A new epoch is emerging with intense research on nutraceuticals, i.e., "food or food product that provides medical or health benefits including the prevention and treatment of diseases", such as Alzheimer's disease (AD). Nutraceuticals contain bioactive principles which act at different biochemical and metabolic levels and there's a growing body of evidence which shows their potent neuroprotective effects, offering thus alternative avenues for a modern innovative medicine in human beings; in particular, these natural compounds are able to provide defence against mitochondrial damage, oxidative stress, toxicity of β-amyloid peptide and tau protein and cell death. More recently, preclinical and clinical studies have demonstrated that a wide range of these substances can also differentially influence the composition of the intestinal microbiota in correlation with the cerebral formation and aggregation of toxic protein aggregates. Further, the routes of interaction between epigenetic mechanisms and the gut–brain axis have been also elucidated, establishing a modulatory role of diet-induced changes on commensal microbial community in shaping the brain function and integrity.
- Alzheimer
- nutraceuticals
- brain
- cell death
- health
- diet
- gut microbiota
- epigenetics
1. Introduction
Fruits and vegetables are largely accepted to be "healthy foods" since they contain bioactive principles of demonstrated efficacy and health security which are undoubtely valuable antagonists of the physiological process of aging. Besides, these natural compounds have been also proved to come in handy in struggle for several age-associated neurodegenerative diseases, including Alzheimer's Disease (AD). Epidemiological studies and double-blind medical trials have confirmed that "functional food" exerts a beneficial impact in human health and, in this framework, every specialist (oncologist, cardiologist, angiologist, neurologist, diabetologist and others) shares this point of view when recommend consuming at least five portions a day of both fruit and vegetables [1].
AD is an age-related, heterogeneous disorder characterized by progressive deterioration of cognitive functions because of selective loss in specific neuronal population located into cerebral limbic regions which control important processes of memory and learning. The diagnosis of AD before age 65 is rare and occurs only into a small percentage of people (2–5% of all cases with autosomal dominant familial forms) which harbor causative genetic mutations in relevant genes, such as Amyloid Pecursor Protein (APP), PreSenilin 1 e 2 (PSEN1, and PSEN2) [2]. Incidence of AD is known to increase with age, and age is the most important risk factor for the development of AD. However, other genetic (for instance, Apoliprotein APOEε4 allele) and non-genetic risk factors depending on lifestyle and the surrounding environment can be also responsible of how our brain changes with declining years [3][4]. Thus, we cannot ignore our parental genetic heritage or the passing time, but we can control some other influencing aspects. Eating wholesome helps delay and/or prevent AD, but a diet that will likely keep this disease away is almost the same that will keep your heart healthy, low cholesterol level, cancer at bay and your glucose levels balanced. In a broad sense: how to eat to live up to 100 years [5][6]. What has been said so far could explain why Popeye is still fit and strong at almost 90 years of age: eating spinach, cabbage and other green leafy vegetables slows down the physio-pathological brain aging and consequent cognitive deterioration. In addition, specific dietary regimens are becoming a widespread curative strategy for AD patients [7][8][9].
Among the vertebrate organs, the high energy-consuming brain, that serves as the center of the nervous system, is particularly susceptible to oxidative damage due to its elevated metabolic activity and to the presence of abundant oxidizable material, in particular the lipid milieu of biomembranes which in large part characterize the shape of neuronal cells and support their biological functions. Therefore, antioxidant foods could have positive effects on neural physiology and metabolism [10][11][12]. To this regard, various types of berries have strong properties of fighting free radicals in our body due to the high presence of tannins, anthocyanins and phenols whose regular intake increases the hippocampal synaptic plasticity and favours an improved performance in the processes of learning and memory. Alpha lipoic acid, which is present in vegetables such as spinach and broccoli, is also important for maintaining the energy homeostasis of mitochondria and stimulating the cerebral activities. Green and black tea, both rich in antioxidants, contain epigallocatechin gallate which reduces the formation of the deleterious aggregates of b-amyloid peptide (Ab) accumulating into brains of AD-affected subjects. Even eggs, as primary source of numerous nutrients including vitamin B6, vitamin B12, choline and folic acid, may help us maintain the brain healthy and properly functioning [13][14][15][16][17][18]. Turmeric, a curcumin (CUR)-rich spice responsible for the yellow color of curry, is able to reduce the memory deficits occurring with the clinical manifestation of AD not only by exerting anti-inflammatory and anti-oxidant activities but also by disaggregating the neurotoxic Ab inclusions. Interestingly, red wine would be more effective than white one because it contains more resveratrol (RSV) with potent anti-oxidant property [19][20][21]. Coconut oil, in addition to Extra Virgin Olive Oil (EVOO), the typical component of the Mediterranean diet (MD), are also beneficial in counteracting AD by protecting against the Ab-induced citotoxicity [22][23]. The salutary role of MD has been long recognized but, recently, it has become even more famous mainly due to the experimental observation that this dietary regimen is able to specifically modulate the "gut microbiome". To this regard, a new study [24] have found that MD successfully modifies the commensal microbita by increasing the abundance of distinct taxa in the digestive tube of elderly subjects that, in turn, show an improved cognitive performance, likely due to reduction in activation of relevant pro-inflammatory markers.
It was Stephen Defelice, chairman of the American organization known as Foundation for Innovation in Medicine, who first coined the term ‘nutraceutical'. This word derives by the crasis between "nutrition" and "pharmaceutical", to indicate food components which not only have beneficial effects on our health but can also be employed in pharmacological-like therapeutic approaches for a broad range of illnesses in humans beings [25][26]. Nutraceuticals, once absorbed and assimilated in the intestinal tract, strongly increase our resistance to harmful environmental agents and, therefore, behave just as drugs with preventive and/or adjuvant action(s) in the healing phase [27]. Mainly present in fruits and vegetables, they are abundantly produced by plants as a natural defense mechanism against predators (i.e. insects) and exhert healthy effects on human beings, even at low dosage [28][29]. Interestingly and more importantly, innovative studies in molecular genetics, epitranscriptomics and molecular biology point to the exciting possibility that the consequences of diet on mental health may be even passed on through generations. Although the molecular mechanisms underlying the "epigenetic"effects of nutritional regimen are still poorly understood, the possibility for dietary components to modulate non-genetic events inducing heritable phenotypic changes, opens thus for the mankind a novel and attractive way of intervention helpful in the management, not just in the control, of several pathologies including the neurological diseases such as AD [30][31].
