AuThis artophagy, or self-eating, isicle is associated with the MDPI International Journal of Molecular Sciences article, which belongs to a Special Issue Plant Cell and Organism Development, “Autophagic conservative process that occursMachinery of Plant Peroxisomes” by Sławomir Borek1*, iSzymon yeast, animalStefaniak1, Jand pl Śliwiński1,2, Manłgorzat cells,a Garnczarska1 and Małgorzata Pietrowska-Borek3
1Deparimarily involves the degratment of Plant Physiology, Faculty of Biology, Adam Mickiewicz University Poznań, Uniwersytetu Poznańskiego 6, 61-614 Poznań, Polandati
2Schoonl of cytoplasmic fragments along with organelles, Medicine, Medical Sciences and Nutrition, University of Aberdeen, Foresterhill, Aberdeen AB25 2ZD, UK
3Deparotein complexes, and other macromolecules. In planttment of Biochemistry and Biotechnology, Faculty of Agronomy and Bioengineering, Poznań University of Life Sciences, Dojazd 11, 60-632 Poznań, Poland
*Corres,pondence: autopborek@amu.edu.pl; (S.B.)
Thagye participates in the circulatiarticle has been published on ofhttps://doi.org/10.3390/ijms20194754, Int. J. Mol. Sci. 2019, 20(19), 4754. cThell components and acts as a quality control mechanism work was financed by the National Science Centre, Poland (grant no. 2016/23/B/NZ3/00735).
- ATG proteins
- autophagy
- macropexophagy
- peroxisome
- peroxins
1. INTRODUCTIntroduction
ON
Despite the fact that autophagy has been known since the 1960s, it was thought until recently that this is a process during which various cell components are degraded in a non-selective manner. Studies from recent years have provided a lot of physiological and molecular information indicating the functioning of selective kinds of autophagy in plant cells. The first data on selective autophagy in plant cells are from 2006 and concern the degradation of protein complexes [1], and the first evidence for the occurrence of autophagic degradation of peroxisomes (pexophagy) in plant cells appeared in the literature at the turn of 2013 and 2014 [2][3][4][5].
Autophagy, or self-eating, is a conservative process that occurs in yeast, animal, and plant cells, and primarily involves the degradation of cytoplasmic fragments along with organelles, protein complexes, and other macromolecules. In plants, autophagy participates in the circulation of cell components and acts as a quality control mechanism. Despite the fact that autophagy has been known since the 1960s, it was thought until recently that this is a process during which various cell components are degraded in a non-selective manner. Studies from recent years have provided a lot of physiological and molecular information indicating the functioning of selective kinds of autophagy in plant cells. The first data on selective autophagy in plant cells are from 2006 and concern the degradation of protein complexes [1], and the first evidence for the occurrence of autophagic degradation of peroxisomes (pexophagy) in plant cells appeared in the literature at the turn of 2013 and 2014 [2–5].
2.DEVELOPMENTALLY Developmentally and AND LON2 Protease/Chaperone Dysfunction-Induced PexophagyROTEASE/CHAPERONE DYSFUNCTION-INDUCED PEXOPHAGY
The occurrence of selective, autophagic degradation of peroxisomes for their recycling is indispensable for the proper development of plant organisms [5], from the germination phase of seeds to the maintenance of homeostasis in mature individuals. Degradation of peroxisomes or some proteins in peroxisomes is very important because during seed germination and seedling growth peroxisomes, also called glyoxysomes, participate in the breakdown of storage lipid (b-oxidation of fatty acids and the glyoxylate cycle), while in the photosynthetic tissues of older plants they are involved in photorespiration. Such peroxisomes are then called leaf peroxisomes. Under such circumstances, some enzymes are no longer needed, and they must be degraded [6]. Experiments performed on
Arabidopsis thaliana atg5-1
and
atg7-2
mutants showed increased accumulation of CFP-SKL (a peroxisomal marker) in hypocotyls of developing seedlings in comparison to wild-type seedlings, and additionally, this peroxisomal marker was localized in the vacuole of wild-type hypocotyls but not in vacuoles of the
atg7-2
mutant. This observation suggested a role of autophagy in the degradation of peroxisomal proteins. It was confirmed for isocitrate lyase and malate synthase, two marker enzymes of the glyoxylate cycle. The degradation of these two enzymes in hypocotyls of
atg
mutants was clearly delayed compared to the degradation in the wild-type seedlings. However, the inhibition of isocitrate lyase and malate synthase degradation in the hypocotyls of mutants was only transient, suggesting the occurrence of degrading processes other than autophagy, for example proteolytic degradation by peroxisomal matrix proteases [3]. Pexophagy in plants is also tissue-dependent. In
Arabidopsis thaliana
, pexophagy was observed in the hypocotyl [3] and leaves [5] but not in the cotyledons [3] and roots [5].
