosanidad 2006, 10, 307–308. (In Spanish)
- Newson, J.; Vandermeer, J.; Perfecto, I. Differential effects of ants as biological control of the coffee berry borer in Puerto Rico. Biol. Control 2021, 160, 104666.
- Perfecto, I.; Vandermeer, J. Antagonism between Anolis spp. and Wasmannia auropunctata in coffee farms on Puerto Rico: Potential complications of biological control of the coffee berry borer. Caribb. J. Sci. 2020, 50, 43–47.
- Gonthier, D.J.; Ennis, K.K.; Philpott, S.M.; Vandermeer, J.; Perfecto, I. Ants defend coffee from berry borer colonization. BioControl 2013, 58, 815–820.
- Armbrecht, I.; Gallego, M.C. Testing ant predation on the coffee berry borer in shaded and sun coffee plantations in Colombia. Entomol. Exp. Appl. 2007, 124, 261–267.
- Orozco, J.; Aristizábal, L. Parasitoides de Origen Africano para el Control de la Broca del Café; 0120-0178; Cenicafé: Chinchiná, Colombia, 1996; p. 8. (In Spanish)
- Lauzière, I.; Brodeur, J.; Pérez-Lachaud, G. Host stage selection and suitability in Cephalonomia stephanoderis Betrem (Hymenoptera: Bethylidae), a parasitoid of the coffee berry borer. Biol. Control 2001, 21, 128–133.
- Benavides, P.; Bustillo, A.; Portilla, M.; Orozco, J. Classical biological control of coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae) in Colombia with African parasitoids. In Proceedings of the 1st International Symposium on Biological Control of Arthropods, Honolulu, HI, USA, 14–18 January 2002; pp. 430–434.
- Infante, F.; Mumford, J.; Baker, P. Life history studies of Prorops nasuta, a parasitoid of the coffee berry borer. BioControl 2005, 50, 259–270.
- Feldhege, M. Rearing techniques and aspects of biology of Phymastichus coffea (Hymenoptera: Eulophidae), a recently described endoparasitoid of the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae). Cafe Cacao Thé 1992, 36, 45–54.
- Espinoza, J.C.; Infante, F.; Castillo, A.; Pérez, J.; Nieto, G.; Pinson, E.P.; Vega, F.E. The biology of Phymastichus coffea LaSalle (Hymenoptera: Eulophidae) under field conditions. Biol. Control 2009, 49, 227–233.
- Abraham, Y.; Moore, D.; Godwin, G. Rearing and aspects of biology of Cephalonomia stephanoderis and Prorops nasuta (Hymenoptera: Bethylidae) parasitoids of the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae). Bull. Entomol. Res. 1990, 80, 121–128.
- Aristizabal, L.F.; Bustillo, A.E.; Baker, P.S.; Orozco, J.; Chaves, B. Efecto depredador del parasitoide Cephalonomia stephanoderis (Hymenoptera: Bethylidae) sobre los estados inmaduros de Hypothenemus hampei (Coleoptera: Scolytidae) en condiciones de campo. Rev. Colomb. Entomol. 1998, 24, 35–41. (In Spanish)
- Pérez-Lachaud, G.; Hardy, I.C.; Lachaud, J.-P. Insect gladiators: Competitive interactions between three species of bethylid wasps attacking the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae). Biol. Control 2002, 25, 231–238.
- Batchelor, T.P.; Hardy, I.C.; Barrera, J.F.; Pérez-Lachaud, G. Insect gladiators II: Competitive interactions within and between bethylid parasitoid species of the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae). Biol. Control 2005, 33, 194–202.
- Jaramillo, J.; Bustillo, A.; Montoya, E.; Borgemeister, C. Biological control of the coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae) by Phymastichus coffea (Hymenoptera: Eulophidae) in Colombia. Bull. Entomol. Res. 2005, 95, 467–472.
- Benassi, V.; Busoli, A.C. Levantamento e índices de Parasitismo de Cephalonomia stephanoderis Betrem (Hymenoptera: Bethylidae), no Norte do Espirito Santo. Available online: http://www.sbicafe.ufv.br/handle/10820/1909 (accessed on 2 March 2023). (In Portuguese).
