Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Julius Gatmaitan.
Atopic dermatitis represents a complex and multidimensional interaction that represents potential fields of preventive and therapeutic management. In addition to the treatment armamentarium available for atopic dermatitis, novel drugs targeting significant molecular pathways in atopic dermatitis biologics and small molecules are also being developed given the condition’s complex pathophysiology. While most of the patients are expecting better efficacy and long-term control, the response to these drugs would still depend on numerous factors such as complex genotype, diverse environmental triggers and microbiome-derived signals, and, most importantly, dynamic immune responses.
- atopic dermatitis
- atopic eczema
- biologic therapy
- cytokine signaling
- treatment
1. Introduction
Atopic dermatitis (AD) is a common chronic inflammatory skin disorder affecting approximately 11 to 20% of children and 5–8% adults. In a retrospective observational study conducted in South Korea by Lee et al., 944,559 pediatric patients and 1,066,453 adults with AD were identified [1,2][1][2]. This condition, described as a heterogenous inflammatory skin disease, consists of various subtypes based on clinical (disease stage/chronicity), demographics (age, ethnicity), and molecular parameters/endotypes. The various differences in the AD endotype contributes to this condition’s complexity [3]. While some patients can be managed through conventional therapies, progress toward an innovative therapeutic approach based on AD subtype is essential to achieve the therapeutic benefits the patient expect. The recurrent eczematous lesions and intense pruritus severely affect the patient’s quality of life and, therefore, have a significant negative impact on their psychological and social well-being. A national, multicenter, non-interventional study in South Korea revealed that out of 1163 patients, the quality of life of 72.3% (840) patients was identified to be moderately to severely affected, indicating the high disease burden of this condition [2]. The mainstay of therapy in AD is topical agents. In patients with mild to moderate AD, the standard medical treatment is anti-inflammatory drugs such as topical corticosteroids and topical calcineurin inhibitors. If the patients cannot achieve disease control and clinical improvement despite the topical therapies, systemic treatments including phototherapy sessions are recommended. If topical agents like emollients, corticosteroids, and calcineurin inhibitors with or without phototherapy still fail to control the clinical condition of the patient, systemic immunomodulatory agents such as cyclosporine, methotrexate, azathioprine, mycophenolate mofetil, and short-term systemic corticosteroids are recommended. Avoidance of triggering factors that exacerbate pruritus such as woolen clothes, emotional stress, and uncomfortable climatic conditions is essential at all times [4]. In addition to the treatment armamentarium available for AD, novel drugs targeting significant molecular pathways in AD such as biologics and small molecules are also introduced given the condition’s complex pathophysiology [5]. Among the challenges faced in this condition are the following: trigger avoidance, treatment adherence due to adverse effects of topical steroids and systemic immunosuppressant agents, and more importantly, the economic burden on some patients due to the cost of biologics and Janus kinases (JAK/STAT )/Signal Transducer and Activator of Transcription (STAT) inhibitors. Like any other drug, these newer biologics and small molecules are not a one-size-fits-all. While most of the patients are expecting better efficacy and long-term control, the response to these drugs would still be influenced by numerous factors such as complex genotype, diverse environmental triggers and microbiome-derived signals, and, most importantly, dynamic immune responses [6].
2. Current and Future Molecular Targets in Atopic Dermatitis
2.1. Modulation of the Skin Microbiome
Numerous studies have shown the important role of the skin’s microbial populations in the promotion of T-cell-driven inflammation in AD. The increase in Staphylococcus aureus colonization, as seen in Figure 1a,b, influences a decrease in other commensal bacterial communities, which are necessary to produce antimicrobial peptides (AMPs) to prevent overgrowth of pathogenic Staphyloccus aureus. AMPs are produced by the skin to inhibit or destroy the growth of microbes. The human body consists of over 20 peptides, those of particular importance in AD of which are cathelicidin, human β-defensin-1, dermcidin, and ribonucleases. The modification in the expression and secretion of AMP may increase the susceptibility of the skin of AD patients to infections by bacteria, viruses, and fungi.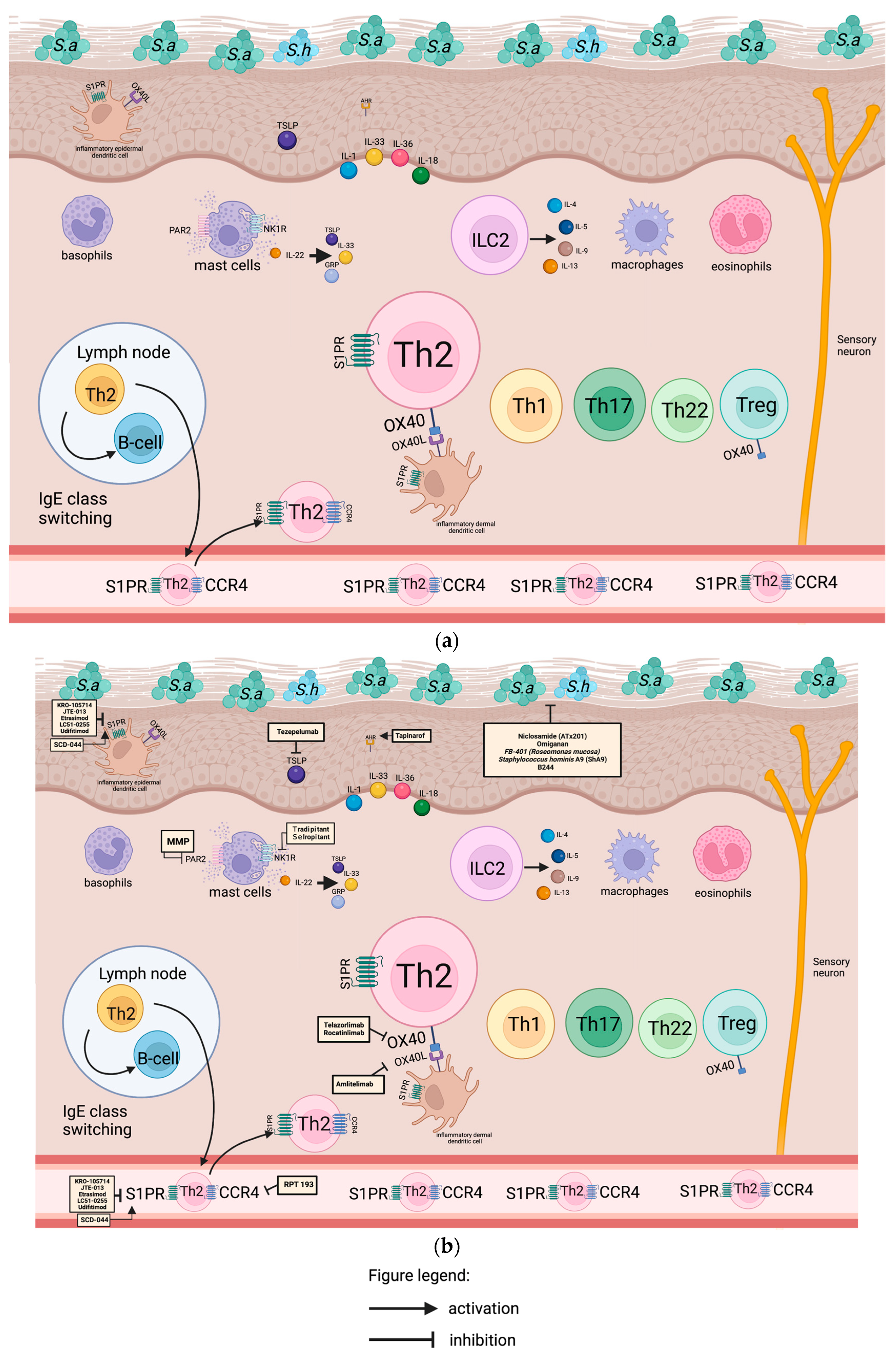
Figure 1. (a) Overview of the skin microbiome and innate immune system in atopic dermatitis. Skin-resident dendritic cells are activated by triggers of atopic dermatitis. Once triggered, dendritic cells (DC) migrate to the local lymph nodes to prime the naive T helper cells and shift these cells toward Th2 polarization. IgE class switching to B cells is also induced by Th2 cells. T-helper 2 cells are recruited back to the skin, along with Th1, Th17, and Th22 generating the “immunological march” of atopic dermatitis. Sa, staphylococcus aureus; Sh, staphylococcus hominis; inflammatory epidermal dendritic cell; S1PR, sphingosine 1-phosphate receptor; TSLP, thymic stromal lymphopoietin; AHR, aryl-hydrocarbon receptor; basophils; PAR2, protease-activated receptor 2; NK1R, neurokinin 1 receptor; GRP, gastrin-releasing peptide; ILC2, innate lymphoid cell 2; macrophages; eosinophils; lymph node, inflammatory dermal dendritic cell; Th2: T-helper 2 cell; OX40; OX40L: OX40 ligand; B-cell; Th1, T-helper 1 cell; Th17, T-helper 17 cell; Treg, regulatory T cell; mast cells; sensory neuron; CCR4: C-C motif chemokine receptor 4. This figure was created with Biorender at www.biorender.com. (b) Mechanism of action of different drugs acting on the skin microbiome and innate immune system in atopic dermatitis. KRO-105714 is a dual antagonist of sphingosylphosphorylcholine and sphingosine-1-phosphate receptor 1 in topical form. JTE-013 is an S1PR2 antagonist in topical form. Etrasimod, LC51-0255, and udifitimod are oral S1PR modulators. Tezepelumab is an anti-TSLP antibody. Tapinarof is an AHR agonist. Niclosamide, omiganan, FB 401 (Roseomonas mucosa), Staphylococcus hominis A9, and B244 are modulators of skin microbiome. VLY-686 and selropitant are NK1R antagonists. Telazorlimab (GBR830) and rocatinlimab are humanized monoclonal anti-OX40 antibodies. Amlitelimab is a monoclonal antibody that targets OX40L. RPT 193 is a CCR4 antagonist. Sa, staphylococcus aureus; Sh, staphylococcus hominis; inflammatory epidermal dendritic cell; S1PR, sphingosine 1-phosphate receptor; TSLP, thymic stromal lymphopoietin; AHR, aryl-hydrocarbon receptor; basophils; PAR2, protease-activated receptor 2; NK1R, neurokinin 1 receptor; GRP, gastrin-releasing peptide; ILC2, innate lymphoid cell 2; macrophages; eosinophils; lymph node, inflammatory dermal dendritic cell; Th2: T-helper 2 cell; OX40; OX40L: OX40 ligand; B-cell; Th1, T-helper 1 cell; Th17, T-helper 17 cell; Treg, regulatory T cell; mast cells; sensory neuron; CCR4: C-C motif chemokine receptor 4. This figure was created with Biorender at www.biorender.com.
2.2. Targeting the Innate Immune System
2.2.1. Innate lymphoid Cells (ILCs)
ILCs functionally resemble T-cells but lack the clonal antigen receptors. These cells require cytokines instead of antigens [15][7]. Group 2 innate lymphoid cells (ILC2) belong to the innate immune system leukocytes known to secrete pro-allergic cytokines such as IL-4, IL-5, IL-9, and IL-13, as depicted in Figure 1a, and are therefore implicated in initiating a Th2 response. Th2 responses with the overexpression of IL-4, IL-5, IL-9, and IL-13 are found increased in both extrinsic and intrinsic AD patients [16][8]. ILC2 can be activated by interleukin 25 (IL-25), interleukin-33 (IL-33), and thymic stromal lymphoprotein (TSLP) or eicosanoids without antigen stimulation. In a steady state, the presence of ILC2 is unique in the skin, keratoconjunctiva, lungs, and intestines. When inflammation occurs, there is discharge of the activated sentinel ILC2s from the skin to the circulatory system like the lymph nodes and peripheral blood [17][9]. Activation of a local innate response such as ILC2 occurs first, followed by the activation of adaptive immunity such as Th2 cells [18,19][10][11].