During the functional transition of glyoxysomes into leaf peroxisomes, obsolete enzymes are degraded inside peroxisomes by LON2 protease, while newly synthesized enzymes are transported into the peroxisome [6]. LON2 protease is a peripheral membrane protein, which associates with the membrane from the matrix side [7] and degrades various peroxisomal proteins, including isocitrate lyase, malate synthase (glyoxylate cycle), and thiolase b-oxidation of fatty acids) [2][6][7]. It has been shown that in
During the functional transition of glyoxysomes into leaf peroxisomes, obsolete enzymes are degraded inside peroxisomes by LON2 protease, while newly synthesized enzymes are transported into the peroxisome [6]. LON2 protease is a peripheral membrane protein, which associates with the membrane from the matrix side [7] and degrades various peroxisomal proteins, including isocitrate lyase, malate synthase (glyoxylate cycle), and thiolase b-oxidation of fatty acids) [2,6,7]. It has been shown that in
Arabidopsis thaliana
peroxisomes with nonfunctional LON2 protease undergo pexophagy. In wild-type plants, when peroxisomes during plant development change their function, isocitrate lyase and malate synthase disappeared and the content of thiolase was significantly reduced. In the
lon2
mutant lacking a functional LON2 protease, it was found that the above-mentioned three enzymes also disappeared, not however due to the LON2 protease activity, but whole peroxisomes were degraded by autophagy. However, in the
lon2atg
double mutant, in which autophagy does not occur, the above-mentioned enzymes were maintained at a constant level. These data suggest that LON2 protease facilitates matrix protein degradation during peroxisome content remodeling, provide evidence for the existence of pexophagy in plant cells, and indicates that peroxisome destruction via autophagy is enhanced when LON2 is absent [2]. The
Arabidopsis thaliana apem10
mutant (impaired in LON2 activity) displayed enlargement of peroxisome size, as well as acceleration of peroxisome degradation, leading to a considerably reduced number of peroxisomes. In the
apem10/peup1
double mutant (
peroxisome unusual positioning 1
), in which peroxisome degradation is arrested due to the defect of ATG2, the peroxisome number increased compared with
apem10
. Additionally, the aggregation of peroxisomes was frequently observed in
apem10/peup1
[7]. Peroxisome aggregation is typical for
peup1
and is caused by oxidative damage of peroxisomal matrix proteins [4]. These results demonstrate that the decrease in peroxisome number is caused by accelerated peroxisome degradation via autophagy [7]. Analyzing the content of isocitrate lyase, malate synthase, and thiolase the authors of this study concluded that glyoxysomal proteins are degraded by two independent pathways. One is the LON2-dependent, and the second is the autophagy-dependent pathway. In contrast to isocitrate lyase and malate synthase, thiolase was not degraded in the
peup1
mutant, indicating that thiolase is not a substrate of LON2 protease, but it is degraded by autophagy during the peroxisomal functional transition [7]. It was also shown that LON2 protein acts as a chaperone. The chaperone function of LON2 suppresses peroxisome degradation by autophagy, but the proteolytic function interferes with the suppression, indicating that modulation between the proteolytic and chaperone functions of LON2 regulates the degradation of peroxisomes by autophagy [7]. If the chaperone function of LON2 can suppress pexophagy, it suggests that misfolded or aggregated matrix proteins may be signals for pexophagy. How peroxisomes with absent or impaired LON2 protease/chaperone are marked for autophagic turnover is not yet clear in plants, but such a signal would presumably traverse the peroxisome membrane to be recognized by a cytosolic pexophagy machinery [8].
