Betel quid (BQ) chewing increased the risk of oral cancer and oral submucous fibrosis (OSMF), an oral premalignant disorder (OPMD) with malignant transformation potential. BQ components such as areca nut (AN), trauma by coarse AN fiber, catechin, copper, alkaloids, stimulated reactive oxygen species (ROS), inflammation and cytotoxicity are suggested to be the contributing factors. In this review, the expression of extracellular matrix (ECM) turnover related genes and proteins in OSMF and the relation to betel quid chewing habit is discussed. Genetic susceptibility of ECM-related genes to OSMF is also mentioned. These results can facilitate our understanding the pathogenesis of OSMF and its possible prevention/treatment in the future.
- Oral Submucous Fibrosis
- betel quid chewing
- genetic susceptibility
- extracellular matrix turnover
1. Definitions
Per
Introduction
Epidemiology and Risk Factor of Oral Submucous Fibrosis (OSMF)
OSMF Jenis J Pindborg and Satyavati Sirsat (1966) (Pathological definition)- 'An defined as an insidious chronic disease affecting any part of the oral cavity and sometimes the pharynx. Although occasionally preceded by and/or associated with vesicle formation, it is always associated with a juxta-epithelial inflammatory reaction followed by a fibro-elastic changeClinically OSMF presents with burning and pain of the lamina propria, with epithelial atrophy leading to stiffness.'[5] Per Mohit Sharmouth, oral ma and Raghu Radhakrishnan (2019) - 'An insidious, chronic potentially malignant fibrotic disorder affecting the entire oral cavity and sometimes the pharynx and oesophagus. Although occasionally preceded by and/or associated with vesicle formation, it is always associated with a juxta-epithelial inflammatory reaction followed by a fibroelastic change of the lamina propria with epithelial atrophy leading to stiffness of the oral mucosa, progressive decrementcosal atrophy with fibrosis of submucosal tissues, mucosal rigidity and reduction in mouth opening and inability to eat'[6] Per Chandra. OSMF is commani More and Naman Rao (2019) (Clinical definition)- 'A debilitating, progressive, irreversible collagen metabolic disorder induced by chronic chewing of areca nut and its commercial preparations; affecting the oral mucosa and occasionally the pharynx and esophagus; leading to mucosal stiffness and functional morbidity;n in India, Sri Lanka, Taiwan and other southeast Asian countries, and has a potential risk of malignant transformation.'[7]
2. Epidemiology
Thstarte incidence of the disease is higher in people from certain parts of the world including South and South East Asian, South Africa and th spreading to Europe and North Ame Mriddle Eastern countriesca [1,2].[8]
3. Symptoms
In the initiTal phase of the diseaseiwan, the mucosa feels leathery with palpable fibrotic bands. In the advanced stage the oral mucosa loses its resiliency and becomes blanched and stiff. The disease is believed to begin in the posterior part of the oral cavity and gradually spread outwardprevalence of OSMF increased from 8.3/100,000 person in 1996 to 16.2/100,000 person in 2013 [3]. OtTher features of the disease include:
- Xerostomia
- Recurrent ulceration
- Pain in the ear or deafness
- Nasal intonation of voice
- Restriction of the movement of the soft palate
- A budlike shrunken uvula
- Thinning and stiffening of the lips
- Pigmentation of the oral mucosa
- Dryness of the mouth and burning sensation (stomatopyrosis)
- Decreased mouth opening and tongue protrusion
4. Causes
Dried products such as paan masala and gutkha have higher co prevalencentrations of areca nut and appear to cause the disease. Other causes include:betel quid
- Immunological diseases
- Extreme climatic conditions
- Prolonged deficiency to iron and vitamins in the diet
5. Pathogenesis
"Exposure to areca nut (ArecaBQ) catechu) containing products with or without tohewing, tobacco (ANCP/T) is currently believed to lead to OSF in individuals with genetic immunologic or nutritional predisposition to the disease."[9] Thsmoking and alcohol drinking habis hypersensitivity reaction results in a juxta-epithelial inflammation that leads to increased fibroblastic activity and decreased breakdown of fibers. The fibroblasts are phenotypically modified, and the fibers they form are more stable, produce thicker bundles that progressively become less elastic. once the original loosely arranged fibrous tissue is replaced by the ongoing fibrosis, the movability of the oral tissues is reduced, there is loss of flexibility and reduced opening of the mouth. These colls in patients with oral premalignant lesions are about 82.9%, 95% and 22.7%, respectively [4]. OSMF mageny fibers are non degradable and the phagocytic activity is minimized. According to occur at a recent cross sectional study the time taken for return of salivary pH to baseline levels after chewing areca nut containing mixtures is significantly longer in habitual users with OSF when compared to unaffected users.