Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Subba Rao Cheekatla.
Due to its immense importance, the progress of novel approaches for the synthesis of indole-based scaffolds has increased steadily. The majority of the macrocycles synthesis proceeds through the macrolactamization and macrolactonization, as well as the C–C bond macrocyclization process described by metal-catalyzed ring-closing metathesis (RCM) and coupling reactions.
- heterocycles
- coupling reactions
- transition-metal catalysis
- indole-based macrocycles
1. Introduction
Macrocycles are versatile motifs that have met considerable attention from synthetic chemists over the last several decades, specifically those who are involved with natural product synthesis. The curiosity aroused in the synthesis of macrocyclic compounds has been raised steadily due to their enormous impact on bioactive natural products, chemical biology, polymers, drug design and development, bioorganic chemistry, supramolecular chemistry, pharmaceutics, and medicinal chemistry [1,2,3,4,5][1][2][3][4][5]. Macrocycles are chemical entities that have a cyclic structure consisting of 12-membered or more atoms with bigger rings. These are very important common privileged scaffolds for drug design and increasing interest in their study continuously. Because of their broad range of biological activities, such as anti-microbial, anti-inflammatory, antileishmanial, anti-cancer and anti-trypanosomatidial properties, these macrocyclic systems are considered an attractive target in pharmaceutical development, as well as in drug discovery [6,7,8,9,10,11][6][7][8][9][10][11]. Some of the macrocycles containing hetero atoms are served as hosts in supramolecular chemistry and act as selective complexing agents and catalysts [12,13][12][13]. The structural topography of macrocycles provides outstanding molecular recognition, as well as useful molecular carriers for delivering drug molecules and therapeutic biomolecules. Macrocyclic scaffolds bearing hetero atoms are worthwhile compounds with a broad spectrum of medicinal and pharmacological activities [14]. Some of the macrocyclic motifs are considered probes or drugs to aim protein–protein interactions, will impart higher metabolic activity, and can improve selectivity and enhance the binding affinity [15,16][15][16]. Basically, these are broadly found in nature and the structure of these macrocycles which performs a degree of conformational pre-organization due to restricted rotation. The macrocyclic ring system possesses a unique structural feature and conformational flexibility, which offers to be selective and highly effective when basic functional groups interact with biological targets [17]. Most of the macrocycles exhibit enhanced lipophilicity and promising drug-like properties, such as better cell membrane permeability, oral bioavailability, well metabolic stability, and good solubility, along with appropriate pharmacokinetic and pharmacodynamic properties [18]. Some of the structures of biologically active natural macrocycles (1–8) are displayed in Figure 1.
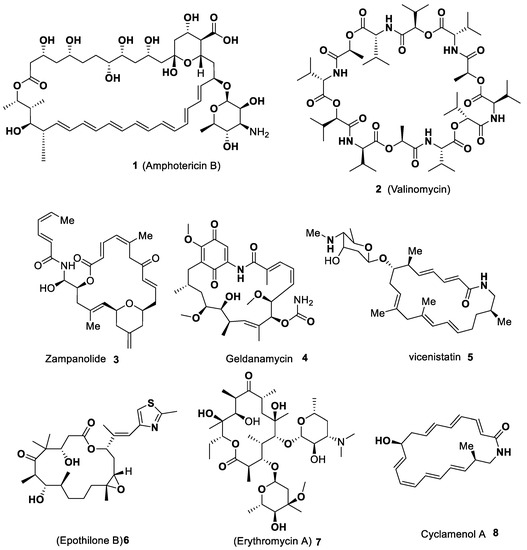
Figure 1. Selected examples of bio-active natural macrocycles (1–8).