3. Sugar Starvation-Induced PexophagyUGAR STARVATION-INDUCED PEXOPHAGY
Pexophagy has been observed in the suspension of BY-2 tobacco (
Nicotiana tabacum
) cells. It was found that a significant peroxisome pool undergoes autophagic degradation under sucrose starvation conditions, and the accumulation of peroxisomes was observed in cells treated with the autophagy inhibitor 3-methyladenine. Additionally, 3-methyladenine caused an increase in peroxisomal proteins and cellular peroxisome numbers in rapidly dividing tobacco cells under nutrient-rich conditions. These data demonstrate that a large fraction of the peroxisome pool is subject to extensive autophagy-mediated turnover under both nutrient starvation and optimal growth conditions [9]. It is also assumed that pexophagy may occur in cells of sugar-starved embryonic axes of germinating seeds of various species of lupin (
Lupinus) [10][11][12]. The main storage compound in lupin seeds is protein, but these seeds may also contain a large amount of lipid (up to 50% of seed dry matter [13]), in the decomposition of which the peroxisomes are involved during seed germination. Under artificially induced sugar starvation, the embryonic axes of lupin contain much more lipid than axes fed with sucrose. This indicates the limitation in lipid breakdown under carbon starvation conditions [11][13]. At the same time, the observations of the ultrastructure of the cells of these organs showed that the autophagic bodies inside the vacuole contain organelles, which may be more or less uniquely identified as peroxisomes [12].
) [10-12]. The main storage compound in lupin seeds is protein, but these seeds may also contain a large amount of lipid (up to 50% of seed dry matter [13]), in the decomposition of which the peroxisomes are involved during seed germination. Under artificially induced sugar starvation, the embryonic axes of lupin contain much more lipid than axes fed with sucrose. This indicates the limitation in lipid breakdown under carbon starvation conditions [11,13]. At the same time, the observations of the ultrastructure of the cells of these organs showed that the autophagic bodies inside the vacuole contain organelles, which may be more or less uniquely identified as peroxisomes [12].
4. Oxidative Damage-Induced PexophagyXIDATIVE DAMAGE-INDUCED PEXOPHAGY
Peroxisomes have intensive oxidative/antioxidative metabolism. Reactive oxygen species (ROS) are generated in these organelles and simultaneously they are detoxified by antioxidant enzymes such as catalase [14][15][16][17]. Such intensive oxidative/antioxidative metabolism causes the peroxisomal proteins to undergo oxidative damage, and they must be continuously removed from peroxisomes. ROS accumulation and oxidative damage require the whole peroxisomes to be degraded via pexophagy, which in plant cells is a part of the peroxisome quality control system [4][5][18][19][20]. In