[9]y age but is frequently seen at the age of 21–30 years Prolonged Alkaline pH induces death fetal fibroblast type and replacement by a profibrotic fibroblast.[9]d. Male to female ratio is The patterns of intraoral fibrotic bands produced by alkaline chemical injury mimic those produced by areca nut chewing.[10] Sharma et al.round 11:1 [5], have equated the pathogenesis of OSF to an over-healing wound, to explain its evolution as well as malignant transformation.[10][11] Increaossibly due to intrinsed mechanical stiffness through YAP/TAZ pathway accelerates the malignant transformation of OSF.[12] The atrophi differenc epithelium in OSF has been attributed to the senescence of basal stem cell layer and the development of hyperplastic epithelium through senescence escape.[11][13]s between genders with/without the BQ
6. Diagnosis
Classification
Oral submucous fibrosis is clinically dividhed into three stages:[14]wing habit.
- Stage 1: Stomatitis
- Stage 2: Fibrosis
- a- Early lesions, blanching of the oral mucosa
- b- Older lesions, vertical and circular palpable fibrous bands in and around the mouth or lips, resulting in a mottled, marble-like appearance of the buccal mucosa
- Stage 3: Sequelae of oral submucous fibrosis
- a- Leukoplakia
- b- Speech and hearing deficits
Khanna and Andrade Min 1995 ddeveloped a group classification system for the surgical management of trismus:[15]le-age chewers
- Group I: Earliest stage without mouth opening limitations with an interincisal distance of greater than 35 mm.
- Group II: Patients with an interincisal distance of 26–35 mm.
- Group III: Moderately advanced cases with an interincisal distance of 15–26 mm. Fibrotic bands are visible at the soft palate, and pterygomandibular raphe and anterior pillars of fauces are present.
- Group IVA: Trismus is severe, with an interincisal distance of less than 15 mm and extensive fibrosis of all the oral mucosa.
- Group IVB: Disease is most advanced, with premalignant and malignant changes throughout the mucosa.tumor necrosis factor alpha and keratin 17 are
inteardependent regulators, they could be used as diagnostic makers and a prognostic mirror of oral submucous fibrosis cases[16] the more
7. Treatment
Biopsy screening although necessary is not mandatory most dentist can visually examine the area and proceed with the proper course of treatment.mmonly involved population Treatment includes:
- Abstention from chewing areca nut (also known as betel nut) and tobacco
- Minimizing consumption of spicy foods, including chiles
- Maintaining proper oral hygiene
- Supplementing the diet with foods rich in vitamins A, B complex, and C and iron
- Forgoing hot fluids like tea, coffee
- Forgoing alcohol
- Employing a dental surgeon to round off sharp teeth and extract third molars
- Interprofessional treatment approach [17]
TreatmeInt also includes following:
- The prescription of chewable pellets of hydrocortisone (Efcorlin); one pellet to be chewed every three to four hours for three to four weeks
- 0.5 ml intralesional injection Hyaluronidase 1500 IU mixed in 1 ml of Lignocaine into each buccal mucosa once a week for 4 weeks or more as per condition
- 0.5 ml intralesional injection of Hyaluronidase 1500 IU and 0.5 ml of injection Hydrocortisone acetate 25 mg/ml in each buccal mucosa once a week alternatively for 4 weeks or more as per condition[18]
- Submucosal injections of hydrocortisone 100 mg once or twice daily depending upon the severity of the disease for two to three weeks
- Submucosal injections of human chorionic gonadotrophins (Placentrax) 2-3 ml per sitting twice or thrice in a week for three to four weeks
- Surgical treatment is recommended in cases of progressive fibrosis when interincisor distance becomes less than 2 centimetres (0.79 in). (Multiple release incisions deep to mucosa, submucosa and fibrotic tissue and suturing the gap or dehiscence so created by mucosal graft obtained from tongue and Z-plasty. In this procedure multiple deep z-shaped incisions are made into fibrotic tissue and then sutured in a straighter fashion.)