2.2.2. Alarmins
Thymic Stromal Lymphopoietin (TSLP)
Keratinocytes, fibroblasts, dendritic cells, stromal cells, and mast cells produce TSLP, as indicated in Figure 1a, to induce a Th2-type immune response, triggered by environmental stimuli like allergens and imbalances in the skin barrier dysfunction [20][12]. The TSLP serum level in the skin of AD patients was found to be elevated in the study of Lee et al. [21][13].Interleukin-1 (IL-1) Family
The interleukin-1 family functions to regulate the immune response and link the innate and adaptive responses. This family is composed of 11 cytokine members, seven agonists (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β, and IL-36γ), and four antagonists (IL-1Ra, IL-36Ra, IL-37, and IL-38) [23][14]. Based on their structural and functional characteristics, the family is further subdivided into subfamilies (IL-1, IL-18, IL-33, and IL-36), with each one having a specific receptor, respectively (IL-1R1, IL-18Rα, IL-33R, and IL-36R). MyD88 is a central signal adaptor involved in the signal transduction of the cytokines of the Interleukin-1 family. Didovic et al. were able to demonstrate its role in the generation of an adaptive and immune response in skin inflammation in an AD-like murine study [24][15].- -
-
Interleukin-1 subfamily
-
Interleukin 1 (IL-1)
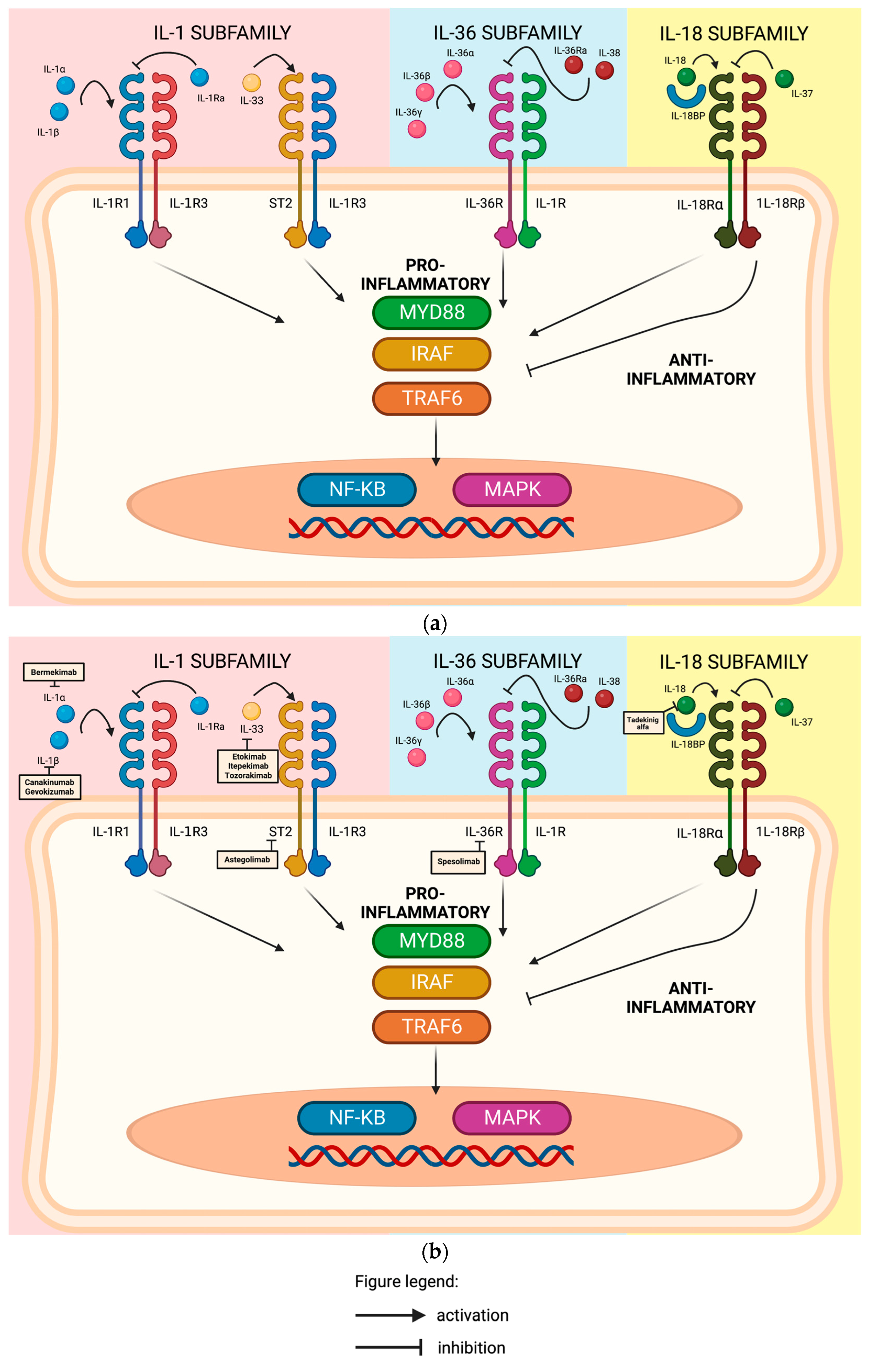 Figure 2. (a) Members of the Interleukin-1 (IL-1) family of cytokines and their receptors inside a keratinocyte. To exert its pro-inflammatory effect, Interleukin-1 alpha (IL-1α) and Interleukin-1 beta (IL-1β) bind to Interleukin-1 receptor type 1 (IL-1R1) and recruits Interleukin-1 receptor accessory protein (IL-1R3). Interleukin-1 receptor antagonist (Il-1Ra) is a natural anti-inflammatory protein that suppresses the pro-inflammatory effect of Interleukin-1. Interleukin-33 (IL-33) binds to receptor ST2 and further recruits Interleukin-1 receptor accessory protein (IL-1R3) to exert its pro-inflammatory effect. Interleukin-36 alpha (IL-36α), Interleukin-36 beta (IL-36β), and Interleukin-36 gamma (IL-36γ) bind to Interleukin-36 receptor (IL-36R) and recruit Interleukin-1 receptor accessory protein (IL-1R3) to induce inflammation, while Interleukin-36 receptor antagonist (IL-36Ra) and Interleukin-18 (IL-18) suppress this binding. IL-18 cytokine binds to interleukin-18 receptor alpha (1L18Rα) and recruits interleukine-18 receptor beta (1L18Rβ) to stimulate the production of IFN-γ, inducing a Th2 and Th1 response. Interleukin-18 binding protein (IL18-BP) regulates its pro-inflammatory activity by creating a negative feedback mechanism by sequestering the cytokine Interleukin 37 (IL-37) to bind to interleukin-18 receptor alpha (1L18Rα) and restrict the interleukin-18 receptor (1L-18R)-dependent inflammation and inhibiting its pro-inflammatory cytokine production. The binding of these cytokines to these specific ligands results in activation of NFKB or MAPK signaling pro-inflammatory gene expression through MyD88, IRAF, and/or TRAF6 signaling mechanisms. MyD88, Myeloid differentiation primary response 88; IRAF, interferon regulatory factor; TRAF6, TNF-receptor-associated factor 6; NFKB, Nuclear factor kappa-light-chain-enhancer of activated B cells; MAPK, mitogen-activated protein kinase 11. This figure was created with Biorender at www.biorender.com. (b) Mechanism of action of different drugs acting on the members of the Interleukin-1 (IL-1) family of cytokines and their receptors inside a keratinocyte. Bermekimab, a fully human monoclonal antibody that targets IL-1α. Canakinumab, a fully human monoclonal antibody against IL-1β. Gevokizumab, a neutralizing humanized monoclonal antibody specific to IL-1β. Etokimab is a humanized anti-IL-33 monoclonal antibody. Itepekimab (REGN3500) is an anti-IL33 antibody. Tozorakimab (MEDI3506) inhibits IL33 signaling via IL33R/ST2 pathway. Astegolimab, a fully human IgG2 monoclonal antibody that binds to ST2. Spesolimab, an antibody against IL36R. Tadekinig alfa is human recombinant IL-18-binding protein that neutralizes IL-18. MyD88, Myeloid differentiation primary response 88; IRAF, interferon regulatory factor; TRAF6, TNF-receptor-associated factor 6; NFKB, Nuclear factor kappa-light-chain-enhancer of activated B cells; MAPK, mitogen-activated protein kinase 11. This figure was created with Biorender at www.biorender.com.Bermekimab, a fully human monoclonal antibody that targets IL-1α (Figure 2b), demonstrated significant and encouraging results in patients with hidradenitis suppurativa and psoriasis [27,28][18][19]. A phase 2b, multicenter, randomized, placebo- and active-comparator-controlled, double-blind study that evaluated the safety and efficacy of subcutaneous bermekimab in adult (18 years and older) participants with moderate-to-severe AD was terminated due to the lack of efficacy (GENESIS) [29][20].Canakinumab, a fully human monoclonal antibody against IL-1β (Figure 2b), has shown promising clinical efficacy and safety in various inflammatory diseases like cryopyrin-associated periodic syndromes (CAPS) and possibly other complex inflammatory diseases, such as rheumatoid arthritis, systemic-onset juvenile idiopathic arthritis (SoJIA), chronic obstructive pulmonary disease, and ocular diseases [30][21].Gevokizumab, a neutralizing humanized monoclonal antibody specific to IL-1β (Figure 2b), given as 60 mg subcutaneously every 4 weeks for 12 weeks, showed its efficacy in a case study of two patients with generalized pustular psoriasis, which resulted in a decrease in generalized pustular psoriasis area and severity index (GPPASI) scores by reductions of 79% and 65% at weeks 4 and 12, respectively [32][22].
Figure 2. (a) Members of the Interleukin-1 (IL-1) family of cytokines and their receptors inside a keratinocyte. To exert its pro-inflammatory effect, Interleukin-1 alpha (IL-1α) and Interleukin-1 beta (IL-1β) bind to Interleukin-1 receptor type 1 (IL-1R1) and recruits Interleukin-1 receptor accessory protein (IL-1R3). Interleukin-1 receptor antagonist (Il-1Ra) is a natural anti-inflammatory protein that suppresses the pro-inflammatory effect of Interleukin-1. Interleukin-33 (IL-33) binds to receptor ST2 and further recruits Interleukin-1 receptor accessory protein (IL-1R3) to exert its pro-inflammatory effect. Interleukin-36 alpha (IL-36α), Interleukin-36 beta (IL-36β), and Interleukin-36 gamma (IL-36γ) bind to Interleukin-36 receptor (IL-36R) and recruit Interleukin-1 receptor accessory protein (IL-1R3) to induce inflammation, while Interleukin-36 receptor antagonist (IL-36Ra) and Interleukin-18 (IL-18) suppress this binding. IL-18 cytokine binds to interleukin-18 receptor alpha (1L18Rα) and recruits interleukine-18 receptor beta (1L18Rβ) to stimulate the production of IFN-γ, inducing a Th2 and Th1 response. Interleukin-18 binding protein (IL18-BP) regulates its pro-inflammatory activity by creating a negative feedback mechanism by sequestering the cytokine Interleukin 37 (IL-37) to bind to interleukin-18 receptor alpha (1L18Rα) and restrict the interleukin-18 receptor (1L-18R)-dependent inflammation and inhibiting its pro-inflammatory cytokine production. The binding of these cytokines to these specific ligands results in activation of NFKB or MAPK signaling pro-inflammatory gene expression through MyD88, IRAF, and/or TRAF6 signaling mechanisms. MyD88, Myeloid differentiation primary response 88; IRAF, interferon regulatory factor; TRAF6, TNF-receptor-associated factor 6; NFKB, Nuclear factor kappa-light-chain-enhancer of activated B cells; MAPK, mitogen-activated protein kinase 11. This figure was created with Biorender at www.biorender.com. (b) Mechanism of action of different drugs acting on the members of the Interleukin-1 (IL-1) family of cytokines and their receptors inside a keratinocyte. Bermekimab, a fully human monoclonal antibody that targets IL-1α. Canakinumab, a fully human monoclonal antibody against IL-1β. Gevokizumab, a neutralizing humanized monoclonal antibody specific to IL-1β. Etokimab is a humanized anti-IL-33 monoclonal antibody. Itepekimab (REGN3500) is an anti-IL33 antibody. Tozorakimab (MEDI3506) inhibits IL33 signaling via IL33R/ST2 pathway. Astegolimab, a fully human IgG2 monoclonal antibody that binds to ST2. Spesolimab, an antibody against IL36R. Tadekinig alfa is human recombinant IL-18-binding protein that neutralizes IL-18. MyD88, Myeloid differentiation primary response 88; IRAF, interferon regulatory factor; TRAF6, TNF-receptor-associated factor 6; NFKB, Nuclear factor kappa-light-chain-enhancer of activated B cells; MAPK, mitogen-activated protein kinase 11. This figure was created with Biorender at www.biorender.com.Bermekimab, a fully human monoclonal antibody that targets IL-1α (Figure 2b), demonstrated significant and encouraging results in patients with hidradenitis suppurativa and psoriasis [27,28][18][19]. A phase 2b, multicenter, randomized, placebo- and active-comparator-controlled, double-blind study that evaluated the safety and efficacy of subcutaneous bermekimab in adult (18 years and older) participants with moderate-to-severe AD was terminated due to the lack of efficacy (GENESIS) [29][20].Canakinumab, a fully human monoclonal antibody against IL-1β (Figure 2b), has shown promising clinical efficacy and safety in various inflammatory diseases like cryopyrin-associated periodic syndromes (CAPS) and possibly other complex inflammatory diseases, such as rheumatoid arthritis, systemic-onset juvenile idiopathic arthritis (SoJIA), chronic obstructive pulmonary disease, and ocular diseases [30][21].Gevokizumab, a neutralizing humanized monoclonal antibody specific to IL-1β (Figure 2b), given as 60 mg subcutaneously every 4 weeks for 12 weeks, showed its efficacy in a case study of two patients with generalized pustular psoriasis, which resulted in a decrease in generalized pustular psoriasis area and severity index (GPPASI) scores by reductions of 79% and 65% at weeks 4 and 12, respectively [32][22].-
Interleukin-36 subfamily (IL-36)
The cytokines are composed of three agonists (IL-36α, IL-36β, and IL-36γ) and two antagonists (IL-36 receptor antagonist (IL36Ra) and IL-38) [33][23]. These cytokines bind to the ligand-binding IL-1R6 receptor and recruits 1L1-1R3 (accessory protein common to other receptor complexes) as a coreceptor, signaling transduction to the nucleus and the transcription of inflammatory genes, as visualized in Figure 2a [34][24].Bissonnette et al. conducted a phase 2a proof-of-concept study involving 51 adult patients with moderate-to-severe AD to evaluate the effectiveness of spesolimab, an anti-IL36R antibody (Figure 2b).- 2.