In Figure 1, wresearche rs described those biological activities, such as Amphotericin B 1 (antifungal medication) has been used for the treatment of invasive fungal infections and leishmaniasis. Valinomycin 2 is an effective natural antibiotic and is employed as an agent to induce apoptosis. It can selectively transport alkali metal ions through biological and synthetic membranes. Zampanolide 3 is a microtubule-stabilizing polyketide owning effective cytotoxicity towards various cancer cell lines. Geldanamycin 4 is a macrocyclic polyketide synthesized by a Type I polyketide synthase. It is a 1,4-benzoquinone ansamycin antitumor antibiotic which inhibits the Hsp90 and induces the degradation of proteins that are mutated or overexpressed in cancer cells. Vicenistatin 5 is a strong polyketide antitumor antibiotic and shows in vitro cytotoxicity toward human promyelocytic leukemia HL-60 and human colon cancer COLO205 cells. Epithilone B 6 has been confirmed to be potent in vivo anticancer activity and inhibits microtubule functions. It prevents cancer cells from dividing by interfering with the tubulin. Erythromycin A 7 is a macrolide that is studied to be an effective and one of the safest antibiotics and broadly utilized in clinical medicine against infections caused by Gram-positive bacteria and pulmonary infections. Cyclamenol A 8 (an anti-inflammatory agent) is one of the macrocyclic polyene lactam natural products that inhibit leukocyte adhesion to endothelial cells [14,15,16,17,18,19][14][15][16][17][18][19].
Natural products having macrocyclic skeletons possess numerous pharmacological properties, and biochemical functions have led to their drug development. These macrocyclic scaffolds are conformationally pre-organized and offer distinct functionality and stereochemical complexity in their architecture, which results in better affinity and good selectivity for protein targets [20]. There are vast benefits of macrocycles, especially when compared with their linear counterparts. The design and development of drug-like macrocycles is always a fascinating area of research in medicinal chemistry and has received immense interest from organic chemists over recent years [6]. The macrocyclization strategy is promising for the design of drugs, as well as it reduces the entropic loss allied with the ligand by adaptation of a favorable conformation, which may lead to improved potency and selectivity. Some of the well-known macrocyclic drugs, such as Rezafungin (for the treatment of candidemia and invasive candidiasis), Lorlatinib (anti-cancer drug), Pacritinib (for the treatment of myelofibrosis), Selepressin (vasodilatory hypotension), Rifaximin (antibiotic), Ciclosporin (immunosuppressant), Tacrolimus (immunosuppressive), Everolimus (antineoplastic chemotherapy drug) are currently being used as drugs in the market [21].
Because of its promising biological activities, nitrogen-bearing heterocycles have always been considered as a desirable target for the synthetic community. Over the past several decades, N-based heterocycles have drawn much attention from synthetic chemists and chemical biologists because of their special ability to bind a variety of receptors, and they are embedded in numerous natural products and medicinally relevant substances [22,23][22][23]. Among a variety of heterocyclic scaffolds, indole is a unique core referred to as a privileged pharmacophore present in the multiple biologically active scaffolds. Some of the indole units are found in natural and synthetic macrocycles with prominent biological functions, and it is a key synthon in the numerous clinically important drugs for the cure of cancer, circulatory disease, Alzheimer’s disease, and neuro disorders [24]. Additionally, indole is one of the most nitrogen heterocycles, particularly in medicinal and pharmaceutics. Macrocycles bearing indole moiety occur in many natural alkaloids and unnatural products [25]. Because of its binding ability, some of the C2-symmetric indole scaffolds are known to exhibit inhibitory activities against Gram-positive bacteria Bacillus subtilis and Micrococcus luteus. Indole-based C2-symmetric new chemical entities (NCEs) are expected to show a distinct role in medicinal chemistry. Based on its potential biological activities and pharmacological applications, intense study and much effort have been dedicated to the design and synthesis of a variety of indole-based analogs [26]. The structure of some important biologically active indole-based macrocycles is shown in Figure 2 [27,28,29,30][27][28][29][30].