- Pentoxifylline (Trental), a methylxanthine derivative that has vasodilating properties and increases mucosal vascularity, is also recommended as an adjunct therapy in the routine management of oral submucous fibrosis.[19]
- IFN-gamma is antifibrotic cytokine which alters collagen synthesis and helps in OSF.[20]
- Colchicine tablets 0.5 mg twice a day[21]
- Lycopene, 16 mg a day helps in improvement of OSF[22]
The treiatment of patients with oral submucous fibrosis depends on the degree of clinical involvement.[23] If the disea[6]. BQ componentse is detected at a very early stage, cessation of the habit is sufficient. Most patients with oral submucous fibrosis present with moderate-to-severe disease. Severe oral submucous fibrosis is irreversible. Moderateare suggested to be the major etiologic factors for OSMF and oral submucous fibrosis is reversible with cessation of habit and mouth opening exercise. Current modern day medical treatments can make the mouth opening to normal minimum levels of 30 mm mouth opening with proper treatment.
8. Research
Requamous cell carcinoma (OSCC), due to their content of inflammatory, genotoxicently, scientists have proven that intralesional injection of autologous bone marrow stem cells is a safe and effective treatment modality in oral sub mucosal fibrosis. It has been shown autologous bone marrow stem cell injections induces angiogenesis in the area of lesion which in turn decreases the extent of fibrosis thereby leading to significant increase in mouth opening.[24][25]
9. History
In 1952carcinogenic and fibrogenic factors such as areca nut (AN), lime, arecoline, catechin, catechol, T.Sheikh coined the termpper, distrophica idiopathica mucosa oris to describe and oral fibrosing disease he discovered in five Indian women from Kenya.[26] S.G. Joreactive oxygen shi subsequpently coined the termed oral submucous fibrosis (OSF) fcies (ROS) [1,7–9].
OSMF is an oral potentially malignant disorder (OPMD) carrying risk for malignant transformation. The malignant transformation rate of OSMF is reported to be about 5.7% after 80.9 months of follow-up [4] or 7–13% [10]. In a total of 1774 cases of OSMF and OSCC in Pakistan, 765 (43.12%) cases were OSMF alone, 472 (26.60%) cases were shown to have OSCC with malignant transformation from OSMF, whereas 537 (30.27%) cases had OSCC without OSMF [11]. A 6.8-year follow-up study also elucidated that alcohol consumption is associated with the malignant transformation of patients with oral precancer [12]. Other factors including BQ chewing habit, smoking habit, environmental heavy metal exposure [13], gender, site of lesion and histological features such as epithelial dysplasia, loss of heterozygosity, aneuploidy of DNA and human papillomavirus (HPV) infection are suggested to stimulate the progression and malignant transformation of OSMF [14].