2. Nutraceuticals and ageing in physiology and pathology: Overview
That food is able to prevent and, even, counteract the disease(s) is a belief proclaimed by our ancestors over the centuries. Since ancient times, the wise Solomon said "Eat honey, my son, because it is good" (Old Testament, proverb 24:13), stressing out the primary importance of honey in nutrition, as a potent remedy for health problems. Similarly ginseng, whose use in China dates back to 2000 years ago, has always been considered a traditional drug as well as cinnamon that, in Ancient Egypt, was appraised as more valuable than gold [27]. Nowadays, the quality of life in terms of income, expenditure and lifestyle has greatly improved on the thrust of economic development. However, in the current historical period, the nutritional regimen [32]: (i) keeps on having a great influence on health of human beings, especially in their later stages of life; (ii) is an important modifiable factor for disease prevention and progression, by contrasting the causative processes associated with its manifestation and by minimizing the potentially-related adverse complications. The worldwide acceptance of these assumptions has seen a boost in the recent market of nutraceuticals on the basis of their in vivo efficacy in lengthening life expectancy, improving quality of life in elderly and -last but not least- containing the overall cost of health care [33]. The biological roles of "functional food" components can vary depending on their metabolites and the exerted effects can be influenced by the gut microbiota itself and can even change from one individual to another [34].
The majority of nutraceuticals are phytochemicals or phytonutrients, natural chemicals mainly contained in foods of plant origin (phyto derives from the Greek ϕυτόν and means plant). These natural substances with nutritional value are endowed with protective properties for the human body owing to their antioxidant, anti-estrogenic, anti-inflammatory, immunomodulatory and anticarcinogenic activities [34]. Moreover nutrients, although exhibit a low potency as bioactive compounds when compared to drugs, can have a significant long-term actions on living organisms, especially in view of the consideration that they are frequently consumed in large amounts as part of their daily diet [35]. Phytonutrients or phytochemicals act against chronic inflammation and are useful in preventing the occurrence of chronic diseases, such as arthritis and atherosclerosis. Among them, flavonoids and carotenoids are the most renowned. These bioactive compounds can also impact on the intestinal environment, by indirectly modulating the composition of the inhabiting local microorganisms or "microbiota". The absorption of phytochemicals is poor and most of the ingested products reach the colon where they are broken down by the intestinal microflora to produce different metabolites. For instance, the transformation of soy isoflavones into equol and desmethyl angolesin, the transformation of hop isoxanthohumol to make prenylnaringenin (much more estrogenic), the transformation of anthocyanins and procyanidins into phenyl acetic and phenyl propionic metabolites, or the transformation of ellagic acid in urolithins are good examples. These metabolic processes are largely influenced by the nature and the abundance of intestinal microflora [36]. Depending on the microorganism strain present in the colon, an individual can be a "producer of equol" or a "producer of non-equols", a "producer of urolithin" or "producer of non-urolithin" and as a consequence, the activity of the phytochemical taken up with the diet can greatly vary [37]. Consistently, the differences in colon microflora between individuals help explain the large inter-individual variability and discrepancies in the outcomes of clinical trials.
However, when dealing with nutraceuticals, it should be kept in mind that the compounds under investigation refer to the original phytochemicals (i.e. those present in the plant and not to the relevant metabolites produced during the digestion and adsorption processes in vivo). It is very intuitive that any extrapolation to human of the preclinical results obtained in animal models is not appropriate due to their distinct physiology and to the differences in specie-specific bioavailability and metabolism of the active compounds. These gaps, which also occur between animal models close in the phylogenetic tree (i.e. between mice and rats), can account for some contradictory experimental results. Furthermore, although the efficacy of several functional foods has been extensively investigated by means of numerous animal and in vitro studies and finally validated, clinical trials in humans turned out to be scarce and inconclusive. To this regard, it's worth noticing that any chemical alteration of the original bioactive compound, occurring during storage and/or digestion, may severely modify the bioavailability as well as the bioactivity of the compound itself. To clarify this concept, it should be kept in mind that, even if the antioxidant activity of a compound is proved in vitro, this quality indicator is not always predictive of its ‘goodness' in vivo. For instance, the high antioxidant power of pomegranate ellagitannins (ETs) makes these natural compounds the most powerful in vitro scavengers of harmful reactive oxygen species (ROS) released by cell metabolism [38]. However, in vivo, ETs are not absorbed and are extensively metabolized by the colon microflora to urolithins which, on the contrary, are no more endowed with their original antioxidant capacity [39]. In addition, the studies investigating the biological activity of phytochemicals have generally been performed using in vitro human cultured cells and/or animal models; thus, the experimental concentrations tested are often unrealistic with respect to the physiological in vivo situation. Moreover, as already stated, the assayed compounds are the phytochemicals originally present in the plant and not the relevant metabolites produced in vivo (see the above pomegranate ellagitannins example). Thus, it is not surprising that results of the human studies often show lack of consistency with a large inter-individual variability. Besides, differences in the chemical composition of tested nutraceuticals and in their pharmaceutical forms of choice (pills, capsules, gels, etc.) -which are known to largely influence the compounds stability and bioavailability– along with the variability in physiological status of enrolled volunteers should be also taken into account. Again, the conversion of phytochemicals into metabolites is strongly affected by the nature and size of the colon microflora. Thus, it follows that the colon microflora per se creates intrinsic divergences among different individuals and can be responsible for the large inter-individual variability causing discrepancies in the outcomes of on-going clinical trials.
Onion, garlic, grapes, rosemary, broccoli, spinach, turmeric, parsley, etc. possess considerable antioxidant activities [38][40] and prevent several age-dependent neurodegenerative diseases, including AD [41]. For example, the odorless aged garlic extract (AGE) which is frequently used in culinary and medicine contains antioxidant chemicals that ward off the ROS damage [42]. Several studies showed that the administration of aged garlic extract can significantly improve the memory deficits by several pathways: (i) by protecting against Aβ-induced neuronal damage [42]; (ii) via reduction of the hyperphosphorylation of tau protein by modulating the activity of glycogen synthase kinase-3β (GSK3b) [42]. From a translational point of view, the neuroprotective effects of AGE on AD-related cognitive dysfunction are of particular relevance because of its ability of passing through the blood–brain barrier (BBB). Conversely, another bioactive compound such as curcumin, which also appears to exert beneficial effects in reducing the signs of AD [27], meets a limited therapeutic use because of its low solubility and poor oral bioavailability [27].
3. Alzheimer's Disease (AD): Molecular Aspects and Causes of the amnestic disorder
Post-mitotic differentiated neurons make us, more or less, what we are and allow us to carry out all the activities of daily life. Therefore, it is not surprising that any damage to brain cells can affect a wide range of the neurological processes [43], including:
-remembering (both short and long term) and recognizing,
-speaking and writing,
-making decisions and solving problems,
-interpreting sensory input,
-orientating and juggling yourself in the world.
