Glycine is an amino acid that our bodies can produce naturally, so we don't necessarily need to get it from our diet. Nevertheless, it plays a vital role in various functions throughout our entire body. Glycine interacts with specific receptors and transporters found in many different types of cells, which allows it to have important effects on our health.
One of the most fascinating things about glycine is its potential to reduce inflammation in our body. Researchers have conducted many studies focusing on this aspect. Glycine has been shown to decrease pro-inflammatory molecules called cytokines, which are responsible for promoting inflammation. Additionally, it can lower the levels of free fatty acids, which are sometimes linked to inflammation in certain situations.
Moreover, glycine seems to positively influence how our body responds to insulin, which is crucial for maintaining stable blood sugar levels. It also appears to bring about other beneficial changes in our body, although scientists are still investigating the specific details of these effects.
Glycine is a remarkable amino acid that offers many health benefits, especially in terms of reducing inflammation and possibly enhancing how our bodies respond to insulin. It's fascinating because it is present throughout our entire body, and we can obtain it through our diet or nutraceuticals, affecting our health in various ways. Scientists continue to study glycine to unlock its full potential and better understand its role in supporting our well-being.
- glycine
- immunomodulator
1. Introduction
Glycine, also called amino acetic acid, is a vital building block for many proteins and plays a key role in creating important biomolecules like creatine and purine nucleotides [1]. It was discovered in 1820 when gelatine was broken down with acid. Its name comes from the Greek word for "sweet," and it's the smallest amino acid with a molecular weight of 75.067 g/mol [1][2,3]. In our bloodstream, glycine makes up about 11.5% of all amino acids and contributes to 20% of the nitrogen in our body's proteins, accounting for 80% of our overall protein content [2][3][4,5]. For a typical daily intake, we need about 1.5–3 grams of glycine from our diet [4][6]. In young men, the rate at which glycine moves through the body is about 34–35 milligrams per kilogram per hour when we're fed, and about half that amount (around 18 mg/kg/h) when we're in the post-absorptive state [5][6][7,8]. Around 35% of the glycine in our body is produced internally (endogenous synthesis), while the rest comes from the food we eat [7][9]. On average, our body generates about 12–15 milligrams of new glycine per kilogram per hour, contributing to about 81% of the total glycine flow in our system [5][8][2,7,10]. The normal glycine concentration in our bloodstream typically ranges from 200 to 300 micromoles per liter. In summary, glycine plays a significant role in our body, and its levels are carefully regulated to support various essential functions [9][11].
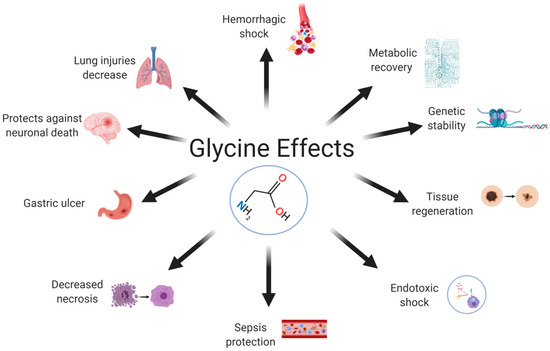
2. Effects of Glycine in the Organism
2.1. Neurotransmission
2.2. Antioxidant
2.3. Immunomodulation
2.4. Low Glycine Plasma Levels Are Associated with Low-Grade Inflammation
3. Effects of Glycine supplementation
In animal models, dietary supplementation with glycine reduces inflammation, morbidity, and mortality from pathogenic infections [59][101]. Betaine supplementation is associated with circulating levels of dimethylglycine. Betaine metabolism may be hampered by the activity of dimethylglycine dehydrogenase (DMGDH), which connects betaine to glycine synthesis via the demethylation of dimethylglycine into sarcosine [60][102]. Glycine supplementation has shown beneficial cardiac effects in a burn model [61][103] and in a skeletal muscle ischemia model, with decreased reperfusion-mediated necrosis and increased metabolic and functional recovery [62][104]. The protective effects of glycine have been reported for several sepsis models [63][64][105,106] and for hemorrhagic shock [65][107]. Normal levels of glycine in the body prevent lipopolysaccharide (LPS)-induced endotoxemia by binding to GlyRs on Kupffer cells [66][108]. Dietary supplementation with glycine decreases adipocyte size, adiposity, and the concentrations of free fatty acids and triglycerides in animal models [67][68][109,110]. It also inhibits the nonenzymatic glycation of proteins and hemoglobin in diabetic rats [69][78] and patients with T2DM [69][70][78,78].References
- Benveniste, M.; Mayer, M.L. Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. . Biophys. J. 1991, 59, 560–573..