- de Souza, M.S.; Almeida, A.; Teixeira, C.A.D.; Costa, J.N.M. Parasitismo na população da broca-do-café Hypothenemus hampei (Ferrari) (Coleoptera: Scolytidae), pelo parasitoide Cephalonomia stephanoderis Betrem (Hymenoptera: Bethylidae). EntomoBrasilis 2014, 7, 178–182. (In Portuguese)
- Barrera, J.F. Investigación sobre la broca del café en México: Logros, retos y perspectivas. In Simposio sobre Situación Actual y Perspectivas de la Investigación y Manejo de la Broca del Café en Costa Rica, Cuba, Guatemala y México; Sociedad Mexicana de Entomología y El Colegio de la Frontera Sur: Tapachula, Chiapas, México, 2005; pp. 1–13. (In Spanish)
- Ruiz, J.G.; Ovilla, A.S.; Valle-Mora, J.; Gerardo, P.J.M. Determinación del establecimiento de parasitoides de la broca del café Hypothenemus hampei (Coleoptera: Curculionidae, Scolytinae) en cafetales del Soconusco, Chiapas, México. Entomotropica 2011, 25, 25–35. (In Spanish)
- Dalvi, L.P.; Pratissoli, D.; Polanczyk, R.A.; Andrade, G.S. Ocorrência de parasitóides associados à broca do café no Sul do Espírito Santo-Brasil. Idesia 2008, 26, 97–98. (In Portuguese)
- Maldonado, C.; Benavides, P. Evaluación del establecimiento de Cephalonomia stephanoderis y Prorops nasuta controladores de Hypothenemus hampei en Colombia. Cenicafé 2007, 58, 333–339. (In Spanish)
- Morales, R.; Bacca, T.; Soto, A. Establecimiento de los parasitoides de origen Africano de la broca del café en la zona cafetera del norte del Departamento de Nariño. Boletín Científico Centro Mus. Mus. Hist. Nat. 2011, 15, 81–93. (In Spanish)
- Benavides, P.; Bustillo, A.; Montoya, E. Avances sobre el uso del parasitoide Cephalonomia stephanoderis para el control de la broca del café, Hypothenemus hampei. Rev. Colomb. Entomol. 1994, 20, 247–253. (In Spanish)
- Salazar, H.M.; Baker, P.S. Impacto de liberaciones de Cephalonomia stephanoderis sobre poblaciones de Hypothenemus hampei. Cenicafé 2002, 53, 306–316. (In Spanish)
- Aristizábal, L.F.; Salazar, H.M.; Mejía, C.G.; Jiménez, M.; Bustillo, A.E.; Arthurs, S.P. Establishment of the exotic parasitioids of the coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae) in Colombia through farmer participatory research. Int. J. Trop. Insect Sci. 2012, 32, 24–31.
- Rivera, N. Estudio Sobre el Parasitismo de Cephalonomia stephanoderis Betrem en la Broca del Cafe (Hypothenemus hampei Ferrari), en Plantaciones de Café con Manejo Orgánico Finca Bella Vista, San Miguel Tucurú, Alta Verapaz. Bachelor’s Thesis, Universidad San Carlos de Guatemala, Departmento de Agonomía, Ciudad de Guatemala, Guatemala, 1998. (In Spanish).
- Mendoza, J.; Quijije, R.; Patiño, M.; Delgado, D. Resultados de Varios Estudios Efectuados con Prorops Nasuta y Cephalonomia Stephanoderis para el Control Biológico de la Broca del Café Hypothenemus Hampei, en Ecuador; El Control Biológico de la Broca del Café en Ecuador: Informe Técnico, INIAP. 1994; Available online: https://www.cabi.org/wp-content/uploads/Mendoza-1994-Biocontrol-of-coffee-berry-borer.pdf (accessed on 9 March 2023). (In Spanish)
- Rodríguez, D.; Cure, J.R.; Gutierrez, A.P.; Cotes, J.M. A coffee agroecosystem model: III. Parasitoids of the coffee berry borer (Hypothenemus hampei). Ecol. Modell 2017, 363, 96–110.
- Aristizábal, L.F.; Jiménez, M.; Bustillo, A.E.; Arthurs, S.P. Introduction of parasitoids of Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae) on small coffee plantations in Colombia through farmer participatory methods development. Fla. Entomol. 2011, 94, 690–693.
- Aristizabal, L.F.; Salazar, H.M.; Mejia, C.; Bustillo, A. Introducción y evaluación de Phymastichus coffea (Hymenoptera: Eulophidae) en fincas de pequeños caficultores, a través de investigación participativa. Rev. Colomb. Entomol. 2004, 30, 219–224. (In Spanish)
- Salazar, H.M.; Mejía, C.G.; Aristizábal, L.F.; Jiménez, M.; Cantor, F. Introducción de Phymastichus coffea parasitoide de la broca en fincas de caficultores experimentadores. Avances Técnicos Cenicafé 2013, 358, 1–8. (In Spanish)
- Vergara, J.; Orozco, J.; Bustillo, A.; Chaves, B. Dispersión de Phymastichus coffea en un lote de café infestado de Hypothenemus hampei. Cenicafé 2001, 52, 104–110. (In Spanish)
- Jaramillo, J.; Borgemeister, C.; Setamou, M. Field superparasitism by Phymastichus coffea, a parasitoid of adult coffee berry borer, Hypothenemus hampei. Entomol. Exp. Appl. 2006, 119, 231–237.
- Infante, F.; Castillo, A.; Pérez, J.; Vega, F.E. Field-cage evaluation of the parasitoid Phymastichus coffea as a natural enemy of the coffee berry borer, Hypothenemus hampei. Biol. Control 2013, 67, 446–450.