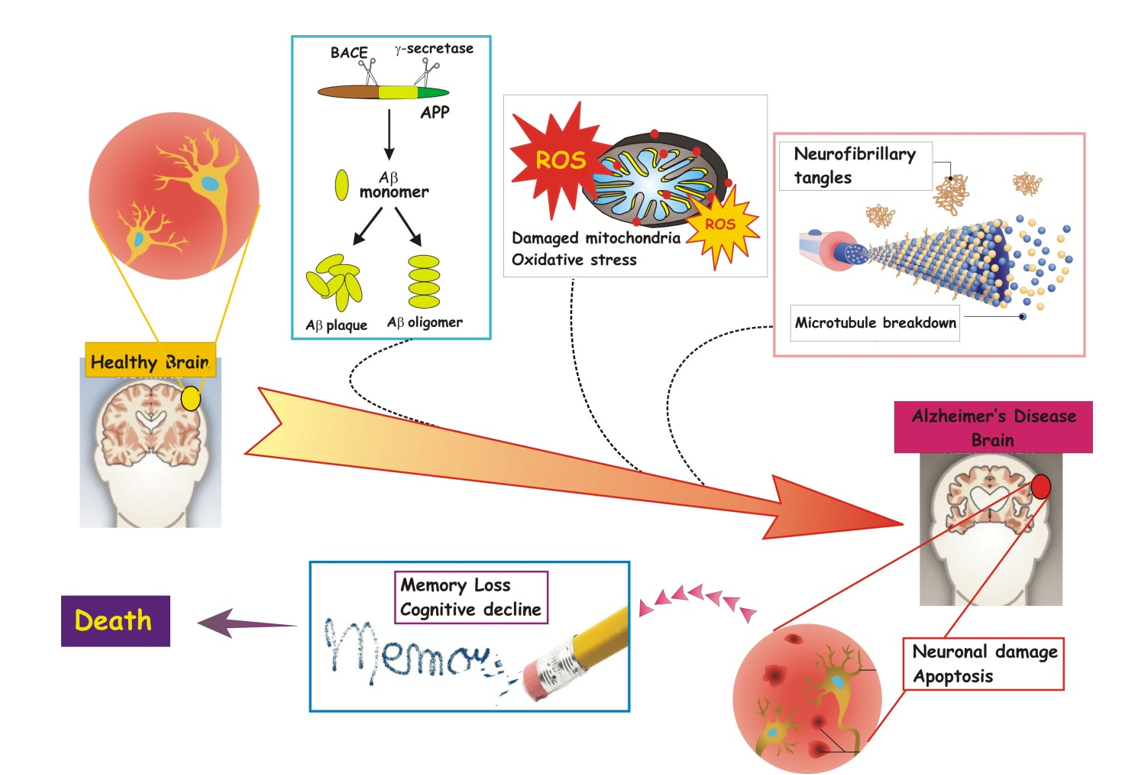
In particular, when insults/injuries cause damage and neurons are not able to compensate enough by activating endogenous homeostatic mechanisms (Figure 1), they die.
Figure 1. Major events, namely oxidative stress and mitochondrial dysfunction, toxicity and aggregation of Aβ peptide(s) and tau protein, neuronal damage and apoptosis, memory loss and cognitive decline, characterizing the onset and progression of AD until death.
As the incidence and prevalence of AD are constantly increasing worldwide due to the progressive ageing of hearth population, in the last years the comprehension of molecular and biochemical mechanisms underlying its etiopathogenesis is gaining the attention of scientifc community. Amnestic AD is a progressive age-related neurodegenerative disease that is clinically characterized by the deterioration of memory, executive function, language and social behavior. Intracellular NeuroFibrillary Tangles (i.e. NFTs) composed of abnormally-phosphorylated tau protein (ptau) and extracellular plaques (i.e. senile plaque, SP) made of Ab species resulting from cleavage of the transmembrane Amyloid Precursor Protein (APP) are the two hallmark neuropathological inclusions found in the AD brain. In particular, these insoluble proteinaceous lesions are distributed all around the hippocampus of affected subjects, a cerebral area which is crucially involved in learning and memory processes. The protein deposition of ptau and Aβ induces neuronal death (i.e. apoptosis) and impairs the normal physiological functions of several cerebral circuits. The etiology of this complex neurodegenerative disease is greatly influenced by reciprocal interaction between environmental factors and genetic determinants [44]. Gene mutations are responsible for about 10% of the AD cases but this percentage can reach 100% when the disorder occurs before age 40-50. In particular, these early-onset forms are caused by mutations in three genes that encode specific proteins, i.e. Amyloid Precursor Protein (APP) on chromosome 21, presenilin-1 (PSEN1) on chromosome 14 and presenilin-2 (PSEN2) on chromosome 1 [45]. Instead, several risk factors can contribute to increase the individual susceptibility of developing the late-onset forms. Among them, it's worth mentioning a genetic variant of apoliprotein E (APOE) gene located on chromosome 19 (i.e. APOEe4 allele) which encodes a protein playing an important function in cholesterol trafficking and metabolism [46][47][48]. No AD-associated mutations are found in the gene encoding for tau, a Microtubule-Associated-Protein (MAP) which promotes the dynamic stability of microtubule cytoskeleton. Of note, the microtubule assembly depends partially on the phosphorylation state of tau proteins: phosphorylated tau proteins are less effective than nonphosphorylated tau on microtubule polymerization. Alterations in metabolism of tau drastically reduce its intrinsic affinity of binding and/or stabilizing tubulins' heterodimers, resulting thus in collapse and disintegration of microtubles. The breakdown of microtuble network leads to disruption in axonal cargo transport, an active mechanism required in neurons for maintaining their polarized morphology and for accurately trafficking specific cargoes towards different signaling pathways. Mitochondrial damage and oxidative stress, overload of calcium, synaptic loss, plasticity changes, reduction in neuronal connectivity and, finally, cell death further contribute to the progressive neural system failure that occurs over decades during the AD stage progression [49]. Nevertheless, although we know the molecular nature of the causative gene mutations and risk factors involved, we still lack the knowledge of the intimate interplay by which Ab peptide(s) and tau protein operate in causing the early synaptic dysfunction and, later on, the anatomopathological changes first identified by Alois Alzheimer in the brain of AD-affected subjects.