-
Interleukin-18 subfamily (IL-18)
Interleukin 33 (IL-33)
IL-33 binds to the Interleukin-33-specific receptor (ST2) and recruits interleukin-1 receptor accessory protein (IL-1RAcP) as the coreceptor, signaling transduction to the nucleus and the transcription of inflammatory genes promoting Th2-type immunity, as depicted in Figure 2a [44][28]. Considered to be a “danger alarmin”, this cytokine detects tissue damage to local immune cells and activates the innate immune system such as ILC2, mast cells, and basophils to secrete type 2 cytokines, chemokines, and pro-inflammatory mediators, as visualized in Figure 1a [45][29]. Upon the activation of this cytokine, the dermal basophils are stimulated to produce interleukin-4, which may contribute to the inflammation process [46][30].2.2.3. Aryl-Hydrocarbon Receptor (AHR)
AHR is a ligand-dependent transcriptional factor that can be found on the cytosol in complex with aryl hydrocarbon interacting protein (AIP), prostaglandin E synthase 3, and heat-shock protein (HSP) 90 kda. After binding, the AHR translocates to the nucleus and binds to DNA to regulate target gene expression [55][31]. These receptors are expressed in immune and non-immune cells and, once activated, can exert both pro- and anti-inflammatory activities depending on various exogenous and endogenous factors. In vitro and in vivo studies have shown that AHR is a ubiquitous ligand (Figure 1a and Figure 3a) and can be found on T regulatory cells (Treg), Type 1 regulatory cells (TR1), T-helper cells 17 (Th17), T-helper cells 22 (Th22), cytotoxic effector cells (CD8+), Gamma delta (γδ) T cells, and innate lymphoid cells (ILC-1, ILC-2, ILC-3) [55][31].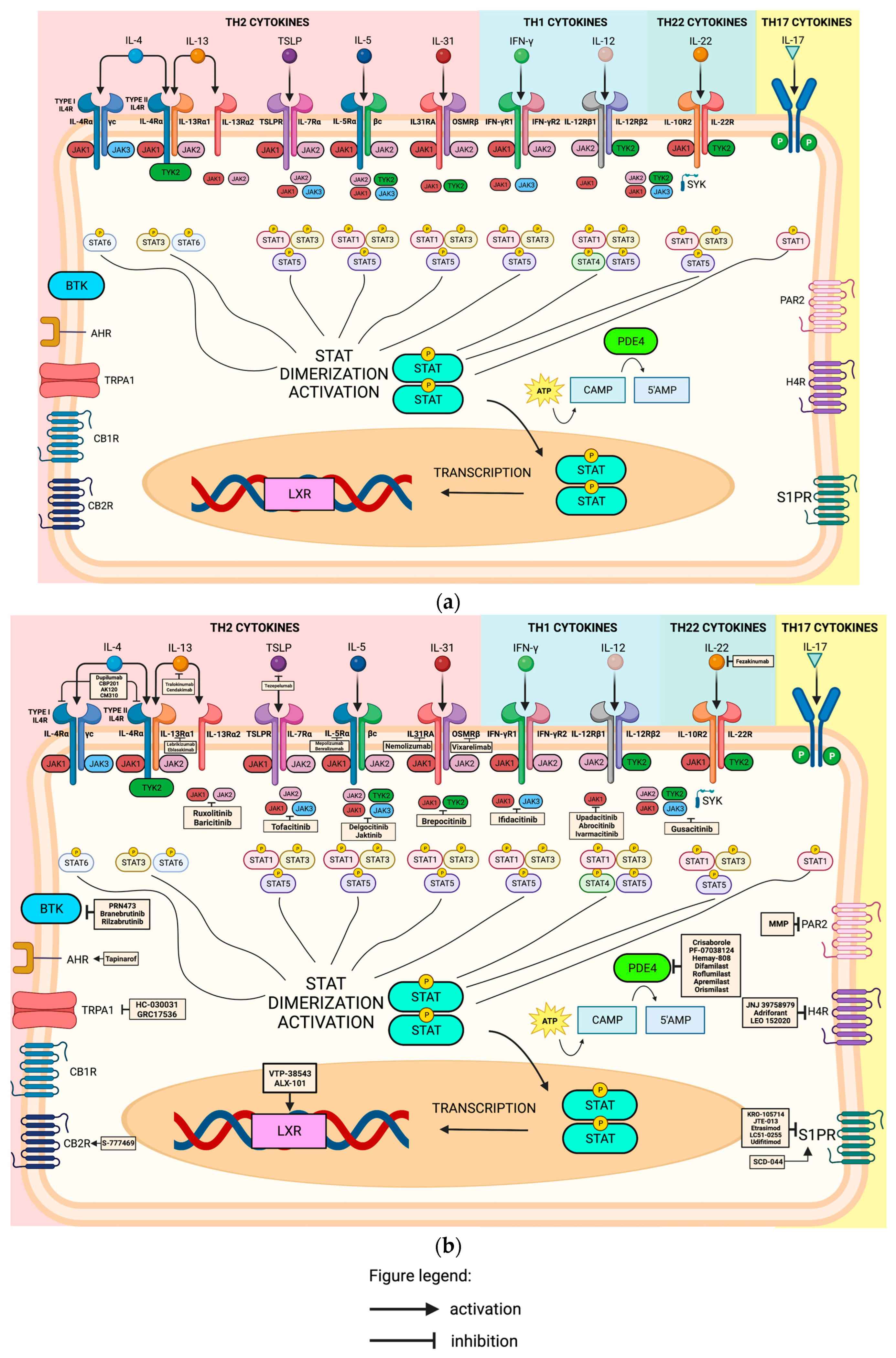 Figure 3. (a) Activation of the adaptive immune system via various receptors, different interleukins and its receptors, and the Janus kinases (JAK) STAT pathway. JAK1, Janus kinase 1; JAK2, Janus kinase 2; JAK3, Janus kinase 3; TYK2, Tyrosine kinase 2; STAT1, Signal transducer and activator of transcription 1; STAT3, Signal transducer and activator of transcription 3; STAT4, Signal transducer and activator of transcription 4; STAT5, Signal transducer and activator of transcription 5; STAT6, Signal transducer and activator of transcription 6; SYK, Spleen tyrosine kinase; BTK, Bruton’s tyrosine kinase; AHR, Aryl-hydrocarbon receptor; TRPA1, Transient receptor cation ankyrin 1; CB1R, Cannabinoid type 1 receptor; CB2R, Cannabinoid type 2 receptor; LXR, Liver X receptor; STAT, Signal transducer and activator of transcription; PDE4, Phosphodiesterase-4; PAR2, Protease-activated receptor 2; H4R, Histamine 4 receptor; S1PR, Sphingosine-1-phosphate receptor. This figure was created with Biorender at www.biorender.com. (b) Mechanism of action of different drugs acting on the adaptive immune system via various receptors, different interleukins and its receptors, and the Janus kinases (JAK) STAT pathway. Dupilumab is a fully humanized monoclonal antibody targeting IL4Rα receptor. CBP201 is a human IgG4 monoclonal antibody that binds to IL-4Rα receptor. AK120 is an antibody against IL4Rα receptor. Tralokinumab and cendakimab, a fully humanized IgG4λ, bind to IL-13 at an epitope that overlaps with the binding site of receptors IL13Rαl subunit and IL13Rα2 subunit. Tezepelumab is an anti-TSLP antibody. Lebrikizumab, a humanized monoclonal IgG4 antibody that selectively blocks IL13 and prevents heterodimerization of type II IL4R receptor composed of IL4Rα and IL13Rαl subunit. Eblasakimab is a fully humanized monoclonal antibody that targets the IL-13Rα1 subunit, therefore inhibiting the signals by IL4 and IL-13. Mepolizumab, a humanized immunoglobulin G monoclonal antibody, binds to interleukin-5, inhibiting its activity. Benralizumab directly binds to the IL-5Rα chain to inhibit the signaling pathway. Nemolizumab, a humanized monoclonal antibody that antagonizes IL-31RA receptor. Vixarelimab, a first-in-class fully human monoclonal antibody that targets oncostatin M receptor. Fezakinumab is an anti-IL-22 monoclonal antibody. Ruxolitinib, baricitinib, and ivarmacitinib inhibit JAK1 and JAK2. Tofacitinib is an inhibitor of JAK1, JAK2, and JAK3. Delgocitinib and jaktinib are pan-jak inhibitors inhibiting JAK1, JAK2, JAK3, and TYK2. Brepocitinib is a selective JAK-1 and TYK-2 inhibitor. Ifidacitinib selectively targets JAK1 and JAK3. Upadacitinib, abrocotinib, and ivarmacitinib are JAK inhibitors with a higher potency for JAK-1. Gusacitinib is a dual JAK-SYK inhibitor. PRN473 is a covalent BTK inhibitor. Branebrutinib is a highly selective BTK inhibitor that covalently binds to the cysteine residue of BTK. Tapinarof is an AHR agonist. HC-030031 and GRC17536 are both TRPA1 antagonists. S-777469 is a CB2R agonist. VTP-38543 and ALX-101 are LXR-β agonists. Crisaborole, PF-07038124, Hemay-808, difamilast, and roflumilast are PDE4 inhibitors in topical forms, while apremilast and orismilast are PDE4 inhibitors in oral form. Methylbenzyl methylbenzimidazole piperidinyl methanone (MMP) is a selective PAR-2 inhibitor in topical form. JNJ 39758979, adriforant, and LEO 152020 are H4R antagonists. KRO-105714 is a dual antagonist of sphingosylphosphorylcholine and sphingosine-1-phosphate receptor 1 in topical form. JTE-013 is an S1PR2 antagonist in topical form. Etrasimod, LC51-0255, and udifitimod are oral S1PR modulators. SCD-044 is an S1PR1 agonist. JAK1, Janus kinase 1; JAK2, Janus kinase 2; JAK3, Janus kinase 3; TYK2, Tyrosine kinase 2; STAT1, Signal transducer and activator of transcription 1; STAT3, Signal transducer and activator of transcription 3; STAT4, Signal transducer and activator of transcription 4; STAT5, Signal transducer and activator of transcription 5; STAT6, Signal transducer and activator of transcription 6; SYK, Spleen tyrosine kinase; BTK, Bruton’s tyrosine kinase; AHR, Aryl-hydrocarbon receptor; TRPA1, Transient receptor cation ankyrin 1; CB1R, Cannabinoid type 1 receptor; CB2R, Cannabinoid type 2 receptor; LXR, Liver X receptor; STAT, Signal transducer and activator of transcription; PDE4, Phosphodiesterase-4; PAR2, Protease-activated receptor 2; H4R, Histamine 4 receptor; S1PR, Sphingosine-1-phosphate receptor. This figure was created with Biorender at www.biorender.com.Tapinarof, a topical AHR agonist (Figure 1b and Figure 3b), has been approved by the FDA for psoriasis. As of this writing, an open-label long-term extension study is currently recruiting to evaluate the safety and efficacy of tapinarof cream 1% in 961 participants (>2 years old) with AD.