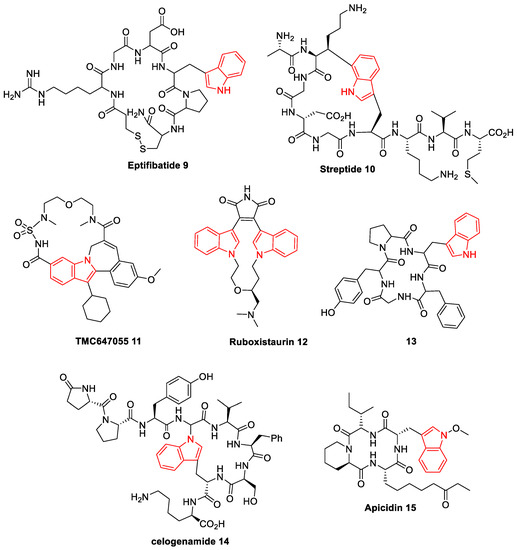
Figure 2. Representative examples of bio-active indole-based macrocycles (9–15).
The macrocyclization efficiency totally varies on the size and structure, as well as the structural pre-organization of the linear substrates. Mostly, the assembly of macrocycles is considered as an exciting task and a crucial step for synthetic chemists. Because of its interesting biological activity, as well as the intractable synthetic complexity of naturally occurring macrocycles, several research groups have diverted their significant efforts to explore highly effective and easiest synthetic methods for the design of macrocycles. From the literature search, a number of reports have been available for the synthesis of different varieties of macrocycles from several macrocyclization strategies [31]. These include the olefin metathesis reactions (RCM) [32], coupling reactions catalyzed by transition metals [33], macrolactonization [34], Cu (I) mediated click chemistry [35], macrolactamization [36], thiol-ene photochemical strategy [37], SN2 & SN2Ar reactions [38], cross-couplings by palladium metal [39], Horner–Emmons olefination [40], Pd (0)-mediated Larock indole annulation [41], intramolecular radical macrocyclization by light source [42], Ugi reaction [43], and IMDAR (intramolecular Diels–Alder reaction) strategies [44].
There are two well-known metal-catalyzed macrocyclizations for the design and synthesis of biologically relevant scaffolds, such as the olefin metathesis (RCM) catalyzed by ruthenium and CuAAC (copper-catalyzed alkyne–azide cycloaddition) which are extensively reviewed in recently [45,46][45][46].
2. Metal Catalyzed Strategies toward the Indole Macrocycles
Advanced catalysts, also called precious metal catalysts, are prepared from gold, silver, platinum, ruthenium, palladium, and rhodium, which speed up chemical reactions without altering themselves. These metal-based catalysts are broadly utilized nowadays in pharmaceutical, refining industries, and various chemical manufacturing units. Additionally, these metal catalysts show many advantages, such as their catalytic activity is high, which can accelerate chemical reactions more effectively. As well as displays better selective performance, good thermal stability, higher surface area, porosity, chemical inertness, sustainability, versatility, and longevity. The usage of metal-free catalysts has been increasing in recent years to develop industrial benefits with respect to more economical, eco-friendly, and environmental and safety considerations. Most metal-free catalysts are based on many forms of carbon sources. The catalyst with Ru center is most popular both in industrial and academic because of its properly stable tolerance to moisture and air, as well as a higher affinity toward olefin instead of other groups. Over the past several decades, synthetic chemists demonstrated various metal-catalyzed approaches toward the synthesis of a variety of heteroaryl-based macrocycles [47]. These strategies provide powerful synthetic protocols with diverse applications, particularly in pharmaceuticals, natural product synthesis, and drug development [48]. In comparison with the standard protocols, these approaches do not require pre-functionalization of substrates which affects on minimization of waste and atom economy. Additionally, these developed synthetic strategies can often be easily applied in the synthesis of other macrocyclic scaffolds. In metal-catalyzed approaches, cross-coupling and RCM reactions are deliberated as one of the most valuable protocols for the easiest formation of C–C bonds [47,48,49][47][48][49]. Here in, wresearchers outlined various metal-catalyzed strategies that can be used to make C–C bonds and ring closures to construct small drug-like molecules and complex architectures via multistep domino sequences.References
- Marsault, E.; Peterson, M.L. Macrocycles Are Great Cycles: Applications, Opportunities, and Challenges of Synthetic Macrocycles in Drug Discovery. J. Med. Chem. 2011, 54, 1961–2004.