4. Nutraceuticals for Neuroprotection and Treatment of Alzheimer's Disease (AD)
Cholinergic deficiency is associated with AD, and various acetylcholinesterase inhibitors have been developed for its treatment. However, their poor efficacy in stopping and/or delaying the progression of disease symptomathology supports the idea that only an early diagnosis and, then, a timely treatment can really safeguard the decline of cognitive functions when the neuronal decay is not widespread and irreversible. The disease onset occurs many years before symptoms truly appear, offering thus an useful therapeutic "window" for effective medical interventions [50]. Considering that multiple factors -genetic, environmental, dietary, or a combination of them- are all crucial initiators and/or contributing factors of disease onset/progression, it is assumed that a certain threshold needs to be surpassed before clinical manifestations arise. That is why the scientific research is greatly focused on the prevention and, in this framework, healthy dietary regimen can come in handy.
For instance, the polyphenols, flanked by the colored carotenoids and other bioactive compounds belonging to plant kingdom, have strong positive effects on neuronal metabolism and brain health maintenance and show the ability to inhibit the activity of β- and γ-secretases and to increase the Aβ degradation [51]. Likewise, mono- (MUFA) and polyunsaturated fatty acids (PUFA, ω-3 and ω-6) -whose main food sources are fish and vegetable oils- and the B vitamins, folic acid, vitamins B6 and B12 are also all substances essential for normal neuronal functioning with beneficial effects on poor cognitive and behavioral performances characterizing the AD symptomatology [52][53]. Besides, polyphenols are endowed with potent effect on oxidative stress occurring in many neurodegenerative diseases, including AD which is characterized by high production of deleterious ROS [32]. Polyphenols are commonly present in fruits, vegetables, cereals, olives, dry legumes, licorice, chocolate and beverages, such as tea, coffee and wine [28][54]. Flavonoids are present in particular foods, such as flavanones in citrus fruits [55], isoflavones in soy [56][57]. Other polyphenols, such as flavan-3-ols (sometimes referred to as flavanols [56] including catechin, epicatechin, epigallocatechin, epigallocatechin gallate) are found in a plethora of vegetable products, as cocoa, chocolate, black and green tea and grapes. Epigallocatechin gallate (EGCG), a polyphenol found in green tea, in vivo decreases the β- and γ-secretase activities and enhanced the α-secretase cleavage of APP, leading to the reduction of Aβ levels with consequent improvement in the memory abilities. Besides EGCG inhibits the in vitro tau aggregation and increased the in vivo clearance of phosphorylated tau. Ferulic acid, a phenolic compound naturally present in numerous fruits and vegetables, is a strong antioxidant and anti-inflammatory compound and reduces in vivo the Aβ production by downregulating the β-secretase activity. Resveratrol, a naturally occurring non-flavonoid polyphenol present in grapes (Vitis vinifera L. (Vitaceae)) and red wine with both anti-inflammatory and anti-oxidant actions, decreased the Aβ levels and plaque levels in AD brain of rats, prevents the tau hyperphosphorylation. Caffeine -which is present in the coffee bean, in some teas and cocoa drinks- has anti-inflammatory and antioxidant properties and reduces the β- and γ-secretase levels, by lowering in vivo the Aβ generation. Studies have revealed that retinoic acid -the metabolite of vitamin A- inhibits the Aβ fibrils formation in vitro and reduces brain Aβ deposition, APP level and tau phosphorylation in AD animal models. β-carotene belonging to the carotenoid family has not only an anti-aggregation activity, by destabilizing Aβ protofibrils, but is also endowed with ability of reducing the production of toxic ROS. Extract of cinnamon (Cinnamomum verum J. Presl., Lauraceae), one of the most used spices, is found to inhibit in vitro tau aggregation and promote the disassembly of its filaments. Docosahexaenoic acid (DHA), a polyunsaturated fatty acid from marine fish and algae, has an antioxidant activity, reduces the Aβ accumulation and plaque burden by downregulating the β- and γ-secretase activities. Finally, noteworthy is extra virgin olive oil (EVOO), the principal ingredient of the MD. In vivo results proved that oleocanthal, which is one of the main active components of EVOO, induces changes in the structure of tau protein, by inhibiting its aggregation and fibrillization, as well as enhances the Aβ clearance, by reducing the amyloid load. An higher adherence to the MD is associated with a trend for reduced risk of developing Mild Cognitive Impairment (MCI) and with reduced risk of MCI conversion to full-blown AD.
5. Nutrients Modulation of Gut Microbiota as Therapeutic Strategy for the Treatment of Alzheimer's Disease (AD)
Microorganisms residing in the human gastrointestinal tract represent a complex ecosystem consisting of around trillions of commensals (such as bacteria, archaea, protozoa and viruses) located in the large intestine. The majority of over 400 bacterial species populating the colon are anaerobes and are present both in the colonic contents and attached to the mucosa. A mutually beneficial relationship occurs between the microorganisms inhabiting the digestive tube and the human host. On one hand, the colonic environment provides nutrients, pH, temperature, and redox potential favorable for the development and growth of the microbial community. On the other hand, the microbial community: (i) acts as a protective barrier against pathogenic bacteria that might colonize the colon; (ii) metabolizes nutrients, drugs and xenobiotics by contributing to the metabolic, nutritional, physiological, and immunological processes of the host.
Although the communities present in our gastrointestinal tract -the "gut microbiota"- are stable, they can be quickly influenced and/or altered by aging, illness as well as by common human external actions/habits such as antibiotic exposure, lifestyle and dietary changes. In this framework, the loss of microbiota homeostasis, or "dysbiosis", is largely accepted to contribute to the clinical progression of several age-dependent neurodegenerative disorders, including AD [58][59][60][61][62][63][64][65][66][67]. An imbalance in intestinal microflora has been found in cognitively-impaired AD patients with dementia compared with healthy, age-matched control subjects [68][69][70][71].
Due to the failure of physiological aging occurring during neurodegeneration, an altered cross-talk between gut microbiota and host occurs with the detrimental shifting toward "unhealthy" population which negatively affects the brain signaling. This situation further exacerbates the disease-associated symptomatology by releasing circulating inflammatory mediators, by activating/modulating oxidative stress and metabolic signaling pathway(s) and by unbalancing the production of key neuroactive molecules. Indeed, gut microbiota is engaged in bidirectional interplay with the Central Nervous System (CNS) by endocrine, neurological and biochemical mechanisms along both direct and indirect pathways. Anatomically, the multimodal and reciprocal gut-brain physiological interaction takes place by means of a complex network including the sympathetic and parasympathetic autonomic nervous system, the hypothalamic–pituitary–adrenal axis system, the immune system and the enteric nervous system. The inflammatory immune mediators such as cytokines and chemokines, the endocrine descending hypothalamic–pituitary–adrenal axis with cortisol secretion, the ascending vagus nerve sensory pathway which connects the intestinal tract to brain, the tryptophan metabolism, the secretion of circulating bacterial metabolites (short-chain fatty acids and neurotransmitters) are all parts of the two-way communication characterizing the Microbiota–Gut–Brain (MGB) axis [72][73][74][75][76].