Figure 3. (a) Activation of the adaptive immune system via various receptors, different interleukins and its receptors, and the Janus kinases (JAK) STAT pathway. JAK1, Janus kinase 1; JAK2, Janus kinase 2; JAK3, Janus kinase 3; TYK2, Tyrosine kinase 2; STAT1, Signal transducer and activator of transcription 1; STAT3, Signal transducer and activator of transcription 3; STAT4, Signal transducer and activator of transcription 4; STAT5, Signal transducer and activator of transcription 5; STAT6, Signal transducer and activator of transcription 6; SYK, Spleen tyrosine kinase; BTK, Bruton’s tyrosine kinase; AHR, Aryl-hydrocarbon receptor; TRPA1, Transient receptor cation ankyrin 1; CB1R, Cannabinoid type 1 receptor; CB2R, Cannabinoid type 2 receptor; LXR, Liver X receptor; STAT, Signal transducer and activator of transcription; PDE4, Phosphodiesterase-4; PAR2, Protease-activated receptor 2; H4R, Histamine 4 receptor; S1PR, Sphingosine-1-phosphate receptor. This figure was created with Biorender at www.biorender.com. (b) Mechanism of action of different drugs acting on the adaptive immune system via various receptors, different interleukins and its receptors, and the Janus kinases (JAK) STAT pathway. Dupilumab is a fully humanized monoclonal antibody targeting IL4Rα receptor. CBP201 is a human IgG4 monoclonal antibody that binds to IL-4Rα receptor. AK120 is an antibody against IL4Rα receptor. Tralokinumab and cendakimab, a fully humanized IgG4λ, bind to IL-13 at an epitope that overlaps with the binding site of receptors IL13Rαl subunit and IL13Rα2 subunit. Tezepelumab is an anti-TSLP antibody. Lebrikizumab, a humanized monoclonal IgG4 antibody that selectively blocks IL13 and prevents heterodimerization of type II IL4R receptor composed of IL4Rα and IL13Rαl subunit. Eblasakimab is a fully humanized monoclonal antibody that targets the IL-13Rα1 subunit, therefore inhibiting the signals by IL4 and IL-13. Mepolizumab, a humanized immunoglobulin G monoclonal antibody, binds to interleukin-5, inhibiting its activity. Benralizumab directly binds to the IL-5Rα chain to inhibit the signaling pathway. Nemolizumab, a humanized monoclonal antibody that antagonizes IL-31RA receptor. Vixarelimab, a first-in-class fully human monoclonal antibody that targets oncostatin M receptor. Fezakinumab is an anti-IL-22 monoclonal antibody. Ruxolitinib, baricitinib, and ivarmacitinib inhibit JAK1 and JAK2. Tofacitinib is an inhibitor of JAK1, JAK2, and JAK3. Delgocitinib and jaktinib are pan-jak inhibitors inhibiting JAK1, JAK2, JAK3, and TYK2. Brepocitinib is a selective JAK-1 and TYK-2 inhibitor. Ifidacitinib selectively targets JAK1 and JAK3. Upadacitinib, abrocotinib, and ivarmacitinib are JAK inhibitors with a higher potency for JAK-1. Gusacitinib is a dual JAK-SYK inhibitor. PRN473 is a covalent BTK inhibitor. Branebrutinib is a highly selective BTK inhibitor that covalently binds to the cysteine residue of BTK. Tapinarof is an AHR agonist. HC-030031 and GRC17536 are both TRPA1 antagonists. S-777469 is a CB2R agonist. VTP-38543 and ALX-101 are LXR-β agonists. Crisaborole, PF-07038124, Hemay-808, difamilast, and roflumilast are PDE4 inhibitors in topical forms, while apremilast and orismilast are PDE4 inhibitors in oral form. Methylbenzyl methylbenzimidazole piperidinyl methanone (MMP) is a selective PAR-2 inhibitor in topical form. JNJ 39758979, adriforant, and LEO 152020 are H4R antagonists. KRO-105714 is a dual antagonist of sphingosylphosphorylcholine and sphingosine-1-phosphate receptor 1 in topical form. JTE-013 is an S1PR2 antagonist in topical form. Etrasimod, LC51-0255, and udifitimod are oral S1PR modulators. SCD-044 is an S1PR1 agonist. JAK1, Janus kinase 1; JAK2, Janus kinase 2; JAK3, Janus kinase 3; TYK2, Tyrosine kinase 2; STAT1, Signal transducer and activator of transcription 1; STAT3, Signal transducer and activator of transcription 3; STAT4, Signal transducer and activator of transcription 4; STAT5, Signal transducer and activator of transcription 5; STAT6, Signal transducer and activator of transcription 6; SYK, Spleen tyrosine kinase; BTK, Bruton’s tyrosine kinase; AHR, Aryl-hydrocarbon receptor; TRPA1, Transient receptor cation ankyrin 1; CB1R, Cannabinoid type 1 receptor; CB2R, Cannabinoid type 2 receptor; LXR, Liver X receptor; STAT, Signal transducer and activator of transcription; PDE4, Phosphodiesterase-4; PAR2, Protease-activated receptor 2; H4R, Histamine 4 receptor; S1PR, Sphingosine-1-phosphate receptor. This figure was created with Biorender at www.biorender.com.Tapinarof, a topical AHR agonist (Figure 1b and Figure 3b), has been approved by the FDA for psoriasis. As of this writing, an open-label long-term extension study is currently recruiting to evaluate the safety and efficacy of tapinarof cream 1% in 961 participants (>2 years old) with AD.2.3. Targeting the Adaptive Immune System
2.3.1. Antigen Presentation through OX40-OX40L
As an essential costimulatory T-cell receptor molecule and member of the TNF superfamily, OX40 is primarily expressed on enhanced effector T-cells (Th1, Th2, Th17, and Th22) and regulatory T-cells [57][32]. OX40 is essential for T-cell expansion, survival, and memory differentiation. Conversely, the ligand of OX40, known as OX40L, is expressed on activated antigen-presenting cells (such as dendritic cells and macrophages) as well as endothelial cells [58][33]. Pro-inflammatory T-cell responses and T-helper memory cells generation are achieved through the engagement and interaction of OX40 with OX40L, as demonstrated in Figure 1a.2.3.2. T-Helper-2-Related Cytokines
Interleukin-4 (IL-4) through Il-4Rα Receptor
Th-2 cytokines, IL-4, and IL-13 are important for chronic pruritus [68][34] and can exert their function by binding to the IL-4 receptor (IL4R). There are two types of IL4R complex according to the components of the heterodimeric protein subunit. IL-4Rα is one subunit of IL4R complex with a specific binding affinity for IL-4. The first type is the Type I IL4R, which is composed of IL-4Rα and γc subunits, and the second type is the Type II IL4R composed of IL-4Rα and IL-13Rαl subunits. IL4 cytokine can either bind to Type I IL4R or Type II IL4R to exert its effect, as illustrated in Figure 3a [69,70,71][35][36][37]. Therefore, based on these studies, it is safe to conclude that the type II IL4R complex serves as a functional receptor for both IL-4 and IL-13 [72][38]. Dupilumab, a fully humanized monoclonal antibody targeting IL4Rα (Figure 3b), is recognized as a primary treatment option for severe, recurrent, or refractory AD. This medication has obtained FDA approval for the management of moderate to severe AD in children (6 months to 6 years), adolescents (6–17 years), and adults [5] CBP201 is a human IgG4 monoclonal antibody that binds to IL-4Rα (Figure 3b) administered subcutaneously or intravenously. A post hoc analysis on a phase 2b, randomized, double-blind, placebo-controlled trial investigated the safety and efficacy of CBP201 in rapid and sustained improvement across all four (head and neck, trunk, upper and lower limb) body regions among 255 adults with moderate-to-severe AD.Interleukin-13 (IL-13)
IL-13 has garnered significant attention as a potential and valid target in the context of AD, owing to its relevance in the skin condition. The binding of either IL-4 or IL-13 to the IL-13Rα1 subunit of the Type II IL4R triggers the recruitment of IL-4Rα, subsequently activating Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2). This activation leads to the phosphorylation of signal transducer and activator of transcription 6 (STAT6), which promotes the polarization of T-helper cells toward the Th2 phenotype. Tralokinumab, a fully humanized IgG4λ, binds to IL-13 at an epitope that overlaps with the binding site of receptors IL-13Rαl subunit and IL-13Rα2 subunit, thereby preventing its interaction and blocking further signaling (Figure 3b) [77][39]. This drug has obtained approval in the United States and Europe for the treatment of moderate-to-severe AD in adults. Currently, a randomized, parallel-group, monotherapy trial to assess the pharmacokinetics and safety of tralokinumab in children (2 to ≤12 years old) with moderate-to-severe AD is being studied. Cendakimab (RPC-4046), a recombinant humanized anti-interleukin 13 monoclonal antibody, binds to IL-13 at an epitope that overlaps with the binding site of receptors IL-13Rαl subunit and IL-13Rα2 subunit, thereby preventing its interaction and blocking further signaling (Figure 3b) [77][39]. This drug is currently under development for the treatment of eosinophilic esophagitis [79][40], eosinophilic gastroenteritis [80][41], and AD [81][42].Interleukin-5 (IL-5) through IL-5rα Receptor
Interleukin 5 cytokine binds to interleukin 5-receptor (IL-5R) consisting of IL-5 receptor alpha subunit (IL5Rα) and a common receptor B subunit (BC), as depicted in Figure 3a. IL-5 specifically binds to the IL5Rα and recruits the beta chain to activate the downstream signaling molecules for the stimulation of B-cells and eosinophils [85][43]. IL-5 plays the most significant role in eosinophil biology, is detectable in the inflammatory infiltrate of AD, and correlates well with the disease severity [6]. Mepolizumab, a humanized immunoglobulin G monoclonal antibody that binds to IL-5 (Figure 3b), was generally safe and well tolerated in a study conducted by Kang et al. in their phase 2 multicenter, randomized, double-blind, placebo-controlled, parallel-group clinical trial investigating the efficacy and safety of subcutaneous injection of mepolizumab 100 mg every 4 weeks for 16 weeks. The primary endpoint of the study was the proportion of patients who achieved an IGA of 0 or 1 and at least a 2-grade improvement at week 16.2.3.3. Interleukin-22 (IL-22)
IL-22 cytokine is produced by mast cells, various in vitro and animal studies propose its role in epidermal hyperplasia, and it contributes to skin barrier damage by the inhibition of keratinocyte differentiation. This cytokine is also responsible for the upregulation of pruritogenic peptide gastrin-releasing peptide (GRP), TSLP, and interleukin 33 in epithelial cells, as shown in Figure 1a and Figure 3a [89][44]. A phase 2a randomized double-blind trial was conducted to evaluate the effectiveness of fezakinumab, an anti-IL-22 monoclonal antibody (Figure 3b). The trial involved intravenous fezakinumab monotherapy administered every 2 weeks for a duration of 10 weeks, with follow-up assessments conducted until 20 weeks.2.3.4. Phosphodiesterase-4 (PDE4)
The phosphodiesterase (PDE) superfamily is composed of 11 isozymes that degrade cyclin nucleotides, particularly cAMP and cGMP (Figure 3a) [91][45]. PDE4 is the most diversified and is expressed in keratinocytes, T-cells, Langerhans cells, and neutrophils. PDE4 includes four subtypes, PDE4A, PDE4B, PDE4C, and PDE4D, of which PDE4B plays a significant role in inflammatory response [92][46]. Inhibition of PDE4 results in the prevention of degradation of intracellular cAMP, thereby activating protein kinase A (PKA), cyclic nucleotide-gated ion channels, and the exchange factor directly activated by cAMP 1 and 2 (Epac1/Epac2) at the same time, promoting the production of anti-inflammatory cytokines through interactions with the cAMP-responsive element binding protein (CREB) [93][47].Topical Phosphodiesterase-4 (PDE4)