BQ and OSMF—Etiology, Clinical and Histologic Features
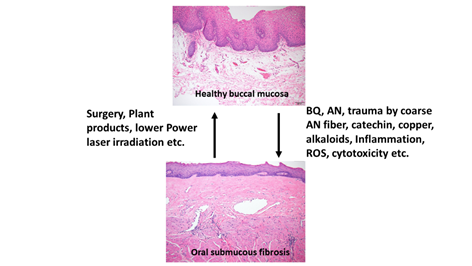
OSMF is a chronic, progressive, high-risk precancerous disease characterized by juxtaepithelial inflammatory reaction, fibrosis of lamina propria, thin parakeratinized squamous epithelium with atrophic change and loss of rete peg. Increased dense collagen fiber deposition in the lamina propria occur through time and, in the end stage, dense hyalinized fibrous tissue occupies the lamina propria and even superficial submucosa, and thus results in varied degrees of mucosal rigidity (Figure 1). OSMF is accompanied by fibroelastic hyperplasia, with/without epithelial hyperplasia/dysplasia over the oral cavity or oropharynx [15,16]. Repeated trauma causes inflammation and aggravates the fibrosis while increased collagen fiber deposition, decreased amount of blood vessels and atrophic change of the epithelium occur. Progression of OSMF, thereby, may lead to loss of tissue mobility, trismus and limited mouth opening [15,16]. However, current treatment strategies are not so effective for attenuation of OSMF. BQ chewing is shown as the major etiologic factor of OSMF [1,2,17]. There are about 200–600 million BQ chewers in the world [1]. The ingredients of BQ vary in different countries. In Taiwan and Papua New Guinea, BQ comprised of AN, and slaked lime with/without inclusion of Piper betle inflorescence or Piper betle leaf (betel leaf) [1,7]. However, in India and Sri Lanka, tobacco is popularly added as one major component of BQ [1]. Among the BQ ingredients, AN components are considered to be the main causative factors in the disease process of OSMF. This is because OSMF is widespread in Taiwan where tobacco is not added into BQ. The roles of lime, betel leaf and other ingredients in the pathogenesis of OSMF await further clarification. AN contains mainly alkaloids (such as arecoline, arecaidine, guvacoline, and guvacine), catechol, catechin, transition metals (Copper, Iron) and fibers [1,7]. The contributory role of AN components to the pathogenesis of OSMF is closely associated with the induction of ROS production [18], chronic mucositis, ulcers caused by mechanical trauma from coarse AN fibers [19,20], activation of the coagulation system [21], cytotoxicity to oral epithelial cells [8], stimulation of fibroblast proliferation/contraction [22,23], collagen synthesis/deposition [22,24], myofibroblast differentiation [10], tissue inflammation [9,25] and the inhibition of collagen degradation and phagocytosis [26,27].These AN components include AN extract (ANE), areca alkaloids (arecoline, arecaidine, guvacoline, guvacine), catechin, catechol and copper (Figures 1 and 2). However, only about 1% to 2% of BQ chewers develop OSMF, suggesting the presence of some predisposition factors toward mild or severe OSMF in these affected patients [17].
Figure 1. Proposed contributing factors (betel quid [BQ], areca nut [AN], coarse fiber of AN, arecoline, catechin, catechol, reactive oxygen species [ROS], inflammation etc.) for oral submucous fibrosis (OSMF) and the treatment methods (surgery, natural products, low power laser irradiation, enzymes, corticosteroid, vasodilator, antioxidants) for OSMF. The histologic pictures of (upper panel) normal buccal mucosa and (lower panel) late stage of OSMF. (H&E stain, A & B: 100×). Normal buccal mucosa is covered by nonkeratinized squamous epithelium with few short rete ridges and supported by lamina propria, which is composed of mainly loose fibrous connective tissue with some collagen fibrils dispersed. In contrast to normal mucosa, the mucosa in late stage OSMF patients shows thin parakeratinized squamous epithelium with atrophic change and a flat junction between epithelium and connective tissue. Dense collagen fiber deposition to hyalinized fibrous tissue involving lamina propria and superficial submucosa is evident. Some chronic inflammatory cell infiltration is frequently seen.
