You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Ali Mirzaei and Version 2 by Peter Tang.
Volatile organic compounds (VOCs) are among the most abundant air pollutants. Their high concentrations can adversely affect the human body, and therefore, early detection of VOCs is of outmost importance.
- acetaldehyde
- chemiresistive gas sensor
- nanostructured metal oxide
- sensing mechanism
1. Introduction
By definition, volatile organic compounds (VOCs) are organic compounds with a low boiling point, (50–100 °C to 240–260 °C) and with saturation vapor pressures higher than 102 kPa at 25 °C [1]. They are air pollutants and mostly are emitted from industrial factories and vehicles. They accelerate the formation of secondary organic aerosols, and under oxidized conditions, they will convert them to particles [2]. Accordingly, they cause different environmental problems and also have detrimental effects on human health [3].
Aldehydes are a class of VOCs that are highly reactive and odorous. They are one of the most common sources of pollution in air because they are not only used in many chemical adhesives such as cigarette adhesives [4] but also are produced in many industrial processes or incomplete combustions [5]. In particular, they can be formed as a product of incomplete wood combustion in fireplaces and woodstoves, pulp and paper factories, internal combustion engines and turbines, and vehicle exhaust fumes [6]. Formaldehyde (HCHO) and acetaldehyde (CH3CHO) are considered two of the most important aldehydes [7][8][7,8]. They are known as carcinogenic and probably carcinogenic agents, respectively [9][10][9,10].
Acetaldehyde, with the systematic name of ethanal [11], is a small molecule comprising of only four hydrogen atoms, two carbon atoms and one oxygen atom with a low molecular weight (44.05 g/mol) [12], as shown in Figure 1. It has an aroma like oranges, low boiling point (20.2 °C), and high solubility in water and lipids [13]. The indoor sources of acetaldehyde include laminates, building materials, wood ceilings, wooden varnished, etc., while its outdoor sources include power plants, wood, trash, oil and gas extraction, cement kilns, refineries, and automobile exhausts [14]. It is also the most abundant carcinogen of tobacco smoke [15]. Acetaldehyde is widely employed to produce acetic acid, acetate esters, pentaerythritol, and pyridine bases [16]. In addition, it is used in the dairy industry as a synthetic flavoring component and food additive [17].
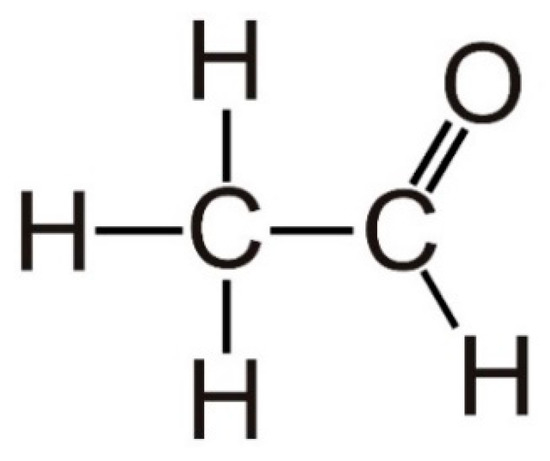
Figure 1.
Structure of acetaldehyde.
Acetaldehyde is also a highly toxic compound [18]. The effects of acetaldehyde on the human body include eye irritation, headache, vomiting, liver diseases, and detrimental effects on the throat, skin, and the respiratory tract [19][20][21][19,20,21]. In particular, because of the pungent odor of acetaldehyde, it is extremely irritating at concentrations above 50 ppm [14]. In addition, it can be a cause of sick building syndrome, so-called SBS, even at ppb levels [22][23][22,23]. Acetaldehyde has a strong electrophilic nature and can damage DNA in humans, and it is considered as a possible human carcinogen [24][25][24,25]. Furthermore, it can easily react with Vitamin B1, leading to B1 deficiency. Accordingly, it can cause mental illness, visual disturbances, and poor memory in human beings. To avoid such problems, 100 ppm as a permissible exposure limit of acetaldehyde has been proposed [26].