- Butler, M.S. Natural products to drugs: Natural product derived compounds in clinical trials. Nat. Prod. Rep. 2005, 22, 162–195.
- Kotz, J. Bringing macrocycles full circle. Sci.-Bus. Exch. 2012, 5, 1176.
- Albericio, F.; Kruger, H.G. Therapeutic peptides. Future Med. Chem. 2012, 4, 1527–1531.
- Giordanetto, F.; Kihlberg, J. Macrocyclic Drugs and Clinical Candidates: What Can Medicinal Chemists Learn from Their Properties? J. Med. Chem. 2014, 57, 278–295.
- Driggers, E.M.; Hale, S.P.; Lee, J.; Terrett, N.F. The exploration of macrocycles for drug discovery—An underexploited structural class. Nat. Rev. Drug Discov. 2008, 7, 608–624.
- Cheekatla, S.R.; Thurakkal, L.; Jose, A.; Barik, D.; Porel, M. Aza-Oxa-Triazole Based Macrocycles with Tunable Properties: Design, Synthesis, and Bioactivity. Molecules 2022, 27, 3409.
- Porel, M.; Thornlow, D.N.; Phan, N.N.; Alabi, C.A. Sequence-defined bioactive macrocycles via an acid-catalysed cascade reaction. Nat. Chem. 2016, 8, 590–596.
- McGeary, R.P.; Fairlie, D.P. Macrocyclic peptidomimetics: Potential for drug development. Curr. Opin. Drug Discov. Dev. 1998, 1, 208–217.
- Levis, J.I. (Ed.) Macrocycles in Drug Discovery; RSC: Cambridge, UK, 2015.
- Mallinson, J.; Collins, I. Macrocycles in New Drug Discovery. Future Med. Chem. 2012, 4, 1409–1438.
- Gokel, G.W.; Leevy, W.M.; Weber, M.E. Crown ethers: Sensors for ions and molecular scaffolds for materials and biological models. Chem. Rev. 2004, 104, 2723–2750.
- Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F. Pillararenes, a New Class of Macrocycles for Supramolecular Chemistry. Acc. Chem. Res. 2012, 45, 1294–1308.
- Yudin, A.K. Macrocycles: Lessons from the distant past, recent developments, and future directions. Chem. Sci. 2015, 6, 30–49.
- DeLorbe, J.E.; Clements, J.H.; Whiddon, B.B.; Martin, S.F. Thermodynamic and Structural Effects of Macrocyclic Constraints in Protein−Ligand Interactions. ACS Med. Chem. Lett. 2010, 1, 448–452.
- Thurakkal, L.; Nanjan, P.; Porel, M. Design, synthesis, and bioactive properties of a class of macrocycles with tunable functional groups and ring size. Sci. Rep. 2022, 12, 4815.
- Martí-Centelles, V.; Pandey, M.D.; Burguete, M.I.; Luis, S.V. Macrocyclization reactions: The importance of conformational, configurational, and template-induced preorganization. Chem. Rev. 2015, 115, 8736–8834.
- Furukawa, A.; Schwochert, J.; Pye, C.R.; Asano, D.; Edmondson, Q.D.; Turmon, A.C.; Klein, V.G.; Ono, S.; Okada, O.; Lokey, R.S. Drug-Like Properties in Macrocycles above MW 1000: Backbone Rigidity versus Side-Chain Lipophilicity. Angew. Chem. Int. Ed. 2020, 59, 21571–21577.