Krautkramer et al. [77] clarified for the first time the tight connection between diet and metabolites produced by gut microbiome. Briefly, the authors compared the intestinal microbiome of control mice fed on a balanced diet with that of mice fed on an unbalanced diet (i.e. low in fiber and complex carbohydrates and rich in simple fats and sugars). In both cases, the microbiome was found profoundly different. In fact, following the unbalanced diet, animals produced a lower level of some metabolites than those provided with healthier diet (i.e. the short chain fatty acids released upon fermentation of insoluble fibers by microorganisms). Interestingly, results similar to those from mice fed on a balanced diet were detected following administration of water containing short-chain fatty acids to control animals without microbiome, demonstrating thus that metabolites per se are responsible for the observed biological changes. Therefore, nutritional approaches which rely on the diet to modulate the composition and diversity of the intestinal bacterial flora are currently under deep investigation. Preclinical and clinical results from animal models and affected elderly people have recently demonstrated that the modulation of the microbiota population residing into the digestive tube represents a novel, attractive option for the clinical management of AD by protecting against neuroinflammation, cerebral amyloidosis, tau deposition, neurotransmission imbalance, oxidative stress, vascular degeneration and neuronal loss [64][78]. The aim of this approach is to induce the local growth of specific bacterial populations along the luminal/mucosal intestinal tract with the intent of alleviating the clinical and histopathological dementia-related signs [79]. Biotherapy based on daily consumption of natural compounds actually ameliorates the mental health and lifestyle of elderly patients suffering from AD. In particular, the nutraceutics from MD have been shown to be the most potent in promoting a peculiar gut microbial profile [80] which correlates with a reduction of the cerebral Aβ accumulation [81] and with a beneficial effect on AD-associated synaptic demise and cognitive decline [82].
6. Bioactive Compound Actions on Epigenetic Mechanisms in Alzheimer's Disease (AD)
Epigenetics –a scientific term introduced in the late 1930s by the English biologist, geneticist and paleontologist Conrad Hal Waddington— investigates that part of genetics that affects the gene expression, otherwise called "phenotype". Epigenetic changes do not involve changes in the DNA sequence itself but, rather, they involve multiple processes such as DNA methylation, histone code modifications and noncoding RNAs biogenesis which, finally, differently regulate the gene expression [83]. These epigenetic factors can determine the selective "ignition" or "shutdown" of genes, also providing an explanation of how the genetic material can adapt and/or compensate, in a short time, for a variety of external insults and environmental changes. Therefore, the real matter is not so much our genetic code, but rather its dynamic expression resulting from integrated and coordinated combination of multiple and cumulative modifications following interaction with environment stimuli. Epigenetics comes to assume a sort of "positive" and "liberating" value compared to the determinism and "condemnation" of pure genetics. Basically, it's not possible to intervene on genes themselves (or, at least, not completely), but on gene expression. Therefore, two individuals with an identical genotype can develop different phenotypes thanks to the differences in their "epigenome". In this context, exemplary are studies conducted on monozygotic twins demonstrating the presence of an epigenetic drift between subjects which share the same nuclear genotype. Ten years ago, the magazine Time dedicated an article on this topic, by using an amazing title: "Why Your DNA Isn't Your Destiny" [84]. The message is extremely alluring: DNA is not your destiny, so you can do something to change it.
At the beginning of the 2000s, only 3 pathologies were considered as unquestionably linked to epigenetics: Rett syndrome, fragile X syndrome and Immunodeficiency with centromeric instability and facial anomalies (ICF) syndrome [85][86][87]. Nevertheless, in the last decade, experimental evidence has shown that most of the multifactorial diseases, including tumors and neurodegenerative syndromes, are -or could be- induced by epigenetic alterations. An interesting aspect is that, unlike genetic mutations, epigenetic modifications do not strictly involve the nucleotide sequence of DNA itself and, thus are, reversible by their nature. In principle, drugs are able to modify/correct epigenetic defects but not genetic alterations [88]. Interestingly, in this context, one of the natural sources of elements impacting on genome is food. In fact dietary nutrients are able to regulate the cellular metabolism, to make us lose or gain weight, to determine whether we get sick or remain healthy right through differential modulation in expression of our nuclear genes.
Concerning the AD, although it is not still clear whether the epigenetic changes observed in affected patients represent a cause or a consequence of the disease, emerging studies point out that these regulatory mechanisms may be important candidate targets in the treatment of AD [83]. To this regard, by analyzing the prefrontal cortex of AD mouse and deceased human donors, a group of researchers recently identified an epigenetic anomaly which negatively affects the expression of the genes coding for the glutamate receptors [89]. Both in animal models and human AD brains, the stability of these transmembrane channels turned out to be greatly diminished, thus leading to changes in synaptic plasticity and diminution in mnestic capacity. Importantly, the scientist team specifically restored the memory skills by treating the cognitively-impaired AD mice with selective histone methyltransferases 1/2 (EHMT1/2) inhibitors. By reversing the histone hyper-methylation of interest genes, these compounds led to recovery of glutamate receptor expression levels and excitatory synaptic function into animals' prefrontal cortex and hippocampus with consequent improvement in their working/spatial memory performances. These fascinating results open new translational opportunities by demonstrating for the first time that the selective pharmacological targeting of the histone methylation enzymes is able to ameliorate the synaptic and cognitive deficits in AD representing an unlooked-for therapeutic strategy to advance the cure of this heterogeneous and multifactorial neurodegenerative disorder.
More recently, Monti et al. [90] discovered the existence of causal association between an epigenetic alteration in the Presenilin1 (PSEN1) gene and AD. In particular, "non-CpG" methylation was found to leave a specific "imprinting" on PSEN1 gene which ends in its anomalous overexpression. Experimental studies, carried out on a mouse model of AD and in human brain tissue specimens, confirmed a significant inverse relationship between the expression rate of PSEN1 gene and the level of DNA methylation. Consistently, when peripheral blood samples of AD patients were compared with those from healthy subjects, the low level of DNA methylation turned out to be inversely correlated with the high expression of PSEN1. This exciting finding prospects the possibility that the quantitative evaluation of a specific epigenetic modification ("non-CpG" methylation in PS1 gene) in blood or other biological fluids might be a non-invasive biomarker to diagnosize the disease.