Peroxisomes have intensive oxidative/antioxidative metabolism. Reactive oxygen species (ROS) are generated in these organelles and simultaneously they are detoxified by antioxidant enzymes such as catalase [14-17]. Such intensive oxidative/antioxidative metabolism causes the peroxisomal proteins to undergo oxidative damage, and they must be continuously removed from peroxisomes. ROS accumulation and oxidative damage require the whole peroxisomes to be degraded via pexophagy, which in plant cells is a part of the peroxisome quality control system [4,5,18–20]. In
Arabidopsis thaliana peup1, peup2
, and
peup4
mutants can increase in the number of peroxisomes and a tendency of the peroxisome to form aggregates has been observed. It was also found that the above-mentioned mutants are identical to the
Arabidopsis thaliana
mutants with impaired autophagy (
atg2, atg18a
, and
atg7
, respectively). The increase in the number of peroxisomes in the peup1 mutant clearly proves that the autophagic degradation of these organelles is impaired. At the same time, aggregated peroxisomes showed an increase in the inactive catalase content, which caused the peroxisomes in the mutants to contain more ROS, and their components were significantly more oxidized than in the wild-type plants. Similar aggregation of peroxisomes can also be induced in wild-type plants by exogenous application of H
2
O
2
. Aggregated peroxisomes also appeared in the
Arabidopsis thaliana
mutant
cat2
with decreased catalase activity [4]. There was also observed (using fluorescence techniques) frequent coexistence of aggregated peroxisomes and ATG8 (a marker of autophagosomes). The above-mentioned data clearly prove that the peroxisomes damaged by H
2
O
2
are selectively degraded by autophagy in plant cells [4]. A team of other researchers [5] also came to identical conclusions, performing research on a different set of
Arabidopsis thaliana
mutants (
atg2, atg5, atg7
, and
atg9
). Considering that peroxisomes with components damaged by ROS undergo pexophagy, it may be assumed that H
2
O
2
generated inside the peroxisomes is the signal triggering the sequence of events leading to the utilization of these organelles in the vacuole [20]. Aggregation of peroxisomes was also observed in tobacco BY-2 suspension cells, where the activity of catalase was artificially inhibited by aminotriazole [21].
5.PEROXISOME Peroxisome Receptor/Adaptor and Scaffold ProteinsRECEPTOR/ADAPTOR AND SCAFFOLD PROTEINS
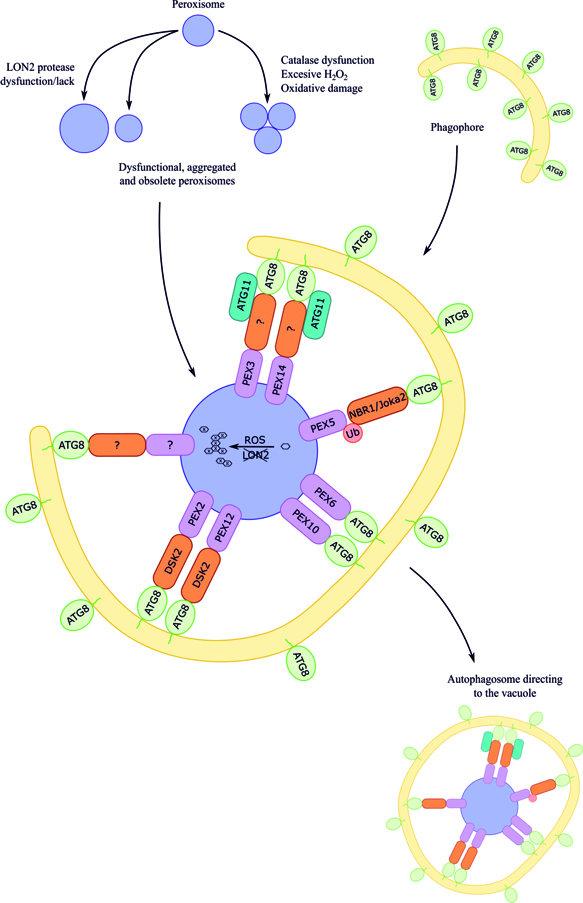
Although pexophagy has been evidenced in plant cells, little is known about receptor/adaptor proteins of peroxisomes targeted for pexophagy in plants. Additionally, little is known about other proteins recruited in autophagic machinery during plant pexophagy. To date, several hypotheses have been formulated, mainly based on knowledge concerning yeasts and mammals. Figure 1 shows the summarized data on macropexophagic machinery in plant cells.
Figure 1.