Genetic Susceptibility and Expression in Tissue/organ Fibrosis
A number of studies have found the association of genetic susceptibility with tissue/organ fibrosis such as pulmonary fibrosis, systemic sclerosis, liver and kidney fibrosis [28–31]. While exogenous factors such as viral hepatitis and alcohol abuse are the common causative factors of liver fibrosis, genetic predisposition may contribute to the progression of fibrosis, cirrhosis, liver failure or hepatic carcinoma [28]. Environmental factors are the key etiologic factors of lung fibrosis, but genetic factors in host defense, aging/senescence and cell-cell adhesion may also increase the risk of pulmonary fibrosis, subsequent disease progression and poor prognosis [30]. Systemic sclerosis as an autoimmune disease may involve vascular abnormalities, immune alterations and fibrosis of skin and other internal organs, where tissue inflammation and genetic susceptibility are present [31].
Several factors including nutritional deficiency, vitamin deficiencies and hypersensitivity to various dietary constituents, may also play a part in the pathogenesis of OSMF. Epidemiological studies strongly indicate that AN is the major etiologic agent that releases alkaloids to promote fibroblastic proliferation and increase collagen formation. However, only a small population of BQ chewers develop the disease, indicating that difference in genetic susceptibility plays a role in this event [32]. About 7–12% of OSMF cases progress into oral malignancy [33]. Additionally, a few individuals developed the disease after only a few contacts with BQ [34]. Therefore, the relationship between gene and OSMF is still uncertain and awaits clarification.
BQ and Collagen Turnover
3.1. Cor llagen-Relathe condition in 1953.[27]
ed Genes
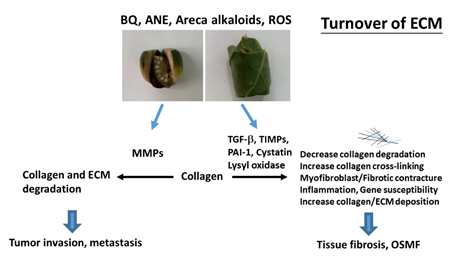
The extracellular matrix (ECM) provides a three-dimensional scaffold for cells via connection of cell surface receptors with various ECM components such as collagen, fibronectin, elastin and nonfibrillar proteins including proteoglycan, hyaluronan and glycoproteins [35]. Hypoxia and collagen-rich conditions also intensify cancer progression [36]. Impairment of ECM and ECM-cell interaction play important roles in various diseases such as osteoarthritis, fibrosis, cancer and genetic diseases [35]. OSMF is such a collagen-related disorder, with dense collagen deposition in the oral submucosa as its main characteristic feature. It has been found that fibroblasts from buccal mucosa exposed to areca alkaloid, due to BQ chewing, may result in the accumulation of collagen [22]. Therefore, it is hypothesized that collagen-related genes might play a role in OSMF pathogenesis. Transforming growth factor-β1 (TGF-β1), lysyl oxidase (LOX), cystatin (CST3), plasminogen activator inhibitor-1 (PAI-1), matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) proved to be involved in the turnover of ECM, wound healing, tumor invasion and metastasis (Figure 2) [37,38]. However, few studies have compared protein expression and polymorphisms of the collagen-related genes situated on different chromosomes between OSMF patients and healthy controls. Some noninvasive markers (serum markers, urinary markers and image tissue stiffness markers) have been developed for evaluation of organ fibrosis, but still with some limitations [39]. Chiu et al. (2002) suggested that polymorphisms of collagen-related-genes can serve as markers of disease susceptibility in patients with OSMF [40]. Further studies on the development of noninvasive early disease markers of OSMF are crucial for disease prevention and treatment in the future.
Figure 2. Molecular mechanisms of changes in turnover of extracellular matrix (ECM) of OSMF and tumor invasion. Collagen and ECM deposition are stimulated by transforming growth factor-β1 (TGF-β1), tissue inhibitors of metalloproteinases (TIMPs), lysyl oxidase, cystatin, and plasminogen activator inhibitor-1 (PAI-1). These events may contribute to decreased ECM degradation, increased collagen cross-linking/stability and increased collagen/ECM deposition, leading to tissue fibrosis and oral submucous fibrosis (OSMF). ECM degradation is mediated mainly via metalloproteinases (MMPs) and contributes to tumor invasion/metastasis.