In the last year, a growing body of evidence have confirmed the hypothesis that nutrients or other bioactive compounds can, directly and/or indirectly, modify the epigenetic marks of cells by impacting on dynamic chromatin remodeling. For instance, plant polyphenols endowed with potent antioxidant activity [91] are reported to interfere with epigenetic modifications of chromatin [92]. A causal link between diet and epigenetic mechanisms is not only clearly demonstrated for dietary methyl donors (e.i. folate, choline and betaine) [93][94] but is also hypothesized for many other dietary molecules which can in some way alter the genomic organization of the eukaryotic nucleus [14]. Besides, it's important noticing that the epigenetic influence of diet can be mediated, at least in part, by the microbiome whose composition depends on age, as well as on the status of health or disease [30]. In conclusion, these studies indicate that: (i) epigenetic histone modifications, which alter the accessibility of DNA to transcription regulators by inducing changes to the structural configuration of nucleosomes, play a causal role in the development of AD; (ii) the complex interplay occurring between the gut microbiota and several dietary nutrients is amenable of modulation through a personalized diet as an alternative and effective strategy for clinical management of AD.
References
- Bergamini, E. Nutraceuticals: a valuable aid to be used cautiously. G. Gerontol. 2010, 58, 255-258.
- Bekris, L.M.; Yu, C.E.; Bird, T.D.; Tsuang, D.W. Genetics of Alzheimer Disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 213–227; doi: 10.1177/0891988710383571.
- Cole, J.H.; Marioni, R.E.; Harris, S.E.; Deary, I.J. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Mol. Psychiatry 2019, 24, 266–281; doi: 10.1038/s41380-018-0098-1.
- Small, G.W.; Ercoli, L.M.; Silverman, D.H.; Huang, S.C.; Komo, S.; Bookheimer, S.Y.; Lavretsky, H.; Miller, K.; Siddarth, P.; Rasgon, N.L.; Mazziotta, J.C.; Saxena, S.; Wu, H.M.; et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. PNAS 2000, 97, 6037-6042; doi: 10.1073/pnas.090106797.
- Brown, G.C. Living too long: the current focus of medical research on increasing the quantity, rather than the quality, of life is damaging our health and harming the economy. EMBO Rep. 2015, 16, 137–141; doi: 10.15252/embr.201439518.
- Crimmins, E.M. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist. 2015, 55, 901–911; doi: 10.1093/geront/gnv130.
- Barnard, N.D.; Bush, A.I.; Ceccarelli, A.; Cooper, J.; de Jager, C.A.; Erickson, K.I.; Fraser, G.; Kesler, S.; Levin, S.M.; Lucey, B.; Morris, M.C.; Squitti, R. Dietary and lifestyle guidelines for the prevention of Alzheimer's disease. Neurobiol Aging. 2014, 35, Suppl 2, S74-S78; doi: 10.1016/j.neurobiolaging.2014.03.033.
- Cremonini, A.L.; Caffa, I.; Cea, M.; Nencioni, A.; Odetti, P.; Monacelli, F. Nutrients in the Prevention of Alzheimer's Disease. Oxid Med Cell Longev. 2019, 2019, 9874159; doi: 10.1155/2019/9874159.
- Amini, Y.; Saif, N.; Greer, C.; Hristov, H.; Isaacson, R. The Role of Nutrition in Individualized Alzheimer’s Risk Reduction. Curr. Nutr. Rep. 2020, 9, 55–63; doi: 10.1007/s13668-020-00311-7.
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, D.M. Antioxidant Therapies for Neuroprotection-A Review. J. Clin. Med. 2019, 8, 1659; doi: 10.3390/jcm8101659.
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid Med Cell Longev. 2019, 9, 2019, 2105607; doi: 10.1155/2019/2105607
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583; doi: 10.3390/molecules24081583.
- Subash, S.; Essa, M.M.; Al-Adawi, S.; Memon, M.A.; Manivasagam, T.; Akbar, M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regen Res. 2014, 9, 1557-1566; doi: 10.4103/1673-5374.139483.
- Dos Santos, S.M.; Romeiro, C.F.R.; Rodrigues, C.A.; Cerqueira, A.R.L.; Monteiro, M.C.; Mitochondrial Dysfunction and Alpha-Lipoic Acid: Beneficial or Harmful in Alzheimer’s Disease? Oxid Med Cell Longev. 2019, 2019, 8409329; doi: 10.1155/2019/8409329.
- Cascella, M.; Bimonte, S.; Muzio, M.R.; Schiavone, V.; Cuomo, A. The efficacy of Epigallocatechin-3-gallate (green tea) in the treatment of Alzheimer’s disease: an overview of pre-clinical studies and translational perspectives in clinical practice. Infect Agent Cancer. 2017, 12, 36; doi: 10.1186/s13027-017-0145-6.
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch Toxicol. 2019, 93, 2491-2513; doi: 10.1007/s00204-019-02538-y.
- Colizzi, C. The protective effects of polyphenols on Alzheimer's disease: A systematic review. Alzheimers Dement (NY). 2018, 5, 184-196; doi: 10.1016/j.trci.2018.09.002.
- Moretti, R.; Peinkhofer, C. B Vitamins and Fatty Acids: What Do They Share with Small Vessel Disease-Related Dementia? Int J Mol Sci. 2019, 20, 5797; doi: 10.3390/ijms20225797.
- Mazzanti, G.; Di Giacomo, S. Curcumin and Resveratrol in the Management of Cognitive Disorders: What Is the Clinical Evidence? Molecules. 2016, 21, 1243; doi: 10.3390/molecules21091243
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front Nutr. 2016, 3, 31; doi: 10.3389/fnut.2016.00031.
- Reale, M.; Costantini, E.; Jagarlapoodi, S.; Khan, H.; Belwal, T.; Cichelli, A. Relationship of Wine Consumption with Alzheimer’s Disease. Nutrients. 2020, 12, 206; doi: 10.3390/nu12010206.
- de la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Sancho Cantus, D.; Rochina, J.M.; Aguilar, M.A.; Hu Yang, I. Improvement of Main Cognitive Functions in Patients With Alzheimer's Disease After Treatment With Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J Alzheimers Dis. 2018, 65, 577-587. doi: 10.3233/JAD-180184.
- Omar, S.H. Mediterranean and MIND Diets Containing Olive Biophenols Reduces the Prevalence of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 2797; doi: 10.3233/JAD-180184.
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; Zoetendal, E.G.; Hermes, G.D.A.; Elodie, C.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228; doi: 10.1136/gutjnl-2019-319654.
- DeFelice, S.L. FIM Rationale and Proposed Guidelines for the Nutraceutical Research & Education Act - NREA, November 10, 2002. Foundation for Innovation in Medicine. Available at: https://fimdefelice.org/fim-rationale-and-proposed-guidelines-for-the-nutraceutical-research-education-act-nrea/
- Kalra, E.K. Nutraceutical - Definition and Introduction. AAPS PharmSci. 2003, 5, E25; doi: 10.1208/ps050325.