Hypothetical working model of macropexophagic apparatus in plant cells [22]. The receptors/adaptors, as well as scaffold proteins, involved in the pexophagy in plant cells are still not known. It is postulated that plant NBR1, Joka2 (a hybrid homolog of mammalian NBR1 and p62), and DSK2 can play the role of receptor/adaptor during pexophagy. The signals coming from the surface or from the matrix of the plant peroxisome are also not known; however, based mainly on the data concerning yeasts and mammals, the following proteins are taken into consideration: peroxisomal membrane proteins PEX3 and PEX14, peroxisomal membrane proteins with the identified Atg8-interacting motif (AIM), PEX6 and PEX10, ubiquitinated PEX5, peroxisomal membrane proteins with RING-finger domain PEX2 and PEX12, and oxidized or aggregated matrix proteins. Additionally, ATG8 can be considered as a receptor that may first recognize the target, bind to peroxisomal membrane proteins by AIM, and then bind to phosphatidylethanolamine in the growing phagophore. Among scaffold proteins, only ATG11 is suggested to be involved in plant pexophagy, but it is not known which peroxisome receptor/adaptor it could interact with.
In plants, the NBR1 homolog was identified in
Arabidopsis thaliana
. NBR1 is accumulated in
Arabidopsis thaliana mutants lacking autophagy [23] and recognizes ubiquitinated cargo. Plant NBR1 is implicated in autophagic degradation of ubiquitinated protein aggregates during heat stress [24][25] and in limiting viral infection by targeting cauliflower mosaic virus capsid proteins [26] and turnip mosaic virus silencing the suppressor HCpro [27] for autophagic degradation. However, NBR1 is not involved in autophagic degradation of proteasomes in
mutants lacking autophagy [23] and recognizes ubiquitinated cargo. Plant NBR1 is implicated in autophagic degradation of ubiquitinated protein aggregates during heat stress [24,25] and in limiting viral infection by targeting cauliflower mosaic virus capsid proteins [26] and turnip mosaic virus silencing the suppressor HCpro [27] for autophagic degradation. However, NBR1 is not involved in autophagic degradation of proteasomes in
Arabidopsis thaliana [28]. In plants, Joka2 protein was also found. This is a hybrid homolog of animal NBR1 and p62, which may be involved in selective autophagy in tobacco BY-2 suspension cells [21][29][30]. Nevertheless, there is no clear evidence that NBR1 and Joka2 are peroxisome receptors/adaptors during plant pexophagy. It is rather probable that NBR1 is not necessary for pexophagy in plant cells because it was shown that overexpression of NBR1 in the
[28]. In plants, Joka2 protein was also found. This is a hybrid homolog of animal NBR1 and p62, which may be involved in selective autophagy in tobacco BY-2 suspension cells [21,29,30]. Nevertheless, there is no clear evidence that NBR1 and Joka2 are peroxisome receptors/adaptors during plant pexophagy. It is rather probable that NBR1 is not necessary for pexophagy in plant cells because it was shown that overexpression of NBR1 in the
Arabidopsis thaliana lon2
mutant is not sufficient to trigger autophagy of seedling peroxisomes, indicating that in this plant species an NBR1-independent mechanism to target peroxisomes for autophagic degradation exists [31].