Crisaborole, a topical phosphodiesterase-4 inhibitor (Figure 3b), has been approved by the FDA for the treatment of mild to moderate AD in pediatric and adult patients. The safety of crisaborole 2% ointment has been established for up to 1 year of treatment, with skin burning being the most commonly reported adverse effect. Several selective phosphodiesterase 4 inhibitors are currently under development. One of these investigational compounds is PF-07038124 ointment, a topical small-molecule PDE4 inhibitor. Currently, a phase 2b randomized, double-blind, vehicle-controlled, parallel-group study is underway to assess the efficacy, safety, tolerability, and pharmacokinetics of multiple dose levels (0.01%, 0.03%, and 0.06%) of PF-07038124 ointment, an oxaborole-based PDE-4 inhibitor (Figure 3b).Oral Phosphodiesterase-4 (PDE4)
Simpson et al. in their phase II, double-blind, placebo-controlled trial evaluated the efficacy, safety, and pharmacodynamics of apremilast in adults with moderate to severe AD. In their study, patients were randomly assigned or received placebo, apremilast 30 mg twice daily, and apremilast 40 mg twice daily for 12 weeks. During weeks 12 to week 24, all patients would receive either apremilast 30 mg or apremilast 40 mg. Among the 185 randomly assigned intention-to-treat patients at week 12, apremilast 40 mg revealed a statistically significant improvement in EASI (31.6%) as compared with placebo and apremilast 30 mg.2.3.5. Histamine Receptors
Histamines are well-known pruritogens and causes the release of mast cells and basophils via the activation of histamine receptors. There exist four subtypes of antihistamines, those of particular importance of which are H1 and H4 antihistamines, which play roles in pruritic skin diseases like AD and urticaria. Human CD4(+) T cells express a functional H4 receptor, which was upregulated under Th2 conditions in a study by Gutzmer et al. [104][48]. Numerous studies demonstrated the anti-pruritic effects of H1R antihistamines in urticaria, and their efficiency in AD is still limited. H4 receptors (H4Rs), the most recently discovered subtype, are expressed on keratinocytes (Figure 3a), neurons (Figure 4a), and various immune cell populations like peripheral mononuclear leukocytes and exert immuno-regulatory effects through the upregulation of IL-31, a T-cell-derived cytokine strongly linked with the development of skin barrier dysfunction, local inflammation, and pruritus [105][49]. Emerging clinical studies specifically targeted the H4R subtype to control the pruritus. In vivo studies demonstrated reduced Th2 cytokine production, associated pruritus, and skin inflammation by H4R antagonists in AD-associated animal models.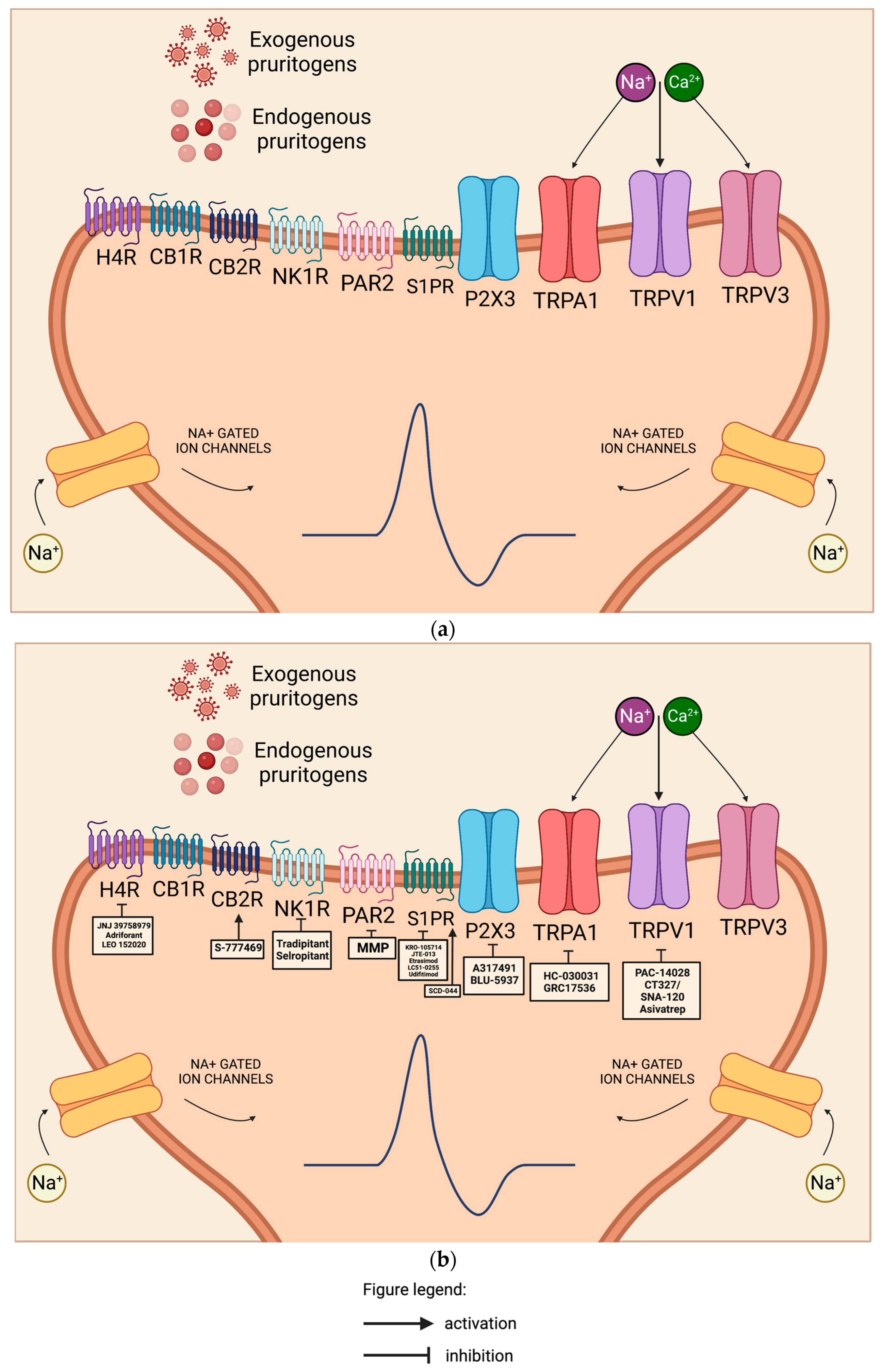 Figure 4. (a) Pyschosomatic aspect of the itch–scratch cycle in atopic dermatitis. This cycle disrupts the epidermal barrier, causes damage to keratinocytes, activates local dendritic cells, and triggers the adaptive response. Exogenous pruritogens like allergens, pathogens, toxins, and irritants, as well as endogenous pruritogens like cytokines, neuropeptides, signaling lipids, and proteases released from keratinocytes and immune cells, bind to receptors on cutaneous itch sensory neurons. These sensory neurons, primarily composed of unmyelinated C-fibers, transmit the itch sensation as action potentials to specific dorsal root ganglia and the central nervous system. The binding of mediators to their respective receptors initiates a signaling cascade involving secondary messengers, which activate TRP family cation channels (particularly TRPA and TRPV), ultimately leading to the opening of voltage-gated sodium channels and neuronal depolarization. Na+, sodium ion; Ca+, calcium ion; H4R, Histamine 4 receptor; CB1R, Cannabinoid type 1 receptor; CB2R, Cannabinoid type 2 receptor; NK1R, neurokinin 1 receptor; PAR2, Protease-activated receptor 2; S1PR, Sphingosine-1-phosphate receptor; P2X, purinoreceptors 3 (P2XR3); TRPA1, Transient receptor cation ankyrin 1; TRPV1, Transient receptor potential channel vanilloid 1; TRPV3, Transient receptor potential channel vanilloid 3. This figure was created with Biorender at www.biorender.com. (b) Mechanism of action of different drugs acting on various receptors expressed in the sensory neuron responsible for the pruritus in atopic dermatitis. JNJ 39758979, adriforant, and LEO 152020 are H4R antagonists. S-777469 is a CB2R agonist. Tradipitant and selropitant are NK1R antagonists. Methylbenzyl methylbenzimidazole piperidinyl methanone (MMP) is a selective PAR-2 inhibitor. KRO-105714 is a dual antagonist of sphingosylphosphorylcholine and sphingosine-1-phosphate receptor 1 in topical form. JTE-013 is an S1PR2 antagonist in topical form. Etrasimod is an oral S1PR modulator. SCD-044 is an S1PR1 agonist. A317491 and BLU-5937 are selective P2XR3 antagonists. HC-030031 is a topical TRPA1 antagonist while GRC17536 is an oral TRPA1 antagonist. PAC-14028, CT327/SNA-120, and asivatrep are all TRPV1 antagonists. Na+, sodium ion; Ca+, calcium ion; H4R, Histamine 4 receptor; CB1R, Cannabinoid type 1 receptor; CB2R, Cannabinoid type 2 receptor; NK1R, neurokinin 1 receptor; PAR2, Protease-activated receptor 2; S1PR, Sphingosine-1-phosphate receptor; P2X purinoreceptors 3, P2XR3; TRPA1, Transient receptor cation ankyrin 1; TRPV1, Transient receptor potential channel vanilloid 1; TRPV3, Transient receptor potential channel vanilloid 3. This figure was created with Biorender at www.biorender.com.The efficacy and safety of JNJ 39758979, a potent and selective H4R antagonist (Figure 3b and Figure 4b), were assessed by Kollmeier et al. in a study involving healthy subjects with histamine-induced pruritus. The researchers concluded that for the treatment of pruritus that is not adequately controlled by H1R antihistamines, novel H4R antagonists like JNJ 39758979 offer a viable alternative treatment option [106][50]. In another study, Kollmeier et al. was able to demonstrate the potential benefit of JNJ-39758979 300 mg on lung function and asthma control in eosinophilic asthma patients [107][51].
Figure 4. (a) Pyschosomatic aspect of the itch–scratch cycle in atopic dermatitis. This cycle disrupts the epidermal barrier, causes damage to keratinocytes, activates local dendritic cells, and triggers the adaptive response. Exogenous pruritogens like allergens, pathogens, toxins, and irritants, as well as endogenous pruritogens like cytokines, neuropeptides, signaling lipids, and proteases released from keratinocytes and immune cells, bind to receptors on cutaneous itch sensory neurons. These sensory neurons, primarily composed of unmyelinated C-fibers, transmit the itch sensation as action potentials to specific dorsal root ganglia and the central nervous system. The binding of mediators to their respective receptors initiates a signaling cascade involving secondary messengers, which activate TRP family cation channels (particularly TRPA and TRPV), ultimately leading to the opening of voltage-gated sodium channels and neuronal depolarization. Na+, sodium ion; Ca+, calcium ion; H4R, Histamine 4 receptor; CB1R, Cannabinoid type 1 receptor; CB2R, Cannabinoid type 2 receptor; NK1R, neurokinin 1 receptor; PAR2, Protease-activated receptor 2; S1PR, Sphingosine-1-phosphate receptor; P2X, purinoreceptors 3 (P2XR3); TRPA1, Transient receptor cation ankyrin 1; TRPV1, Transient receptor potential channel vanilloid 1; TRPV3, Transient receptor potential channel vanilloid 3. This figure was created with Biorender at www.biorender.com. (b) Mechanism of action of different drugs acting on various receptors expressed in the sensory neuron responsible for the pruritus in atopic dermatitis. JNJ 39758979, adriforant, and LEO 152020 are H4R antagonists. S-777469 is a CB2R agonist. Tradipitant and selropitant are NK1R antagonists. Methylbenzyl methylbenzimidazole piperidinyl methanone (MMP) is a selective PAR-2 inhibitor. KRO-105714 is a dual antagonist of sphingosylphosphorylcholine and sphingosine-1-phosphate receptor 1 in topical form. JTE-013 is an S1PR2 antagonist in topical form. Etrasimod is an oral S1PR modulator. SCD-044 is an S1PR1 agonist. A317491 and BLU-5937 are selective P2XR3 antagonists. HC-030031 is a topical TRPA1 antagonist while GRC17536 is an oral TRPA1 antagonist. PAC-14028, CT327/SNA-120, and asivatrep are all TRPV1 antagonists. Na+, sodium ion; Ca+, calcium ion; H4R, Histamine 4 receptor; CB1R, Cannabinoid type 1 receptor; CB2R, Cannabinoid type 2 receptor; NK1R, neurokinin 1 receptor; PAR2, Protease-activated receptor 2; S1PR, Sphingosine-1-phosphate receptor; P2X purinoreceptors 3, P2XR3; TRPA1, Transient receptor cation ankyrin 1; TRPV1, Transient receptor potential channel vanilloid 1; TRPV3, Transient receptor potential channel vanilloid 3. This figure was created with Biorender at www.biorender.com.The efficacy and safety of JNJ 39758979, a potent and selective H4R antagonist (Figure 3b and Figure 4b), were assessed by Kollmeier et al. in a study involving healthy subjects with histamine-induced pruritus. The researchers concluded that for the treatment of pruritus that is not adequately controlled by H1R antihistamines, novel H4R antagonists like JNJ 39758979 offer a viable alternative treatment option [106][50]. In another study, Kollmeier et al. was able to demonstrate the potential benefit of JNJ-39758979 300 mg on lung function and asthma control in eosinophilic asthma patients [107][51].2.3.6. Molecules Involved in Migration of T-Cells
Circulating Memory CLA+ T Lymphocytes
CLA+ T cells are produced during the initiation of the AD lesion. These cells require chemo-attractant stimuli to function through their chemokine receptors CCR4 and CCR10. Thymus and activation-regulated chemokine (TARC/CCL17) and macrophage-derived chemokine (MDC/CCL22) are the chemokine ligands specific for CCR4 (Figure 1a), while the cutaneous T-cell-attracting chemokine (CTACK/CCL27) is specific for CCR10 [112][52]. Some chemokines may be responsible for the selective recruitment of Th2 cells in the Th2-predominant inflammatory diseases like AD, as demonstrated in Figure 1.Sphingosine-1-Phosphate (S1P)
Pro-inflammatory lipid sphingosine 1-phosphate (S1P) is a bioactive lipid mediator released from red blood cells and acts as a second messenger that is involved in several immunological processes like angiogenesis, cytoskeleton organization, trafficking of immune cells, mitogenesis, and apoptosis through acting on either one of the five sphingosine-1-phosphate-specific G-protein coupled receptors (S1PRs) (Figure 1a, Figure 3a and Figure 4a): sphingosine-1-phosphate receptor 1 (S1PR1), sphingosine-1-phosphate receptor 2 (S1PR2), sphingosine-1-phosphate receptor 3 (S1PR3), sphingosine-1-phosphate receptor 4 (S1PR4), and sphingosine-1-phosphate receptor 5 (S1PR5). S1PR1, S1PR2, S1PR3, S1PR4, and S1PR5 are expressed in keratinocytes, while Langerhans cells express S1PR1, S1PR2, and S1PR4, as reflected in Figure 1. S1P plays a diverse role in different cell types, but S1PR1 is expressed by most immune cells [116][53].-
Topical S1PR antagonist
- 2.