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent Pat Drug Deliv Formul. 2019, 13, 105-156; doi: 10.2174/1872211313666190503112040.
- Pandey, K.B.; Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009, 2, 270–278; doi: 10.4161/oxim.2.5.9498.
- Lelario, F.; Scrano, L.; De Franchi, S.; Bonomo, M.G.; Salzano, G.; Milan, S.; Milella, L.; Bufo, S.A. Identification and antimicrobial activity of most representative secondary metabolites from different plant species. Chem. Biol. Technol. Agric. 2018, 5, 13; doi: 10.1186/s40538-018-0125-0.
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int J Mol Sci. 2018, 19, 3425; doi: 10.3390/ijms19113425.
- Franzago, M.; Santurbano, D.; Vitacolonna, E.; Stuppia, L. Genes and Diet in the Prevention of Chronic Diseases in Future Generations. Int. J. Mol. Sci. 2020, 21, 2633; doi:10.3390/ijms21072633.
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J Pharm Biomed Anal. 2019, 25, 175, 112774; doi: 10.1016/j.jpba.2019.07.022.
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci Tech 2013, 31, 118–129; doi: 10.1016/j.tifs.2013.03.006
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol Res. 2010, 61, 219-25; doi: 10.1016/j.phrs.2009.11.001.
- Tanna, B.; Mishra, A. Metabolites Unravel Nutraceutical Potential of Edible Seaweeds: An Emerging Source of Functional Food. Compr. Rev. Food Sci. F. 2018, 17, 1613-1624; doi: 0.1111/1541-4337.12396.
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008; doi: 10.1016/j.phytochem.2007.09.014.
- Cerdà, B.; Tomàs-Barberàn, F.; Espìn, J.C. Metabolism of chemopreventive and antioxidant ellagitannins from strawberries, raspberries, walnuts and oak-aged wines in humans: identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235.
- Gil, M.I.; Tomàs-Barberàn, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589; doi: 10.1021/jf000404a.
- Cerdà, B.; Espìn, J.C.; Parra, A.; Martìnez, P.; Tomàs-Barberàn, F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolized into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur. J. Nutr. 2004, 43, 205–220; doi: 10.1007/s00394-004-0461-7.
- Kaur, S. Free radicals and antioxidant (nutraceuticals). Book to human health. Int. J. Nat. Product Sci. 2012, 1,175
- Dutta, S.; Ali, K.M.; Dash, S.K.; Giri, B. Role of nutraceuticals on health promotion and disease prevention: a review. J. Drug Deliv.Ther. 2018, 8, 42-47; doi: 10.22270/jddt.v8i4.1759.
- Song, H.; Cui, J.; Mossine, V.V.; Greenlief, C.M.; Fritsche, K.; Sun, G.Y.; Gu, Z. Bioactive components from garlic on brain resiliency against neuroinflammation and neurodegeneration (Review). Exp Ther Med. 2020, 19, 1554-1559; doi: 10.3892/etm.2019.8389.
- Abate, G.; Marziano, M.; Rungratanawanich, W.; Memo, M.; Uberti, D. Nutrition and AGE-ing: Focusing on Alzheimer’s disease. Oxid Med Cell Longev. 2017, 2017, 7039816; doi: 10.1155/2017/7039816.
- Nicolia, V.; Lucarelli, M.; Fuso, A. Environment, epigenetics and neurodegeneration: Focus on nutrition in Alzheimer’s disease. Exp Gerontol. 2015, 68, 8-12; doi: 10.1016/j.exger.2014.10.006
- . Checler, F. Presenilins: Multifunctional Proteins Involved in Alzheimer’s Disease Pathology. IUBMB Life 1999, 48, 33-39.
- 46-. Bird, T.D. Genetic aspects of Alzheimer disease. Genetics in Medicine 2008, 10, 231–239.
- Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nat Rev Neurol. 2013, 9, 106–118; doi: 10.1038/nrneurol.2012.263
- Zhou, X.; Chen, Y.; Mok, K.Y.; Kwok, T.C.Y.; Mok, V.C.T.; Guo, Q.; Ip, F.C.; Chen, Y.; Mullapudi, N.; et al. Non-coding variability at the APOE locus contributes to the Alzheimer’s risk. Nat. Commun. 2019, 10, 3310; doi: 10.1038/s41467-019-10945-z.
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; Kumar, S.; Wang R.; et al. Protective Effects of Indian Spice Curcumin Against Amyloid Beta in Alzheimer’s Disease. J Alzheimers Dis. 2018, 61, 843–866; doi:10.3233/JAD-170512.
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013, 12, 357–367; doi: 10.1016/S1474-4422(13)70044-9.
- Singh, R. Current Alzheimer’s management with berries fruits therapy. J Public Health Nutr 2018,1, 17-24; doi:10.35841/public-health-nutrition.1.2.17-24.
- Rathod, R.; Kale, A.; Joshi, S. Novel insights into the effect of vitamin B12 and omega-3 fatty acids on brain function. J Biomed Sci. 2016, 23, 17; doi: 10.1186/s12929-016-0241-8.
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy-A Review. Nutrients 2016, 8, 68; doi: 10.3390/nu8020068.
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009, 2, 270–278; doi: 10.4161/oxim.2.5.9498.
- Mecocci, P.; Tinarelli, C.; Schulz, R.J.; Polidori, M.C. Nutraceuticals in cognitive impairment and Alzheimer’s disease. Front Pharmacol. 2014, 5, 147; doi: 10.3389/fphar.2014.00147.
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006, 47(Suppl.2), S215–S220; doi:10.1097/00005344-200606001-00018.
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.; Zhua, X. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease. Biochim Biophys Acta. 2014, 1842, 1240–1247; doi: 10.1016/j.bbadis.2013.10.015.
- Giau, V.V.; Wu, S.Y.; Jamerlan, A.; An, S.S.A.; Kim, S.Y.; Hulme, J. Gut Microbiota and Their Neuroinflammatory Implications in Alzheimer's Disease. Nutrients 2018, 14, 10, 1765; doi: 10.3390/nu10111765.
- Alkasir, R.; Li, J.; Li, X.; Jin, M.; Zhu, B. Human gut microbiota: the links with dementia development. Protein Cell 2017, 8, 90–102; doi: 10.1007/s13238-016-0338-6.
- Harach, T.; Marungruang, N.; Duthilleul, N.; et al. Reduction of A amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017, 7, 41802; doi: 10.1038/srep41802.
- Minter, M.R.; Zhang, C.; Leone, V.; et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016, 6, 30028; doi: 10.1038/srep30028.
- Minter, M.R.; Hinterleitner, R.; Meisel, M.; et al. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1∆E9 murine model of Alzheimer’s disease. Sci Rep. 2017, 7, 10411; doi: 10.1038/s41598-017-11047-w.