It is suggested that a marker of peroxisomes targeted for pexophagy in plant cells may be ATG8, because the coexistence of ATG8 and peroxisomes, particularly aggregated peroxisomes, has been observed in
Arabidopsis thaliana [3][4][5]. This finding supports the model of selective autophagy, in which ATG8 first recognizes a target by direct interaction with an ATG8-family-interacting motif (AIM) and then is conjugated to phosphatidylethanolamine to recruit and expand the phagophore in the proximity of the target [5]. Such data constitute strong grounds for recognizing ATG8 as a marker of peroxisome, which is targeted for autophagic degradation in plant cells, but it is not known to which peroxisomal protein (or proteins) ATG8 can bind [3][4][5][19]. However, it is known that most of the proteins that are specifically turned over by selective autophagy are recognized by the presence of short AIMs that facilitate their association with the autophagy apparatus. Applying bioinformatics methods it was possible to predict in silico AIMs in AIM-containing proteins on a genome-wide scale in various organisms. Such analysis identified nine peroxisomal PEX proteins in
[3-5]. This finding supports the model of selective autophagy, in which ATG8 first recognizes a target by direct interaction with an ATG8-family-interacting motif (AIM) and then is conjugated to phosphatidylethanolamine to recruit and expand the phagophore in the proximity of the target [5]. Such data constitute strong grounds for recognizing ATG8 as a marker of peroxisome, which is targeted for autophagic degradation in plant cells, but it is not known to which peroxisomal protein (or proteins) ATG8 can bind [3-5,19]. However, it is known that most of the proteins that are specifically turned over by selective autophagy are recognized by the presence of short AIMs that facilitate their association with the autophagy apparatus. Applying bioinformatics methods it was possible to predict in silico AIMs in AIM-containing proteins on a genome-wide scale in various organisms. Such analysis identified nine peroxisomal PEX proteins in
Arabidopsis thaliana
that contain AIMs, among which AtPEX1, AtPEX6, and AtPEX10 possess evolutionarily conserved AIMs. Bimolecular fluorescence complementation results verified that AtPEX6 and AtPEX10 indeed interact with ATG8 in plants, suggesting that they could drive pexophagy [32]. If ATG8 would bind directly to peroxisomal membrane proteins, a bridging receptor/adaptor would not be necessary.
The ubiquitin-binding protein DSK2 (dominant suppressor of KAR2) is another pexophagy receptor/adaptor candidate in plants. DSK2 is a member of the ubiquitin receptor family known to function as shuttle factors ferrying polyubiquitinated substrates to the proteasome for degradation [33].
Arabidopsis thaliana
DSK2 binds to the transcription factor BES1 (BRI1-EMS Suppressor 1), which functions as a master regulator in the brassinosteroid pathway that promotes plant growth. BES1 interacts with the ubiquitin receptor protein DSK2 and is targeted to the autophagy pathway during stress via the interaction of DSK2 with ATG8 through AIMs [34]. DSK2 also interacts with two peroxisomal membrane proteins, PEX2 and PEX12, through the RING (really interesting new gene) finger domain. These results suggest that
Arabidopsis thaliana peroxins containing the RING domain can act together with DSK2 in the peroxisomal membrane-associated protein degradation system [33]. However, the involvement of DSK2 in plant pexophagy has not been confirmed yet.
peroxins containing the RING domain can act together with DSK2 in the peroxisomal membrane-associated protein degradation system [33]. However, the involvement of DSK2 in plant pexophagy has not been confirmed yet.
REFERENCES
- Toyooka, K.; Moriyasu, Y.; Goto, Y.; Takeuchi, M.; Fakuda, H.; Matsuoka, K. Protein aggregates are transported to vacuoles by a macroautophagic mechanism in nutrient-starved plant cells. Autophagy 2006, 2, 96–106.
- Farmer, L.M.; Rinaldi, M.A.; Young, P.G.; Danan, C.H.; Burkhart, S.E.; Bartel, B. Disrupting autophagy restores peroxisome function to an Arabidopsis lon2 mutant and reveals a role for the LON2 protease in peroxisomal matrix protein degradation. Plant Cell 2013, 25, 4085–4100.
- Kim, J.; Lee, H.; Lee, H.N.; Kim, S.H.; Shin, K.D.; Chung, T. Autophagy-related proteins are required for degradation of peroxisomes in Arabidopsis hypocotyls during seedling growth. Plant Cell 2013, 25, 4956–4966.
- Shibata, M.; Oikawa, K.; Yoshimoto, K.; Kondo, M.; Mano, S.; Yamada, K.; Hayashi, M.; Sakamoto, W.; Ohsumi, Y.; Nishimura, M. Highly oxidized peroxisomes are selectively degraded via autophagy in Plant Cell 2013, 25, 4967–4983.