2. Chemiresistive Gas Sensors
Nowadays, gas sensors are widely utilized in different areas including public safety, industrial processes, domestic safety, underground mining, and monitoring of environmental pollution and air quality in vehicles [27]. So far, different types of gas sensors such as surface acoustic wave [28], optical [29], gasochromic [30], thermoelectric [31], electrochemical [32]. and chemiresistive [33][34][35][33,34,35] gas sensors for detection of VOCs have been introduced. Among them, chemiresistive gas sensors are very popular owing to their high sensitivity, short response time, high stability, reproducibility, simple fabrication and operation, and low cost [36][37][38][36,37,38]. Chemiresistive gas sensors, in which the resistance of the sensing layer changes upon exposure to the target gas, were introduced for the first time about sixty years ago [39]. Depending on the increase or decrease of the resistance and the magnitude of the resistance change, the gas type and its concentration can be estimated [40][41][40,41]. In chemiresistive gas sensors, surface area, morphology, chemical composition, and sensing temperature are the main factors affecting the gas response [42][43][42,43]. A typical resistive-based gas sensor consists of a sensitive layer that is deposited on the surface of interdigitated electrodes printed on an (a) insulating ceramic, (b) plastic, or (c) Si substrate [44]. A heater can also be used on the back of the substrate to raise the sensor temperature up to desired sensing temperatures. However, in some cases, the sensor will be put in a gas chamber in a tubular furnace, where the temperature can be precisely controlled [45].2.1. General Sensing Mechanism
2.1. General Sensing Mechanism
Figure 2 schematically shows the acetaldehyde sensing mechanism in both pristine n- or p-type metal oxide chemiresistive sensors. For n-types such as ZnO, SnO2, initially in air, due to abstraction of the electrons by oxygen species, a so-called electron depletion layer will be created on the surface. Because of this, the resistance in the electron depletion layer is higher than that of the core-region parts of the sensing material (Figure 2a,b). Upon exposure to acetaldehyde gas, it reacts with already adsorbed oxygen ions and the released electrons come back to the surface of the gas sensor. As a result, the resistance of the gas sensor increases, leading to the appearance of a sensing signal. For p-type metal oxides, such as CuO and Cr2O3 [46], initially in air, due to extraction of the electrons by oxygen species, a hole accumulation layer will appear on the surface of the gas sensor (Figure 2c,d). Since in p-type metal oxides the main charge carriers are the holes, the resistance in the hole accumulation layer is lower than the core part. In acetaldehyde atmosphere, the released electrons come back to the surface of the gas sensor, leading to higher resistance of the gas sensor and appearance of a sensing signal. A widely used strategy to enhance the gas sensitivity and selectivity is noble metal decoration. The promising effects of noble metals on acetaldehyde detection, which are known as electronic sensitization and chemical sensitization, respectively [47][48][47,48], are schematically shown in Figure 2e,f. In electronic sensitization, due to the difference between the work functions of noble metals and metal oxides, often electrons from the sensing layer will be moved to the noble metals to equate the Fermi levels. Therefore, in contact areas between the noble metal and metal oxide, the width of the electron depletion layer increases, leading to greater resistance modulation upon exposure to the acetaldehyde gas. In addition, noble metals can act as catalysts for the decomposition of oxygen molecules and target gases. Therefore, initially the gas will be adsorbed on the surface of noble metals, then it will be decomposed into smaller molecules or atoms, and finally. in a so-called spillover effect, it will be moved to the surface of the neighboring sensing layer. Thus, the noble metals can enhance the sensitivity and selectivity of the gas sensors.
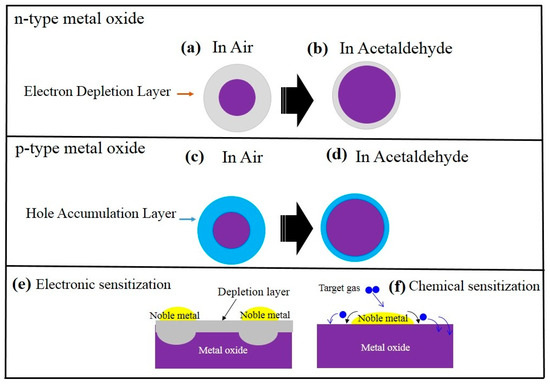

Figure 2. Schematic of acetaldehyde-sensing mechanism in (a,b) n-type metal oxide and (c,d) p-type metal oxide. Effect of noble metals on the gas sensing enhancement: (e) electronic sensitization (f) chemical sensitization.
3. Acetaldehyde Detection Using Chemiresistive-Based Gas Sensors
Traditional strategies to determine the concentration of acetaldehyde are the use of gas chromatography, chemiluminescence, cataluminescence, etc. [49]. Even though such techniques are highly sensitive and accurate, they have some disadvantages for online monitoring, need expert operators, and are bulky as well as expensive. Therefore, sensitive, selective, stable, fast, portable, and simply operated sensors are greatly needed for acetaldehyde detection [6][50][6,50]. For practical applications, an acetaldehyde gas sensor should have following merits: (i) high sensitivity; (ii) high selectivity; (iii) fast dynamics; (iv) long-term stability; (v) reproducibility; (vi) low power consumption; and (vi) low cost. Even though metal-oxide-based gas sensors have most of the above merits, their selectivity and power consumption is a challenge and more research is needed to realize a high acetaldehyde gas sensor for practical applications.