- Hu, X.; Wang, T.; Jin, F. Alzheimer’s disease and gut microbiota. Sci China Life Sci2016, 59, 1006-1023; doi: 10.1007/s11427-016-5083-9.
- Raval, U.; Harary, J.M.; Zeng, E.; Pasinetti, G.M. The Dichotomous Role of the Gut Microbiome in Exacerbating and Ameliorating Neurodegenerative Disorders. Expert Rev Neurother 2020, 27, 1-14; doi: 10.1080/14737175.2020.1775585.
- Friedland, R.P. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J Alzheimers Dis. 2015, 45, 349-362; doi: 10.3233/JAD-142841.
- Köhler, C.A.; Maes, M.; Slyepchenko, A.; Berk, M.; Solmi, M.; Lanctôt, K.L.; Carvalho, A.F. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer's Disease. Curr Pharm Des. 2016, 22, 6152-6166; doi: 10.2174/1381612822666160907093807.
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer's Disease-a Critical Review. Mol Neurobiol 2019, 56, 1841-1851; doi: 10.1007/s12035-018-1188-4.
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; Bendlin, B.B.; Rey, F.E. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017, 7, 13537; doi: 10.1038/s41598-017-13601-y.
- Saji, N.; Niida, S.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Toba, K.; Sakurai, T. Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci Rep. 2019, 9, 1008; doi: 10.1038/s41598-018-38218-7.
- Nguyen, T.T.; Fujimura, Y.; Mimura, I.; Fujii, Y.; Nguyen, N.L.; Arakawa, K.; Morita, H. Cultivable butyrate-producing bacteria of elderly Japanese diagnosed with Alzheimer’s disease. J Microbiol. 2018, 56, 760–771; doi:10.1007/s12275-018-8297-7.
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun. 2019, 80, 633–643; doi:10.1016/j.bbi.2019.05.008.
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915-932; doi: 10.1016/j.cell.2016.10.027.
- Bruce-Keller, A.J.; Salbaum, J.M.; Berthoud, H.R. Harnessing Gut Microbes for Mental Health: Getting From Here to There. Biol Psychiatry. 2018, 83, 214-223; doi:10.1016/j.biopsych.2017.08.014.
- Szczechowiak, K.; Diniz, B.S.; Leszek, J. Diet and Alzheimer's dementia - Nutritional approach to modulate inflammation. Pharmacol Biochem Behav. 2019, 184, 172743; doi: 10.1016/j.pbb.2019.172743.
- Cryan, J.F.; O'Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179-194; doi: 10.1016/S1474-4422(19)30356-4.
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012, 10, 735-742; doi: 10.1038/nrmicro2876.
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; et al. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol Cell. 2016, 64, 982-992; doi: 10.1016/j.molcel.2016.10.025.
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer's Disease. J Alzheimers Dis. 2017, 58, 1-15; doi: 10.3233/JAD-161141.
- Bostanciklioğlu, M. The role of gut microbiota in pathogenesis of Alzheimer's disease. J. Appl Microbiol. 2019, 127, 954-967; doi: 10.1111/jam.14264.
- Szablewski, L. Human Gut Microbiota in Health and Alzheimer's Disease. J Alzheimers Dis. 2018, 62, 549-560; doi: 10.3233/JAD-170908.
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; et al. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics inan adult population. Br. J. Nutr. 2017, 117, 1645–1655; doi: 10.1017/S0007114517001593.
- Rainey-Smith, S.R.; Gu, Y.; Gardener, S.L.; Doecke, J.D.; Villemagne, V.L.; Brown, B.M.; Taddei, K.; Laws, S.M.; Sohrabi, H.R.; et al. Mediterranean Diet Adherence and Rate of Cerebral Aβ-amyloid Accumulation: Data From the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Transl Psychiatry 2018, 8, 238; doi: 10.1038/s41398-018-0293-5.
- Pasinetti, G.M. Novel Role of Red Wine-Derived Polyphenols in the Prevention of Alzheimer's Disease Dementia and Brain Pathology: Experimental Approaches and Clinical Implications. Planta Med 2012, 78, 1614-9; doi: 10.1055/s-0032-1315377.
- Lange, K.W.; Guo, J.; Kanaya, S.; Lange, K.M.; Nakamura, Y.; Li, S. Medical foods in Alzheimer’s disease. Food Science and Human Wellness 2019, 8, 1–7; doi:10.1016/j.fshw.2019.02.002.
- Cloud, J. Why Your DNA Isn't Your Destiny. Time 2010.
- Robertson, K.D. DNA methylation and human disease. Nature Reviews Genetics 2005, 6, 597–610; doi: 10.1038/nrg1655.
- Robertson, K.D.; Wolffe, A.P. DNA methylation in health and disease. Nature Reviews Genetics 2000, 1, 11–19; doi: 10.1038/35049533.
- Zoghbi, H.Y.; Beaudet, A.L. Epigenetics and Human Disease. Cold Spring Harb Perspect Biol. 2016, 8, a019497; doi: 10.1101/cshperspect.a019497.
- Korzus, E. Manipulating the brain with epigenetics. Nat. Neurosci. 2010, 13, 405–406; doi: 10.1038/nn0410-405.
- Zheng, Y.; Liu, A.; Wang, Z.J.; Cao, Q.; Wang, W.; Lin, L.; Ma, K.; Zhang, F.; Wei, J. et al. Inhibition of EHMT1/2 Rescues Synaptic and Cognitive Functions for Alzheimer's Disease. Brain 2019, 142, 787-807; doi: 10.1093/brain/awy354.
- Monti, N.; Cavallaro, R.A.; Stoccoro, A.; Nicolia, V.; Scarpa, S.; Kovacs, G.G.; Fiorenza, M.T.; Lucarelli, M.; et al. CpG and non-CpG Presenilin1 methylation pattern in course of neurodevelopment and neurodegeneration is associated with gene expression in human and murine brain. Epigenetics 2020, 5, 1-19; doi: 10.1080/15592294.2020.1722917.
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250; doi:10.3390/ijms21041250.
- Ayissi, V.B.O.; Ebrahimi, A.; Schluesenner, H. Epigenetic effects of natural polyphenols: A focus on SIRT1-mediated mechanisms. Mol. Nutr. Food Res. 2014, 58, 22–32; doi: 10.1002/mnfr.201300195.
- Kouzarides, T.; Berger, S.L. Chromatin modifications and their mechanism of action. In: Allis CD, Jenuwein T, Reinberg D (eds) Epigenetics. Cold Spring Harbor Press, Cold Spring Harbor, 2007, 191–209.