- Yoshimoto, K.; Shibata, M.; Kondo, M.; Oikawa, K.; Sato, M.; Toyooka, K.; Shirasu, K.; Nishimura, M.; Ohsumi, Y. Organ-specific quality control of plant peroxisomes is mediated by autophagy. Cell Sci. 2014, 127, 1161–1168.
- Goto-Yamada, S.; Mano, S.; Yamada, K.; Oikawa, K.; Hosokawa, Y.; Hara-Nishimura, I.; Nishimura, M. Dynamics of the light-dependent transition of plant peroxisomes. Plant Cell Physiol. 2015, 56, 1264–1271.
- Goto-Yamada, S.; Mano, S.; Nakamori, C.; Kondo, M.; Yamawaki, R.; Kato, A.; Nishimura, M. Chaperone and protease functions of LON2 modulate the peroxisomal transition and degradation via autophagy. Plant Cell Physiol. 2014, 55, 482–496.
- Marshall, R.S.; Vierstra, R.D. Autophagy: The master of bulk and selective recycling. Rev. Plant Biol. 2018, 69, 173–208.
- Voitsekhovskaja, O.V.; Schiermeyer, A.; Reumann, S. Plant peroxisomes are degraded by starvation-induced and constitutive autophagy in tobacco BY-2 suspension-cultured cells. Plant Sci. 2014.
- Borek, S.; Ratajczak, W.; Ratajczak, L. Regulation of storage lipid metabolism in developing and germinating lupin (Lupinus spp.) Acta Physiol. Plant. 2015, 37.
- Borek, S.; Kubala, S.; Kubala, S.; Ratajczak, L. Comparative study of storage compound breakdown in germinating seeds of three lupine species. Acta Physiol. Plant. 2011, 33, 1953–1968.
- Borek, S.; Paluch-Lubawa, E.; Pukacka, S.; Pietrowska-Borek, M.; Ratajczak, L. Asparagine slows down the breakdown of storage lipid and degradation of autophagic bodies in sugar-starved embryo axes of germinating lupin seeds. Plant Physiol. 2017, 209, 51–67.
- Borek, S.; Pukacka, S.; Michalski, K. Regulation by sucrose of storage compounds breakdown in germinating seeds of yellow lupine (Lupinus luteus L.), white lupine (Lupinus albus L.) and Andean lupine (Lupinus mutabilis Sweet). II. Mobilization of storage lipid. Acta Physiol. Plant. 2012, 34, 1199–1206.
- Corpas, F.J.; del Río, L.A.; Palma, J.M. Plant peroxisomes at the crossroad of NO and H2O2 J. Integr. Plant Biol. 2019, 61, 803–816.
- Sandalio, L.M.; Romero-Puertas, M.C. Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Bot. 2015, 116, 475–485.
- Kao, Y.T.; Gonzalez, K.L.; Bartel, B. Peroxisome function, biogenesis, and dynamics in plants. Plant Physiol. 2018, 176, 162–177.
- Corpas, F.J.; Barroso, J.B. Peroxisomal plant metabolism—An update on nitric oxide, Ca2+ and the NADPH recycling network. Cell Sci. 2018, 131.
- Veljanovski, V.; Batoko, H. Selective autophagy of non-ubiquitylated targets in plants: Looking for cognate receptor/adaptor proteins. Plant Sci. 2014.
- Avin-Wittenberg, T.; Fernie, A.R. At long last: Evidence for pexophagy in plants. Plant 2014, 7, 1257–1260.
- Lee, H.N.; Kim, J.; Chung, T. Degradation of plant peroxisomes by autophagy. Plant Sci. 2014.
- Tyutereva, E.V.; Dobryakova, K.S.; Schiermeyer, A.; Shishova, F.; Pawlowski, K.; Demidchik, V.; Reumann, S.; Voitsekhovskaja, O.V. The levels of peroxisomal catalase protein and activity modulate the onset of cell death in tobacco BY-2 cells via reactive oxygen species levels and autophagy. Plant Biol. 2018, 45, 247–258.