- Abreu, E.; Gallardo, F. Presencia de Cephalonomia stephanoderis (Hymenoptera: Bethylidae), parasidoide de la broca del cafe Hypothenemus hampei (Coleoptera: Curculionidae), en Puerto Rico. In Proceedings of the Reunion cientifica Anual Sociedad Puertorriquena de Ciencias Agricolas SOPCA, Ponce, Puerto Rico, 19 November 2011. (In Spanish).
- Gallardo, F. Biological control of insect pests in Puerto Rico. J. Agric. Univ. P. R. 2017, 101, 153–163.
- Gallardo, F. The Introduction and Establishment of Parasitoids for the Biological Control of the Coffee Berry Borer, Hypothenemus hampei. Available online: https://reeis.usda.gov/web/crisprojectpages/0225040-the-introduction-and-establishment-of-parasitoids-for-the-biocontrol-of-the-coffee-berry-borer-hypothenemus-hampei.html (accessed on 29 March 2023).
- Yousuf, F.; Follet, P.A.; Gillett, C.P.; Honsberger, D.; Chamorro, L.; Giraldo-Jaramillo, M.; Benavides, P.; Wright, M.G. Limited host range in the idiobiont parasitoid Phymastichus coffea, a prospective biological control agent of the coffee pest Hypothenemus hampei in Hawaii. J. Pest Sci. 2021, 94, 1183–1194.
- Allard, G.; Moore, D. Heterorhabditis sp. nematodes as control agents for coffee berry borer, Hypothenemus hampei (Scolytidae). J. Invertebr. Pathol. 1989, 54, 45–48.
- Castillo, A.; Marbán-Mendoza, N. Evaluación en laboratorio de nematodos entomopatógenos para el control biológico de la broca del café Hypothenemus hampei. Nematropica 1996, 26, 101–109. (In Spanish)
- Lara, J.C.; Lopez, J.C.; Bustillo, A.E. Efecto de entomonematodos sobre poblaciones de la broca del café, Hypothenemus hampei (Coleoptera: Scolytidae), en frutos en el suelo. Rev. Colomb. Entomol. 2004, 30, 179–185. (In Spanish)
- Molina, J.P.; Lopez, J.C. Desplazamiento y parasitismo de los entomonematodos Steinernema feltiae (Rhabditida: Steinernematidae) y Heterorhabditis bacteriophora (Rhabditida: Heterorhabditidae) hacia frutos infestados con la broca del café Hypothenemus hampei (Coleoptera: Scolytidae). Rev. Colomb. Entomol. 2002, 28, 145–151. (In Spanish)
- Manton, J.L.; Hollingsworth, R.G.; Cabos, R.Y. Potential of Steinernema carpocapsae (Rhabditida: Steinernematidae) against Hypothenemus hampei (Coleoptera: Curculionidae) in Hawai’i. Fla. Entomol. 2012, 95, 1194–1197.
- Vega, F.; Benavides, P.; Stuart, J.; O’Neill, S. Wolbachia infection in the coffee berry borer (Coleoptera: Curculionidae). Ann. Entomol. Soc. Am. 2002, 95, 374–378.
- Mariño, Y.A.; Verle Rodrigues, J.C.; Bayman, P. Wolbachia affects reproduction and population dynamics of the coffee berry borer (Hypothenemus hampei): Implications for biological control. Insects 2017, 8, 8.
- Mariño, Y.A.; Ospina, O.E.; Verle Rodrigues, J.; Bayman, P. High diversity and variability in the bacterial microbiota of the coffee berry borer (Coleoptera: Curculionidae), with emphasis on Wolbachia. J. Appl. Microbiol. 2018, 125, 528–543.
- Bourtzis, K. Wolbachia-based technologies for insect pest population control. Adv. Exp. Med. Biol. 2008, 627, 104–113.
- Brun, L.O.; Marcillaud, C.; Gaudichon, V.; Suckling, D.M. Endosulfan resistance in Hypothenemus hampei (Coleoptera: Scolytidae) in New Caledonia. J. Econ. Entomol. 1989, 82, 1311–1316.
- Villalba, D.; Bustillo, A.E.; Chaves, B. Evaluación de insecticidas para el control de la broca del café en Colombia. Cenicafé 1995, 46, 152–163. (In Spanish)
- Johnson, M.A.; Ruiz-Diaz, C.P.; Manoukis, N.C.; Verle Rodrigues, J.C. Coffee berry borer (Hypothenemus hampei), a global pest of coffee: Perspectives from historical and recent invasions, and future priorities. Insects 2020, 11, 882.
- Aristizábal, L.F.; Shriner, S.; Hollingsworth, R.; Mascarin, G.M.; Chaves, B.; Matsumoto, T.; Arthurs, S.P. Field sampling strategies for coffee berry borer (Coleoptera: Curculionidae: Scolytinae) infesting berries in coffee farms in Hawaii. Int. J. Trop. Insect Sci. 2018, 38, 418–426.
- Kawabata, A.; Myers, R.; Miyahira, M.; Yamauchi, N.; Nakamoto, S.T. Field Efficacy of Spinetoram for the Management of Coffee Berry Borer (Hypothenemus hampei). Insects 2023, 14, 287.
More