- Gibson, S.E.; Lecci, C. Amino acid derived macrocycles—An area driven by synthesis or application? Angew. Chem. Int. Ed. 2006, 45, 1364–1377.
- Begnini, F.; Poongavanam, V.; Over, B.; Castaldo, M.; Geschwindner, S.; Johansson, P.; Tyagi, M.; Tyrchan, C.; Wissler, L.; Sjo, P.; et al. Mining Natural Products for Macrocycles to Drug Difficult Targets. J. Med. Chem. 2021, 64, 1054–1072.
- Garcia Jimenez, D.; Poongavanam, V.; Kihlberg, J. Macrocycles in Drug Discovery—Learning from the Past for the Future. J. Med. Chem. 2023, 66, 5377–5396.
- Tahlan, S.; Kumar, S.; Narasimhan, B. Pharmacological significance of heterocyclic 1H-benzimidazole scaffolds: A review. BMC Chem. 2019, 13, 101.
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909.
- Gribble, G.W. Indole Ring Synthesis: From Natural Products to Drug Discovery; Wiley: Weinheim, Germany, 2016.
- Gribble, G.W. (Ed.) Heterocyclic Scaffolds II: Reactions and Applications of Indoles. In Topics in Heterocyclic Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; Volume 26, ISBN 978-3-642-15733-2.
- Kumar, S.; Ritika. A brief review of the biological potential of indole derivatives. Future J. Pharm. Sci. 2020, 6, 121.
- Cummings, M.D.; Lin, T.-I.; Hu, L.; Tahri, A.; McGowan, D.; Amssoms, K.; Last, S.; Devogelaere, B.; Rouan, M.-C.; Vijgen, L.; et al. Discovery and Early Development of TMC647055, a Non-Nucleoside Inhibitor of the Hepatitis C Virus NS5B Polymerase. J. Med. Chem. 2014, 57, 1880–1892.
- Ueda, T.; Takai, N.; Nishida, M.; Nasu, K.; Narahara, H. Apicidin, a novel histone deacetylase inhibitor, has profound anti-growth activity in human endometrial and ovarian cancer cells. Int. J. Mol. Med. 2007, 19, 301–308.
- Bedini, A.; Di Cesare Mannelli, L.; Micheli, L.; Baiula, M.; Vaca, G.; De Marco, R.; Gentilucci, L.; Ghelardini, C.; Spampinato, S. Functional Selectivity and Antinociceptive Effects of a Novel KOPr Agonist. Front. Pharmacol. 2020, 11, 188.
- Smolyar, I.V.; Yudin, A.K.; Nenajdenko, V.G. Heteroaryl Rings in Peptide Macrocycles. Chem. Rev. 2019, 119, 10032–10240.
- Mortensen, K.T.; Osberger, T.J.; King, T.A.; Sore, H.F.; Spring, D.R. Strategies for the diversity-oriented synthesis of macrocycles. Chem. Rev. 2019, 119, 10288–10317.
- Yu, M.; Wang, C.; Kyle, A.F.; Jakubec, P.; Dixon, D.; Schrock, R.R.; Hoveyda, A.H. Synthesis of macrocyclic natural products by catalyst-controlled stereoselective ring-closing metathesis. Nature 2011, 479, 88–93.
- Jiang, B.; Zhao, M.; Li, S.-S.; Xu, Y.-H.; Loh, T.-P. Macrolide synthesis through intramolecular oxidative cross-coupling of alkenes. Angew. Chem. Int. Ed. 2018, 57, 555–559.
- Parenty, A.; Moreau, X.; Campagne, J.-M. Macrolactonizations in the total synthesis of natural products. Chem. Rev. 2006, 106, 911–939.
- Jagasia, R.; Holub, J.M.; Bollinger, M.; Kirshenbaum, K.; Finn, M.G. Peptide Cyclization and Cyclodimerization by CuIMediated Azide-Alkyne Cycloaddition. J. Org. Chem. 2009, 74, 2964–2974.