- Borek, S.; Stefaniak, S.; Śliwiński, J.; Garnczarska, M.; Pietrowska-Borek, M. Autophagic Machinery of Plant Peroxisomes. Int J Mol Sci. 2019, 20, 4754.
- Svenning, S.; Lamark, T.; Krause, K.; Johansen, T. Plant Nbr1 is a selective autophagy substrate and a functional hybrid of the mammalian autophagic adapters Nbr1 and P62/Sqstm1. Autophagy 2011, 7, 993–1010.
- Zhou, J.; Wang, J.; Cheng, Y.; Chi, Y.J.; Fan, B.; Yu, J.Q.; Chen, Z. NBR1-mediated selective autophagy targets insoluble ubiquitinated protein aggregates in plant stress responses. PLoS Genet. 2013.
- Zhou, J.; Zhang, Y.; Qi, J.; Chi, Y.; Fan, B.; Yu, J.Q.; Chen, Z. E3 ubiquitin ligase CHIP and NBR1-mediated selective autophagy protect additively against proteotoxicity in plant stress responses. PLoS Genet. 2014.
- Hafrén, A.; Macia, J.L.; Love, A.J.; Milner, J.J.; Drucker, M.; Hofius, D. Selective autophagy limits cauliflower mosaic virus infection by NBR1-mediated targeting of viral capsid protein and particles. Natl. Acad. Sci. USA 2017, 114.
- Hafrén, A.; Üstün, S.; Hochmuth, A.; Svenning, S.; Johansen, T.; Hofius, D. Turnip mosaic virus counteracts selective autophagy of the viral silencing suppressor HCpro. Plant Physiol. 2018, 176, 649–662.
- Marshall, R.S.; Li, F.; Gemperline, D.C.; Book, A.J.; Vierstra, R.D. Autophagic degradation of the 26S proteasome is mediated by the dual ATG8/ubiquitin receptor RPN10 in Arabidopsis. Cell 2015, 58, 1053–1066.
- Zientara-Rytter, K.; Łukomska, J.; Moniuszko, G.; Gwozdecki, R.; Surowiecki, P.; Lewandowska, M.; Liszewska, F.; Wawrzynska, A.; Sirko, A. Identification and functional analysis of Joka2, a tobacco member of the family of selective autophagy cargo receptors. Autophagy 2011.
- Zientara-Rytter, K.; Sirko, A. Significant role of PB1 and UBA domains in multimerization of Joka2, a selective autophagy cargo receptor from tobacco. Plant Sci. 2014.
- Young, P.G.; Passalacqua, M.J.; Chappell, K.; Llinas, R.J.; Bartel, B. A facile forward-genetic screen for Arabidopsis autophagy mutants reveals twenty-one loss-of-function mutations disrupting six ATG genes. Autophagy 2019, 15, 941–959.
- Xie, Q.; Tzfadia, O.; Levy, M.; Weithorn, E.; Peled-Zehavi, H.; Van Parys, T.; Van de Peer, Y.; Galili, G. hfAIM: A reliable bioinformatics approach for in silico genome-wide identification of autophagy-associated Atg8-interacting motifs in various organisms. Autophagy 2016, 12, 876–887.
- Kaur, N.; Zhao, Q.; Xie, Q.; Hu, J. Arabidopsis RING peroxins are E3 ubiquitin ligases that interact with two homologous ubiquitin receptor proteins. Integr. Plant Biol. 2013, 55, 108–120.
- Nolan, T.M.; Brennan, B.; Yang, M.; Chen, J.; Zhang, M.; Li, Z.;Wang, X.; Bassham, D.C.;Walley, J.; Yin, Y. Selective autophagy of BES1 mediated by DSK2 balances plant growth and survival. Cell 2017.