-
Oral S1PR modulator
2.3.7. Bruton’s Tyrosine Kinase (BTK)
Expressed in all hematopoietic cells except T cells, Bruton’s tyrosine kinase (BTK), a member of the TEC kinase family of non-receptor tyrosine kinases, has been a promising target for immunological disorders like AD and psoriasis, as depicted in Figure 3a [127][58]. BTK is a multi-component signaling protein that not only aids in the differentiation of B-cells but contributes with innate and adaptive immunity and, more importantly, cytokine production. BTK inhibitors (BTKis) suppress B-cell receptor- and myeloid-fragment-crystallizable-receptor-mediated signaling, thus inhibiting B-cell activation, antibody class-switching, expansion, and cytokine production [127][58]. Branebrutinib is an oral and highly selective BTK inhibitor (Figure 3b) that covalently binds to the cysteine residue of BTK [130][59]. A phase 2, randomized, double-blind, placebo-controlled, 5-parallel-group study trial was designed to evaluate the efficacy, safety, and tolerability of oral branebrutinib for 16 weeks in 17 adult (18 to 65) participants with moderate to severe AD. No results are available yet [124][60].2.3.8. Liver X Receptor (LXR)
Liver x receptor (LXR), a member of the nuclear receptor superfamily, is expressed in keratinocytes (Figure 3a) and fibroblasts and exists in alpha and beta isoforms. LXR forms heterodimers with RXR, enabling it to regulate various functions such as maintaining epidermal homeostasis and reducing inflammatory responses [133][61]. Being able to exert these functions makes these receptors potential targets for pharmacological intervention in AD. In a phase II study with a double-blind, placebo-controlled design, involving 104 patients with mild-to-moderate AD, the topical LXR-β agonist VTP-38543 (Figure 3b) was administered twice daily for 28 days at varying concentrations (0.05%, 0.15%, and 1.0%). Although the drug successfully enhanced the mRNA expression of structural proteins such as loricrin and filaggrin, and led to a reduction in epidermal thickness, it did not demonstrate the ability to downregulate the Th2/Th17 markers [134][62].2.4. Targeting the Itch–Scratch Cycle
2.4.1. Interleukin-31 (IL-31)
Coined as the “itchy” cytokine, IL-31 has been associated with acute itch in an article by Oetjen et al. [68][34] and has been an important mediator of pruritus in chronic conditions like AD and prurigo nodularis [137][63]. Once pro-inflammatory IL-31 cytokine binds to either of the heterodimer receptors IL-31 receptor A (IL31RA) and oncostatin M receptor beta subunit (OSMRβ), it exerts its role in inflammation, pruritus, immune defense, and tissue hemostasis, as showcased in Figure 3a [138][64]. IL-31RA is expressed in epithelial and neuronal cell types, while OSMRβ is widely expressed throughout the mammalian body [138][64].In a post hoc analysis of a Phase III randomized controlled trial conducted in Japan, nemolizumab, a humanized monoclonal antibody that antagonizes IL-31RA (Figure 3b), was evaluated in two parts (Part A and Part B). Part A involved the subcutaneous administration of nemolizumab at a dose of 60 mg every 4 weeks for 16 weeks, followed by a long-term follow-up period of up to 68 weeks (Part B). The study included patients aged 13 years and above with AD. The primary endpoint of Part A was the mean percent change from baseline in the pruritus visual analog scale (VAS) score at week 16. The analysis revealed a reduction of 42.8% in the nemolizumab group receiving topical agents, compared to 21.4% in the placebo group receiving topical agents. Additionally, the percentage change in the EASI score from baseline to week 16 was 45.9% in the nemolizumab group with topical agents, and 33.2% in the placebo group with topical agents. These results demonstrated that nemolizumab led to a greater reduction in pruritus compared to placebo, achieving the primary endpoint of the study [140][65].2.4.2. Neurokinin 1 Receptor (NK1R)
NK1Rs are channels located on mast cells and the dorsal horn of the spinal cord, as pictured in Figure 1a and Figure 4a [143][66]. Once activated by substance P, it leads to the sensitization of mast cells, which leads to increased expression of TNFα, which sensitizes type C nociceptors [144][67]. The blocking of substance P may be beneficial to interfering with the cross-talk between the mast cells and the nerves, which is responsible for the pruritus [145][68]. A phase 3 randomized, placebo-controlled, double-blind clinical trial, known as the EPIONE study, investigated the efficacy of oral tradipitant (VLY-686), a novel NK-1 receptor antagonist (Figure 4b), in reducing chronic pruritus in adults with mild to moderate AD. Although the study did not achieve its primary endpoint of pruritus reduction, statistically significant improvements were observed in itch after one day of treatment and in sleep after two days of treatment in the population with mild AD. The findings suggest the need for future studies to refine treatment recommendations for patients with mild lesions who experience significant pruritus [146][69].2.4.3. P2X Purinoreceptor3 (P2XR3)
Extracellular adenosine 5′-triphosphate (ATP) is released from cells under physiologic or pathologic conditions. These ATPs are mediated by cell-surface receptors called P2R, which are divided into two families: P2XR and P2YR. P2XR has several heterotrimers, among which P2XR3 is particularly important, which are cationic channels mainly expressed in sensory neurons, as illustrated in Figure 4b. These receptors play a role by the localized release of pro-inflammatory neuropeptides via the axon reflex, resulting in coughing, peripheral irritation, pain sensation [148][70], and possibly itch.2.4.4. Transient Receptor Potential Channel (TRP)
The TRP channel family is divided into eight subfamilies, including TRPA (ankyrin), TRPC (canonical), TRPM (melastatin), TRPML (mucolipin), TRPN (Drosophila NOMPC), TRPP (polycystin), TRPV (vanilloid), and TRPY (yeast) according to their amino acid sequence. These receptors are involved in transmitting sensory inputs such as heat, pain, and taste. These channels are highly expressed in the keratinocytes, mast cells, cutaneous sensory neurons, and T-cells, as showcased in Figure 3b and Figure 4b [151][71]. TRPA and TRPV channels hold significance in the context of AD.Transient Receptor Potential Channel Ankyrin (TRPA)
Transient receptor cation ankyrin 1 (TRPA1) is a novel channel that is believed to be responsible for the histamine-independent itch pathway in AD. Aside from the TRPA1 receptor being a cold-sensitive calcium channel in keratinocytes in response to temperatures less than 17 °C, these channels are also activated by various endogenous (leukotriene B4, TSLP, serotonin, IL-13, and IL-31) and exogenous pruritogens (chloroquine, cowhage, allyl isothiocyanate, cinnamaldehyde, allicin, and carvacrol). Once activated, it triggers the different pruritogenic pathways, as seen in Figure 3a and Figure 4a [152][72]. Topical TRPA1 antagonist HC-03003 (Figure 3b and Figure 4b), applied after irradiation, blocked the development of mechanical and thermal allodynia in a murine study by Fialho et al. [154][73]. Another TRPA1 antagonist, oral GRC17536 (Figure 3b and Figure 4b), was able to finish a phase II clinical trial as a promising treatment for diabetic neuropathy but was not able to advance to phase III, due to its limited bioavailability and pharmacokinetic profile [155][74].Transient Receptor Potential Channel Vanilloid (TRPV)
TRPV channels have six members, TRPV1, TRPV2, TRPV3, TRPV4, TRPV5, and TRPV6. Vanillin, vanillic acid, and capsaicin are among the substances that trigger these channels [156][75]. TRPV 1–4 are non-selective cation channels sensitive to temperature, while TRPVs 5–6 are highly calcium-selective channels not sensitive to temperature [157][76].-
Transient receptor potential channel vanilloid 1 (TRPV1)
-
Transient receptor potential channel vanilloid 3 (TRPV3)
2.4.5. Cannabinoid Receptors (CBRs)
The endocannabinoid system (ECS) is an evolutionary complex intercellular signaling network, which plays roles in the modulation of the immune and nervous systems. The two main receptors for endocannabinoids (ECB) are the cannabinoid type 1 (CB1R) and cannabinoid type 2 (CB2R) receptors, which are G-coupled proteins expressed in epidermal keratinocytes, melanocytes, dermal cells, mast cells, sweat glands, hair follicles, and cutaneous nerve fibers, as shown in Figure 3a and Figure 4a [164,165][79][80]. In the epidermal keratinocytes, the CB1R located in the stratum granulosum and stratum spinosum, once activated, triggers cytokine storms, which intensifies the generation of reactive oxygen species (ROS) and TNFα.2.4.6. Protease-Activated Receptor 2 (PAR2)
The family of protease-activated receptors (PARs) includes protease-activated receptor-1 (PAR-1), protease-activated receptor-2 (PAR-2), protease-activated receptor-3 (PAR-3), and protease-activated receptor-4 (PAR-4). In vivo and in vitro studies suggest the roles of PARs in the regulation of epidermal permeability and barrier function [172][81]. Protease-activated receptor-2 (PAR-2), a G-protein coupled receptor expressed in keratinocytes, neurons (Figure 4a), and inflammatory cells such as mast cells (Figure 1a) and T-cells, has been demonstrated to be of significance in AD [173][82]. PAR-2 overexpression in murine studies exhibited an increased density of nerve fibers [174][83] and skin remodeling [175][84]. During skin inflammation, proteolytic enzymes trypsin and tryptase signal pro-inflammatory factors through PAR-2, leading to the production of chemokines and cytokines like TNFα, IL-4, and TSLP [173,176][82][85]. In addition, the activation of PAR-2 on keratinocytes and endothelia stimulates the NF-κb signaling pathway, which has been suspected to be linked with AD. In the study of Steinhoff et al., PAR-2 was significantly enhanced on skin biopsies of 38 patients with AD. On the other hand, tryptase (endogenous PAR-2 agonist) was increased up to fourfold [177][86].2.5. Suppression of the Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT) Pathway, along with the Activation of Suppressor of Cytokine Signaling (SOCS), Is Being Explored
2.5.1. JAK Inhibitors
Topical JAK Inhibitors
After demonstrating both safety and efficacy in multiple clinical trials, ruxolitinib 1.5% cream, an inhibitor of JAK1 and JAK2 (Figure 3b), received approval from the US-FDA in September 2021 for the treatment of mild-to-moderate AD in adolescent patients (≥12 years) and adults [182,183][87][88]. In a phase IIa study conducted by Bisonnette et al., the topical (ointment) form of tofacitinib, an inhibitor of JAK1, JAK2, and JAK3 (Figure 3b), demonstrated both safety and efficacy in treating mild-to-moderate AD. The study involved 69 adult patients who were randomly assigned to receive either tofacitinib 2% ointment or a placebo. The treatment was applied twice daily for a duration of 4 weeks. The results showed that tofacitinib exhibited a greater mean percentage change in EASI from baseline compared to the placebo group across all endpoints. Based on these findings, tofacitinib shows potential as a therapeutic target for AD [184][89].Oral JAK Inhibitors
Oral tofacitinib (Figure 3b) was used by Levy et al. in an open-label study on patients with moderate to severe AD. Improvements in pruritus score, sleep loss score, and SCORAD index were noted with tofactinib 5 mg once or twice daily in addition to topical treatment for 29 weeks. In their study, the drug was tolerated, with no adverse effects such as infections, cytopenias, transaminitis, decreased renal function, or elevated lipid levels [190][90]. Oral tofacitinib is a promising drug; however, recently, the FDA issued a warning black box due to the increased risk of serious cardiovascular problems [191][91]. The European Medicines Agency (EMA) has granted approval for the use of baricitinib, an oral selective Janus kinase 1 (JAK1) and Janus kinase 2 (JAK 2) inhibitor (Figure 3b), in adult patients with AD who are suitable candidates for biologics. The effectiveness and safety of baricitinib in combination with topical corticosteroids were evaluated in the BREEZE-AD4 study, which was a multicenter, double-blind, randomized, placebo-controlled clinical trial. This trial specifically focused on patients with moderate-to-severe AD who had an inadequate response, intolerance, or contraindication to cyclosporine. The study successfully demonstrated the superiority of baricitinib at a dosage of 4 mg in conjunction with topical corticosteroids compared to placebo plus topical corticosteroids. The primary endpoint of achieving at least a 75% improvement in EASI score at week 16 was reached. The US FDA has granted approval for upadacitinib, an oral reversible JAK inhibitor with higher potency for JAK1 (Figure 3b), available in 15 mg and 30 mg doses, as well as Abrocitinib, a selective JAK1 inhibitor (Figure 3b) available in 100 mg and 200 mg doses, for the treatment of AD. The goal for these drugs is to limit the blocking of cytokine axes involved for a much safer profile. A 24-week, head-to-head, phase 3b, multicenter, randomized, double-blinded, double-dummy, active-controlled clinical trial comparing the safety and efficacy of upadacitinib 30 mg daily with subcutaneous dupilumab 300 mg every other week among 692 adults with moderate-to-severe AD who were candidates for systemic therapy demonstrated the superiority of upadacitinib over dupilumab.2.5.2. Suppressor of Cytokine Signaling (SOCS)
The family of suppressors of cytokine signaling (SOCS) comprises several members, including SOCS-1, SOCS-2, SOCS-3, SOCS-4, SOCS-5, SOCS-6, SOCS-7, and cytokine-inducible SH2 protein (CIS). As their name suggests, these molecules act as negative feedback stimulators, targeting the downstream components of the JAK-STAT pathway. In the context of AD pathophysiology, the role of SOCS-1, SOCS-3, and SOCS-5 as negative modulators is particularly significant. The modulation of the JAK/STAT/SOCS pathway using natural biomolecules may hold potential as a therapeutic target for future drug development.Suppressor of Cytokine Signaling-1 (SOCS-1)
In vitro, in vivo, and human studies have shown that SOCS-1 inhibits IFN-γ signaling by binding to JAK1 and JAK2. This results in the inhibition of STAT1 phosphorylation of STAT1. Disabled Jak1 and Jak2 cannot mediate STAT1 phosphorylation, which is necessary for the activation of γ-activated sequence (GAS) inflammatory genes [206][92]. A recent study by Coelho et al. reported that SOCS-1 controls inflammation by specifically targeting the p65 nuclear factor-κb (NF-κB) in inactivated macrophages, thereby suppressing the pro-inflammatory transcription [207][93]. A short motif called the Kinase Inhibitory Region (KIR) was found in SOCS-1 and, through its direct binding with JAK2, this interaction was able to inhibit the tyrosine kinase activity of JAK2 [208][94].Suppressor of Cytokine Signaling-3 (SOCS-3)
Increased expression of SOCS-3 was observed in the skin of AD patients than in healthy individuals. In a mouse model, the overexpression of the SOCS-3 protein impaired Th1 differentiation and elevated levels of Th2 responses [209,210][95][96]. SOCS-3 inhibited the IL-6 pathway through STAT3 activation [211][97] and inhibited IL-12 through STAT4 activation. The results of the molecular studies performed by Babon et al. showed that SOCS-3 directly inhibited JAK1, JAK2, and TYK2 but did not inhibit JAK3 [212][98]. SOCS-3 also contains a Kinase Inhibitory Region (KIR), which competitively inhibits the activity JAK kinase. The role of SOCS-3 in the Th2-mediated allergic response might be a potential target in the treatment of AD.Suppressor of Cytokine Signaling-5 (SOCS-5)
While little is known about the function of SOCS-5, it has been shown to be involved in a variety of allergic disease states such as AD and asthma. Seki et al. concluded that SOCS-5 inhibits IL-4-dependent signaling toward the control of Th2 differentiation. In the mouse study of Sharma et al., the group was able to demonstrate the role of SOCS-5 in regulating the activation of STAT proteins and the phosphorylation of JAK1/2/3 but not TYK2 [213][99]. SOCS protein mimetics found in various natural biomolecules are currently being developed in vitro and may provide novel insights in the regulation of Th2 and Th1 immune responses.2.6. The Hippo-YAP Pathway
It is established that the Th1/Th2 imbalance is involved in the pathophysiology of AD. However, recent studies have shown that the Th17 and Treg cells play important roles in AD [225][100]. Yes-associated protein (YAP) is a key downstream member of Hippo signaling and may be an essential factor for the maintenance of Th17/Treg cell equilibrium. The importance of the Hippo-YAP pathway and its role in Th17 and Treg cell differentiation has been cited in numerous studies. In the study of Xia et al., 35 patients with AD and 24 healthy controls were enrolled. Peripheral venous blood was collected from patients and healthy controls to isolate serum or separate the peripheral blood mononuclear cells. Also in their study, an AD mouse model was constructed using 2,4-dinitrofluorobenzene, and an AD-like inflammatory cell model was constructed using TNF-α/IFN-γ-activated HaCaT cells to detect Th1/Th2/Th17/Treg cell imbalances using flow cytometry. The group found out that a high expression of YAP was found in healthy individuals and mice, suggesting its role in the function and maintenance of Tregs [226][101]. Their study was similar to that of Ni et al., where the isolated Treg cells from the peripheral blood of healthy humans or mice spleen expressed a high expression of YAP compared with Th1, Th2, and Th17 cells [227][102]. AD being a state of constant inflammation may be a result of the downregulation of YAP expression in Tregs.