- Ishihara, K.; Kuroki, Y.; Hanaki, N.; Ohara, S.; Yamamoto, H. Antimony-templated macrolactamization of tetraamino esters. Facile synthesis of macrocyclic spermine alkaloids, (±)-buchnerine, (±)-verbacine, (±)-verbaskine, and (±)-verbascenine. J. Am. Chem. Soc. 1996, 118, 1569–1570.
- Aimetti, A.A.; Shoemaker, R.K.; Lin, C.-C.; Anseth, K.S. On-resin peptide macrocyclization using thiol–ene click chemistry. Chem. Commun. 2010, 46, 4061–4063.
- Feng, Y.; Pattarawarapan, M.; Wang, Z.; Burgess, K. Solid-Phase SN2 Macrocyclization Reactions To Form β-Turn Mimics. Org. Lett. 1999, 1, 121–124.
- Wang, X.; Lu, M.-Z.; Loh, T.-P. Transition-Metal-Catalyzed C–C Bond Macrocyclization via Intramolecular C–H Bond Activation. Catalysts 2023, 13, 438.
- Larsen, B.J.; Sun, Z.; Nagorny, P. Synthesis of Eukaryotic Translation Elongation Inhibitor Lactimidomycin via Zn(II)-Mediated Horner–Wadsworth–Emmons Macrocyclization. Org. Lett. 2013, 15, 2998–3001.
- Breazzano, S.P.; Poudel, Y.B.; Boger, D.L. A Pd(0)-Mediated Indole (Macro) Cyclization Reaction. J. Am. Chem. Soc. 2013, 135, 1600–1606.
- Nishikawa, K.; Yoshimi, Y.; Maeda, K.; Morita, T.; Takahashi, I.; Itou, T.; Inagaki, S.; Hatanaka, M. Radical Photocyclization Route for Macrocyclic Lactone Ring Expansion and Conversion to Macrocyclic Lactams and Ketones. J. Org. Chem. 2012, 78, 582–589.
- Abdelraheem, E.M.M.; Khaksar, S.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Shaabani, S.; Dömling, A. Two-Step Macrocycle Synthesis by Classical Ugi Reaction. J. Org. Chem. 2018, 83, 1441–1447.
- Zapf, C.W.; Harrison, B.A.; Drahl, C.; Sorensen, E.J. A Diels-Alder Macrocyclization Enables an Efficient Asymmetric Synthesis of the Antibacterial Natural Product Abyssomicin C. Angew. Chem. 2005, 117, 6691–6695.
- Gradillas, A.; Pérez-Castells, J. Macrocyclization by ring-closing metathesis in the total synthesis of natural products: Reaction conditions and limitations. Angew. Chem. Int. Ed. 2006, 45, 6086–6101.
- Zakharova, E.A.; Shmatova, O.I.; Kutovaya, I.V.; Khrustalev, V.N.; Nenajdenko, V.G. Synthesis of macrocyclic peptidomimetics via the Ugi-click-strategy. Org. Biomol. Chem. 2019, 17, 3433–3445.
- Lu, X.; He, S.-J.; Cheng, W.-M.; Shi, J. Transition-metal-catalyzed C–H functionalization for late-stage modification of peptides and proteins. Chin. Chem. Lett. 2018, 29, 1001–1008.
- Chouhan, G.; James, K. Efficient Construction of Proline-Containing β-Turn Mimetic Cyclic Tetrapeptides via CuAAC Macrocyclization. Org. Lett. 2013, 15, 1206–1209.
- Cai, C.; Wang, F.; Xiao, X.; Sheng, W.; Liu, S.; Chen, J.; Zheng, J.; Xie, R.; Bai, Z.; Wang, H. Macrocyclization of bioactive peptides with internal thiazole motifs via palladium-catalyzed C–H olefination. Chem. Commun. 2022, 58, 4861–4864.
More