3.1. Morphology-Engineered Nanostructures as Acetaldehyde Gas Sensors
It is well-known that morphology engineering is one of the best strategies to improve the sensing capabilities of metal oxide gas sensors [51][52][51,52]. For example, regarding zinc oxide (ZnO), which is one of the most used sensing materials [37][53][54][37,53,54], different morphologies of ZnO such as nanoparticles (NPs), tetrapods, nanobeads, and nanotubes have been used for gas-sensing studies.
In Table 1, some ZnO-based sensors with different morphologies reported for the sensing of acetaldehyde in the literature is presented (Table 1). As can be seen, ZnO-based sensors with different morphologies are able to detect low and high concentrations of acetaldehyde gas at different sensing temperatures.
Table 1.
Acetaldehyde sensing properties of some ZnO-based gas sensors reported in the literature.
|
Morphology |
Concentration (ppm) |
Operating Temperature (°C) |
Response (Ra/Rg) |
Ref. |
|---|---|---|---|---|
|
Al-doped ZnO |
10 |
500 |
2250 |
[55] |
|
ZnO powders |
2 |
450 |
5.73 |
[14] |
|
ZnO-NiCo2O4 nanofibers |
100 |
250 |
~3 |
[56] |
|
0.15 mol% Au-ZnO NPs |
100 |
377 |
~7 |
[57] |
|
ZnO particles |
250 |
400 |
∼45 |
[58] |
|
ZnO tetrapods |
50 |
47.5 |
[5] |
|
|
ZnO nanoaggregates |
200 |
25 |
~8 |
[59] |
|
ZnO sheets |
1 |
220 |
77 |
[60] |
|
ZnO rods |
250 |
400 |
5.30 |
[61] |
|
ZnO flowers |
8 |
[62] |
||
|
ZnO petals |
100 |
25 |
14 |
|
|
ZnO branched nanorods |
10 |
2.85 |
[26] |
|
|
Co-doped ZnO branched nanorods |
10 |
800 |
For example, ZnO tetrapods with nanosized dimensions were synthesized with a vapor phase method for aldehyde detection [5]. The employed method was catalyst-free and offered very high yield (a few grams were easily produced). On average, the diameter of tetrapods was 60 nm, and their length was 1 mm. The dynamic gas responses were obtained at different temperatures, showing that response/recovery times decreased when the temperature increased. At 400 °C, the sensor revealed a higher response to propionaldehyde (CH3CH2CHO) compared to acetaldehyde due to the higher number of electrons returning back to the ZnO when the molecule was completely oxidized [5]:
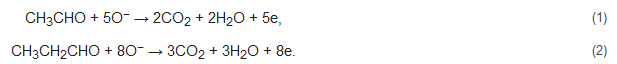
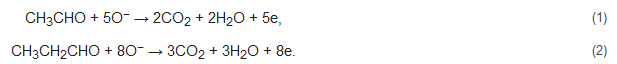
As shown in the above equations, the reaction of acetaldehyde and propionaldehyde with adsorbed electrons releases five and eight electrons, respectively. Accordingly, the response to acetaldehyde was slightly lower than the response to propionaldehyde. Interestingly, it was found that at T > 350 °C, the response was independent of the relative humidity (RH) value (0–75%).
In another study, flower-like ZnO nanostructures comprised of ZnO nanorods were fabricated through a hydrothermal synthesis method [62]. The hydrothermal method is a cost-effective and versatile method with the possibility of morphology control and is widely used for synthesis of metal oxides for sensing applications [63]. The high response the gas sensor to acetaldehyde compared to CO gas was related to the electron-donating effect of acetaldehyde (10 electrons), which was greater than that of the CO gas (two electrons).
ZnO nanosheets with two-dimensional morphology have attracted a lot of attention for sensing studies due to their high surface areas. In this regard, a fast acetaldehyde gas sensor was introduced using nanosheet-like ZnO nanostructures synthesized through a sonochemical method followed by subsequent etching [64]. The ZnO nanosheets had a high surface area, resulting in an enhanced sensing performance down to the ppb level. At the optimal sensing temperature of 220 °C, the sensor was able to detect even 50 ppb of acetaldehyde gas. Furthermore, the sensor revealed a linear response to acetaldehyde along with fast response and recovery times. The response and recovery times of the sensor to acetaldehyde were 8 and 60 s, respectively, which is very fast for practical applications. In fact, the presence of abundant channels and open space between the ZnO nanosheets accelerated the gas diffusion and decreased the response and recovery times.