References
- Lee, J.H.; Choi, A.; Noh, Y.; Oh, I.S.; Jeon, J.Y.; Yoo, H.J.; Shin, J.Y.; Son, S.W. Real-world treatment patterns for atopic dermatitis in South Korea. Sci. Rep. 2022, 12, 13626.
- Son, S.W.; Lee, J.H.; Ahn, J.; Chang, S.E.; Choi, E.H.; Han, T.Y.; Jang, Y.H.; Kim, H.O.; Kim, M.B.; Kim, Y.C.; et al. Assessment of Disease Severity and Quality of Life in Patients with Atopic Dermatitis from South Korea. Ann. Dermatol. 2022, 34, 419–430.
- Tokura, Y.; Hayano, S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol. Int. 2022, 71, 14–24.
- Lee, J.H.; Son, S.W.; Cho, S.H. A Comprehensive Review of the Treatment of Atopic Eczema. Allergy Asthma Immunol. Res. 2016, 8, 181–190.
- Naik, P.P. Treatment-resistant atopic dermatitis: Novel therapeutics, digital tools, and precision medicine. Asia Pac. Allergy 2022, 12, e20.
- Bieber, T. Atopic dermatitis: An expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov. 2022, 21, 21–40.
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019, 96, 2–7.
- Tsoi, L.C.; Rodriguez, E.; Degenhardt, F.; Baurecht, H.; Wehkamp, U.; Volks, N.; Szymczak, S.; Swindell, W.R.; Sarkar, M.K.; Raja, K.; et al. Atopic Dermatitis Is an IL-13-Dominant Disease with Greater Molecular Heterogeneity Compared to Psoriasis. J. Investig. Dermatol. 2019, 139, 1480–1489.
- Ricardo-Gonzalez, R.R.; Schneider, C.; Liao, C.; Lee, J.; Liang, H.E.; Locksley, R.M. Tissue-specific pathways extrude activated ILC2s to disseminate type 2 immunity. J. Exp. Med. 2020, 217, e20191172.
- Leyva-Castillo, J.M.; Geha, R.S. Cutaneous Type 2 Innate Lymphoid Cells Come in Distinct Flavors. JID Innov. 2021, 1, 100059.
- Dominguez-Huttinger, E.; Christodoulides, P.; Miyauchi, K.; Irvine, A.D.; Okada-Hatakeyama, M.; Kubo, M.; Tanaka, R.J. Mathematical modeling of atopic dermatitis reveals "double-switch" mechanisms underlying 4 common disease phenotypes. J. Allergy Clin. Immunol. 2017, 139, 1861–1872.e1867.
- Luo, J.; Zhu, Z.; Zhai, Y.; Zeng, J.; Li, L.; Wang, D.; Deng, F.; Chang, B.; Zhou, J.; Sun, L. The Role of TSLP in Atopic Dermatitis: From Pathogenetic Molecule to Therapeutical Target. Mediat. Inflamm 2023, 2023, 7697699.
- Lee, E.B.; Kim, K.W.; Hong, J.Y.; Jee, H.M.; Sohn, M.H.; Kim, K.E. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr. Allergy Immunol. 2010, 21, e457–e460.
- Boutet, M.A.; Nerviani, A.; Pitzalis, C. IL-36, IL-37, and IL-38 Cytokines in Skin and Joint Inflammation: A Comprehensive Review of Their Therapeutic Potential. Int. J. Mol. Sci. 2019, 20, 1257.
- Didovic, S.; Opitz, F.V.; Holzmann, B.; Forster, I.; Weighardt, H. Requirement of MyD88 signaling in keratinocytes for Langerhans cell migration and initiation of atopic dermatitis-like symptoms in mice. Eur. J. Immunol. 2016, 46, 981–992.
- Iznardo, H.; Puig, L. IL-1 Family Cytokines in Inflammatory Dermatoses: Pathogenetic Role and Potential Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 9479.
- Hojen, J.F.; Kristensen, M.L.V.; McKee, A.S.; Wade, M.T.; Azam, T.; Lunding, L.P.; de Graaf, D.M.; Swartzwelter, B.J.; Wegmann, M.; Tolstrup, M.; et al. IL-1R3 blockade broadly attenuates the functions of six members of the IL-1 family, revealing their contribution to models of disease. Nat. Immunol. 2019, 20, 1138–1149.
- Kanni, T.; Argyropoulou, M.; Dinarello, C.A.; Simard, J.; Giamarellos-Bourboulis, E.J. MABp1 targeting interleukin-1alpha in hidradenitis suppurativa ineligible for adalimumab treatment: Results of the open-label extension period. Clin. Exp. Dermatol. 2021, 46, 162–163.
- Coleman, K.M.; Gudjonsson, J.E.; Stecher, M. Open-Label Trial of MABp1, a True Human Monoclonal Antibody Targeting Interleukin 1alpha, for the Treatment of Psoriasis. JAMA Dermatol. 2015, 151, 555–556.
- A Phase 2b, Multicenter, Randomized, Placebo- and Active-Comparator-Controlled, Double-Blind Study to Evaluate the Safety and Efficacy of Bermekimab (JNJ-77474462) for the Treatment of Participants with Moderate to Severe Atopic Dermatitis. Available online: https://clinicaltrials.gov/ct2/show/NCT04791319 (accessed on 14 April 2023).
- Church, L.D.; McDermott, M.F. Canakinumab, a fully-human mAb against IL-1beta for the potential treatment of inflammatory disorders. Curr. Opin. Mol. Ther. 2009, 11, 81–89.
- Krueger, J.; Puig, L.; Thaci, D. Treatment Options and Goals for Patients with Generalized Pustular Psoriasis. Am. J. Clin. Dermatol. 2022, 23, 51–64.
- Fukaura, R.; Akiyama, M. Targeting IL-36 in Inflammatory Skin Diseases. BioDrugs 2023, 37, 279–293.
- Boraschi, D.; Italiani, P.; Weil, S.; Martin, M.U. The family of the interleukin-1 receptors. Immunol. Rev. 2018, 281, 197–232.
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 2013, 4, 289.
- Lee, J.H.; Cho, D.H.; Park, H.J. IL-18 and Cutaneous Inflammatory Diseases. Int. J. Mol. Sci. 2015, 16, 29357–29369.
- Novak, N.; Valenta, R.; Bohle, B.; Laffer, S.; Haberstok, J.; Kraft, S.; Bieber, T. FcepsilonRI engagement of Langerhans cell-like dendritic cells and inflammatory dendritic epidermal cell-like dendritic cells induces chemotactic signals and different T-cell phenotypes in vitro. J. Allergy Clin. Immunol. 2004, 113, 949–957.
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimaki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A.; et al. IL-33 and ST2 in atopic dermatitis: Expression profiles and modulation by triggering factors. J. Investig. Dermatol. 2012, 132, 1392–1400.
- Cayrol, C. IL-33, an Alarmin of the IL-1 Family Involved in Allergic and Non Allergic Inflammation: Focus on the Mechanisms of Regulation of Its Activity. Cells 2021, 11, 107.
- Kim, B.S.; Wang, K.; Siracusa, M.C.; Saenz, S.A.; Brestoff, J.R.; Monticelli, L.A.; Noti, M.; Tait Wojno, E.D.; Fung, T.C.; Kubo, M.; et al. Basophils promote innate lymphoid cell responses in inflamed skin. J. Immunol. 2014, 193, 3717–3725.
- Helm, E.Y.; Zhou, L. Transcriptional regulation of innate lymphoid cells and T cells by aryl hydrocarbon receptor. Front. Immunol. 2023, 14, 1056267.
- Lé, A.M.; Torres, T. OX40-OX40L Inhibition for the Treatment of Atopic Dermatitis-Focus on Rocatinlimab and Amlitelimab. Pharmaceutics 2022, 14, 2753.
- Iriki, H.; Takahashi, H.; Amagai, M. Diverse Role of OX40 on T Cells as a Therapeutic Target for Skin Diseases. J. Investig. Dermatol. 2023, 143, 545–553.
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017, 171, 217–228.e213.
- McCormick, S.M.; Heller, N.M. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015, 75, 38–50.
- Vadevoo, S.M.P.; Kim, J.E.; Gunassekaran, G.R.; Jung, H.K.; Chi, L.; Kim, D.E.; Lee, S.H.; Im, S.H.; Lee, B. IL4 Receptor-Targeted Proapoptotic Peptide Blocks Tumor Growth and Metastasis by Enhancing Antitumor Immunity. Mol. Cancer Ther. 2017, 16, 2803–2816.
- LaPorte, S.L.; Juo, Z.S.; Vaclavikova, J.; Colf, L.A.; Qi, X.; Heller, N.M.; Keegan, A.D.; Garcia, K.C. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell 2008, 132, 259–272.
- Hershey, G.K. IL-13 receptors and signaling pathways: An evolving web. J. Allergy Clin. Immunol. 2003, 111, 677–690, quiz 691.
- Bieber, T. Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy 2020, 75, 54–62.
- A Phase 3, Multicenter, Multinational, Open-Label Extension Study to Evaluate the Long-Term Safety of CC-93538 in Adult and Adolescent Subjects with Eosinophilic Esophagitis. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04991935 (accessed on 1 May 2023).
- A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled In-duction and Maintenance Study to Evaluate the Efficacy and Safety of CC-93538 in Adult and Adolescent Japanese Subjects with Eosinophilic Gastroenteritis. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05214768 (accessed on 1 May 2023).
- A Phase 2, Multicenter, Global, Randomized, Double-blind, Place-bo-Controlled, Parallel-Group Study to Evaluate the Safety and Efficacy of Cendakimab (CC-93538) in Adult Subjects with Moderate to Severe Atopic Dermatitis. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04800315 (accessed on 1 May 2023).
- Kusano, S.; Kukimoto-Niino, M.; Hino, N.; Ohsawa, N.; Ikutani, M.; Takaki, S.; Sakamoto, K.; Hara-Yokoyama, M.; Shirouzu, M.; Takatsu, K.; et al. Structural basis of interleukin-5 dimer recognition by its alpha receptor. Protein. Sci. 2012, 21, 850–864.
- Fujita, H. The role of IL-22 and Th22 cells in human skin diseases. J. Dermatol. Sci. 2013, 72, 3–8.
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520.
- Manning, C.D.; Burman, M.; Christensen, S.B.; Cieslinski, L.B.; Essayan, D.M.; Grous, M.; Torphy, T.J.; Barnette, M.S. Suppression of human inflammatory cell function by subtype-selective PDE4 inhibitors correlates with inhibition of PDE4A and PDE4B. Br. J. Pharmacol. 1999, 128, 1393–1398.
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1048.
- Gutzmer, R.; Mommert, S.; Gschwandtner, M.; Zwingmann, K.; Stark, H.; Werfel, T. The histamine H4 receptor is functionally expressed on T(H)2 cells. J. Allergy Clin. Immunol. 2009, 123, 619–625.
- Mehta, P.; Miszta, P.; Rzodkiewicz, P.; Michalak, O.; Krzeczyński, P.; Filipek, S. Enigmatic Histamine Receptor H(4) for Potential Treatment of Multiple Inflammatory, Autoimmune, and Related Diseases. Life 2020, 10, 50.
- Kollmeier, A.; Francke, K.; Chen, B.; Dunford, P.J.; Greenspan, A.J.; Xia, Y.; Xu, X.L.; Zhou, B.; Thurmond, R.L. The histamine H(4) receptor antagonist, JNJ 39758979, is effective in reducing histamine-induced pruritus in a randomized clinical study in healthy subjects. J. Pharmacol. Exp. Ther. 2014, 350, 181–187.
- Kollmeier, A.P.; Greenspan, A.; Xu, X.L.; Silkoff, P.E.; Barnathan, E.S.; Loza, M.J.; Jiang, J.; Zhou, B.; Chen, B.; Thurmond, R.L. Phase 2a, randomized, double-blind, placebo-controlled, multicentre, parallel-group study of an H(4) R-antagonist (JNJ-39758979) in adults with uncontrolled asthma. Clin. Exp. Allergy 2018, 48, 957–969.
- Ferran, M.; Santamaria-Babi, L.F. Pathological mechanisms of skin homing T cells in atopic dermatitis. World Allergy Organ J. 2010, 3, 44–47.
- Song, J.; Dagan, A.; Yakhtin, Z.; Gatt, S.; Riley, S.; Rosen, H.; Or, R.; Almogi-Hazan, O. The novel sphingosine-1-phosphate receptors antagonist AD2900 affects lymphocyte activation and inhibits T-cell entry into the lymph nodes. Oncotarget 2017, 8, 53563–53580.
- Yoon, S.B.; Lee, C.H.; Kim, H.Y.; Jeong, D.; Jeon, M.K.; Cho, S.A.; Kim, K.; Lee, T.; Yang, J.Y.; Gong, Y.D.; et al. A novel sphingosylphosphorylcholine and sphingosine-1-phosphate receptor 1 antagonist, KRO-105714, for alleviating atopic dermatitis. J. Inflamm. 2020, 17, 20.
- Kang, J.; Lee, J.H.; Im, D.S. Topical Application of S1P(2) Antagonist JTE-013 Attenuates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis in Mice. Biomol. Ther. 2020, 28, 537–541.
- Silverberg, J.I.; Bissonnette, R.; Kircik, L.; Murrell, D.F.; Selfridge, A.; Liu, K.; Ahluwalia, G.; Guttman-Yassky, E. Efficacy and safety of etrasimod, a sphingosine 1-phosphate receptor modulator, in adults with moderate-to-severe atopic dermatitis (ADVISE). J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1366–1374.
- Won Lee, S.; Hwang, I.; Oh, J.; Lee, S.; Jang, I.J.; Yu, K.S. Single-dose of LC51-0255, a selective S1P(1) receptor modulator, showed dose-dependent and reversible reduction of absolute lymphocyte count in humans. Clin. Transl. Sci. 2022, 15, 1074–1083.
- Satterthwaite, A.B.; Witte, O.N. The role of Bruton’s tyrosine kinase in B-cell development and function: A genetic perspective. Immunol. Rev. 2000, 175, 120–127.
- Watterson, S.H.; Liu, Q.; Beaudoin Bertrand, M.; Batt, D.G.; Li, L.; Pattoli, M.A.; Skala, S.; Cheng, L.; Obermeier, M.T.; Moore, R.; et al. Discovery of Branebrutinib (BMS-986195): A Strategy for Identifying a Highly Potent and Selective Covalent Inhibitor Providing Rapid in Vivo Inactivation of Bruton’s Tyrosine Kinase (BTK). J. Med. Chem. 2019, 62, 3228–3250.
- A Phase 2, Randomized, Double-Blinded, Placebo-Controlled, 5 Parallel-Group Study of BMS-986166 or Branebrutinib for the Treatment of Patients with Moderate to Severe Atopic Dermatitis. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05014438 (accessed on 1 May 2023).
- Czarnowicki, T.; Dohlman, A.B.; Malik, K.; Antonini, D.; Bissonnette, R.; Chan, T.C.; Zhou, L.; Wen, H.C.; Estrada, Y.; Xu, H.; et al. Effect of short-term liver X receptor activation on epidermal barrier features in mild to moderate atopic dermatitis: A randomized controlled trial. Ann. Allergy Asthma Immunol. 2018, 120, 631–640.e611.
- Pinto, L.M.; Chiricozzi, A.; Calabrese, L.; Mannino, M.; Peris, K. Novel Therapeutic Strategies in the Topical Treatment of Atopic Dermatitis. Pharmaceutics 2022, 14, 2767.
- Datsi, A.; Steinhoff, M.; Ahmad, F.; Alam, M.; Buddenkotte, J. Interleukin-31: The “itchy” cytokine in inflammation and therapy. Allergy 2021, 76, 2982–2997.
- Bagci, I.S.; Ruzicka, T. IL-31: A new key player in dermatology and beyond. J. Allergy Clin. Immunol. 2018, 141, 858–866.
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N. Engl. J. Med. 2020, 383, 141–150.
- Tey, H.L.; Yosipovitch, G. Targeted treatment of pruritus: A look into the future. Br. J. Dermatol. 2011, 165, 5–17.
- Junger, H.; Sorkin, L.S. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain 2000, 85, 145–151.
- Yosipovitch, G.; Greaves, M.W.; Schmelz, M. Itch. Lancet 2003, 361, 690–694.
- Welsh, S.E.; Xiao, C.; Kaden, A.R.; Brzezynski, J.L.; Mohrman, M.A.; Wang, J.; Smieszek, S.P.; Przychodzen, B.; Ständer, S.; Polymeropoulos, C.; et al. Neurokinin-1 receptor antagonist tradipitant has mixed effects on itch in atopic dermatitis: Results from EPIONE, a randomized clinical trial. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e338–e340.
- Pelleg, A.; Sirtori, E.; Rolland, J.F.; Mahadevan, A. DT-0111: A novel P2X3 receptor antagonist. Purinergic. Signal. 2023, 1–13.
- Bonchak, J.G.; Swerlick, R.A. Emerging therapies for atopic dermatitis: TRPV1 antagonists. J. Am. Acad. Dermatol. 2018, 78, S63–S66.
- Mahmoud, O.; Soares, G.B.; Yosipovitch, G. Transient Receptor Potential Channels and Itch. Int. J. Mol. Sci. 2022, 24, 420.
- Fialho, M.F.P.; Brum, E.D.S.; Pegoraro, N.S.; Couto, A.C.G.; Trevisan, G.; Cruz, L.; Oliveira, S.M. Topical transient receptor potential ankyrin 1 antagonist treatment attenuates nociception and inflammation in an ultraviolet B radiation-induced burn model in mice. J. Dermatol. Sci. 2020, 97, 135–142.
- Xie, Z.; Hu, H. TRP Channels as Drug Targets to Relieve Itch. Pharmaceuticals 2018, 11, 100.
- Karki, T.; Tojkander, S. TRPV Protein Family-From Mechanosensing to Cancer Invasion. Biomolecules 2021, 11, 1019.
- Pumroy, R.A.; Fluck, E.C., 3rd; Ahmed, T.; Moiseenkova-Bell, V.Y. Structural insights into the gating mechanisms of TRPV channels. Cell Calcium. 2020, 87, 102168.
- Naert, R.; López-Requena, A.; Talavera, K. TRPA1 Expression and Pathophysiology in Immune Cells. Int. J. Mol. Sci. 2021, 22, 11460.
- Seo, S.H.; Kim, S.; Kim, S.E.; Chung, S.; Lee, S.E. Enhanced Thermal Sensitivity of TRPV3 in Keratinocytes Underlies Heat-Induced Pruritogen Release and Pruritus in Atopic Dermatitis. J. Investig. Dermatol. 2020, 140, 2199–2209.e2196.
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942.
- Stander, S.; Schmelz, M.; Metze, D.; Luger, T.; Rukwied, R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J. Dermatol. Sci. 2005, 38, 177–188.
- Cork, M.J.; Danby, S.G.; Vasilopoulos, Y.; Hadgraft, J.; Lane, M.E.; Moustafa, M.; Guy, R.H.; Macgowan, A.L.; Tazi-Ahnini, R.; Ward, S.J. Epidermal barrier dysfunction in atopic dermatitis. J. Investig. Dermatol. 2009, 129, 1892–1908.
- Zhao, J.; Munanairi, A.; Liu, X.Y.; Zhang, J.; Hu, L.; Hu, M.; Bu, D.; Liu, L.; Xie, Z.; Kim, B.S.; et al. PAR2 Mediates Itch via TRPV3 Signaling in Keratinocytes. J. Investig. Dermatol. 2020, 140, 1524–1532.
- Smith, L.; Gatault, S.; Casals-Diaz, L.; Kelly, P.A.; Camerer, E.; Metais, C.; Knaus, U.G.; Eissner, G.; Steinhoff, M. House dust mite-treated PAR2 over-expressor mouse: A novel model of atopic dermatitis. Exp. Dermatol. 2019, 28, 1298–1308.
- Buhl, T.; Ikoma, A.; Kempkes, C.; Cevikbas, F.; Sulk, M.; Buddenkotte, J.; Akiyama, T.; Crumrine, D.; Camerer, E.; Carstens, E.; et al. Protease-Activated Receptor-2 Regulates Neuro-Epidermal Communication in Atopic Dermatitis. Front. Immunol. 2020, 11, 1740.
- Nishimoto, R.; Kodama, C.; Yamashita, H.; Hattori, F. Human Induced Pluripotent Stem Cell-Derived Keratinocyte-Like Cells for Research on Protease-Activated Receptor 2 in Nonhistaminergic Cascades of Atopic Dermatitis. J. Pharmacol. Exp. Ther. 2023, 384, 248–253.
- Steinhoff, M.; Neisius, U.; Ikoma, A.; Fartasch, M.; Heyer, G.; Skov, P.S.; Luger, T.A.; Schmelz, M. Proteinase-activated receptor-2 mediates itch: A novel pathway for pruritus in human skin. J. Neurosci. 2003, 23, 6176–6180.
- Owji, S.; Caldas, S.A.; Ungar, B. Management of Atopic Dermatitis: Clinical Utility of Ruxolitinib. J. Asthma Allergy 2022, 15, 1527–1537.
- Sideris, N.; Paschou, E.; Bakirtzi, K.; Kiritsi, D.; Papadimitriou, I.; Tsentemeidou, A.; Sotiriou, E.; Vakirlis, E. New and Upcoming Topical Treatments for Atopic Dermatitis: A Review of the Literature. J. Clin. Med. 2022, 11, 4974.
- Bissonnette, R.; Papp, K.A.; Poulin, Y.; Gooderham, M.; Raman, M.; Mallbris, L.; Wang, C.; Purohit, V.; Mamolo, C.; Papacharalambous, J.; et al. Topical tofacitinib for atopic dermatitis: A phase IIa randomized trial. Br. J. Dermatol. 2016, 175, 902–911.
- Levy, L.L.; Urban, J.; King, B.A. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J. Am. Acad. Dermatol. 2015, 73, 395–399.
- Administration (FDA). FDA Drug Safety Communication: FDA Approves Boxed Warning about Increased Risk of Blood Clots and Death with Higher Dose of Arthritis and Ulcerative Colitis Medicine Tofacitinib (Xeljanz, Xeljanz XR). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death (accessed on 1 May 2023).
- Federici, M.; Giustizieri, M.L.; Scarponi, C.; Girolomoni, G.; Albanesi, C. Impaired IFN-gamma-dependent inflammatory responses in human keratinocytes overexpressing the suppressor of cytokine signaling 1. J. Immunol. 2002, 169, 434–442.
- Coelho, D.R.; Palma, F.R.; Paviani, V.; LaFond, K.M.; Huang, Y.; Wang, D.; Wray, B.; Rao, S.; Yue, F.; Bonini, M.G.; et al. SOCS1 regulates a subset of NFkappaB-target genes through direct chromatin binding and defines macrophage functional phenotypes. iScience 2023, 26, 106442.
- Giordanetto, F.; Kroemer, R.T. A three-dimensional model of Suppressor Of Cytokine Signalling 1 (SOCS-1). Protein. Eng. 2003, 16, 115–124.
- Seki, Y.; Inoue, H.; Nagata, N.; Hayashi, K.; Fukuyama, S.; Matsumoto, K.; Komine, O.; Hamano, S.; Himeno, K.; Inagaki-Ohara, K.; et al. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat. Med. 2003, 9, 1047–1054.
- Kinjyo, I.; Inoue, H.; Hamano, S.; Fukuyama, S.; Yoshimura, T.; Koga, K.; Takaki, H.; Himeno, K.; Takaesu, G.; Kobayashi, T.; et al. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-beta 1. J. Exp. Med. 2006, 203, 1021–1031.
- Berlato, C.; Cassatella, M.A.; Kinjyo, I.; Gatto, L.; Yoshimura, A.; Bazzoni, F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J. Immunol. 2002, 168, 6404–6411.
- Babon, J.J.; Kershaw, N.J.; Murphy, J.M.; Varghese, L.N.; Laktyushin, A.; Young, S.N.; Lucet, I.S.; Norton, R.S.; Nicola, N.A. Suppression of cytokine signaling by SOCS3: Characterization of the mode of inhibition and the basis of its specificity. Immunity 2012, 36, 239–250.
- Sharma, N.D.; Nickl, C.K.; Kang, H.; Ornatowski, W.; Brown, R.; Ness, S.A.; Loh, M.L.; Mullighan, C.G.; Winter, S.S.; Hunger, S.P.; et al. Epigenetic silencing of SOCS5 potentiates JAK-STAT signaling and progression of T-cell acute lymphoblastic leukemia. Cancer Sci. 2019, 110, 1931–1946.
- Koga, C.; Kabashima, K.; Shiraishi, N.; Kobayashi, M.; Tokura, Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Investig. Dermatol. 2008, 128, 2625–2630.
- Jia, J.; Mo, X.; Yan, F.; Liu, J.; Ye, S.; Zhang, Y.; Lin, Y.; Li, H.; Chen, D. Role of YAP-related T cell imbalance and epidermal keratinocyte dysfunction in the pathogenesis of atopic dermatitis. J. Dermatol. Sci. 2021, 101, 164–173.
- Ni, X.; Tao, J.; Barbi, J.; Chen, Q.; Park, B.V.; Li, Z.; Zhang, N.; Lebid, A.; Ramaswamy, A.; Wei, P.; et al. YAP Is Essential for Treg-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2018, 8, 1026–1043.
More