- Jackson, A.A.; Badaloo, A.V.; Forrester, T.; Hibbert, J.M.; Persaud, C. Urinary excretion of 5-oxoproline (pyroglutamic aciduria) as an index of glycine insufficiency in normal man. Br. J. Nutr. 1987, 58, 207–214.
- Alves, A.; Bassot, A.; Bulteau, A.L.; Pirola, L.; Morio, B. Glycine metabolism and its alterations in obesity and metabolic diseases. Nutrients 2019, 11, 1356.
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37.
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477.
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312.
- Robert, J.J.; Bier, D.M.; Zhao, X.H.; Matthews, D.E.; Young, V.R. Glucose and insulin effects on de novo amino acid synthesis in young men: Studies with stable isotope labeled alanine, glycine, leucine, and lysine. Metabolism 1982, 31, 1210–1218.
- Lamers, Y.; Williamson, J.; Gilbert, L.R.; Stacpoole, P.W.; Gregory, J.F., III. Glycine turnover and decarboxylation rate quantified in healthy men and women using primed, constant infusions of glycine and leucine. J. Nutr. 2007, 137, 2647–2652.
- Yu, Y.M.; Yang, R.D.; Matthews, D.E.; Wen, Z.M.; Burke, J.F.; Bier, D.M.; Young, V.R. Quantitative aspects of glycine and alanine nitrogen metabolism in postabsorptive young men: Effects of level of nitrogen and dispensable amino acid intake. J. Nutr. 1985, 115, 399–410.
- Meléndez-Hevia, E.; de Paz-Lugo, P.; Cornish-Bowden, A.; Cárdenas, M.L. A weak link in metabolism: The metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J. Biosci. 2009, 34, 853–872.
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158.
- Zhang, Y.; Jia, H.; Jin, Y.; Liu, N.; Chen, J.; Yang, Y.; Dai, Z.; Wang, C.; Wu, G.; Wu, Z. Glycine attenuates LPS-induced apoptosis and inflammatory cell infiltration in mouse liver. J. Nutr. 2020, 150, 1116–1125. Wang, W.; Wu, Z.; Lin, G.; Hu, S.; Wang, B.; Dai, Z.; Wu, G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J. Nutr. 2014, 144, 1540–1548.
- Eulenburg, V.S. Glycine transporters: Essential regulators of synaptic transmission. Eur. Neuropsychopharmacol. 2011, 21, S230. Zhang, Y.; Jia, H.; Jin, Y.; Liu, N.; Chen, J.; Yang, Y.; Dai, Z.; Wang, C.; Wu, G.; Wu, Z. Glycine attenuates LPS-induced apoptosis and inflammatory cell infiltration in mouse liver. J. Nutr. 2020, 150, 1116–1125.
- Lynch, J.W. Native glycine receptor subtypes and their physiological roles. Neuropharmacology 2009, 56, 303–309. Eulenburg, V.S. Glycine transporters: Essential regulators of synaptic transmission. Eur. Neuropsychopharmacol. 2011, 21, S230.
- Martínez-Chantar, M.L.; Vázquez-Chantada, M.; Ariz, U.; Martínez, N.; Varela, M.; Luka, Z.; Capdevila, A.; Rodríguez, J.; Aransay, A.M.; Matthiesen, R.; et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology 2008, 47, 1191–1199. Lynch, J.W. Native glycine receptor subtypes and their physiological roles. Neuropharmacology 2009, 56, 303–309.
- Walrand, S.; Chiotelli, E.; Noirt, F.; Mwewa, S.; Lassel, T. Consumption of a functional fermented milk containing collagen hydrolysate improves the concentration of collagen-specific amino acids in plasma. J. Agric. Food Chem. 2008, 56, 7790–7795. Foster, E.; Wildner, H.; Tudeau, L.; Haueter, S.; Ralvenius, W.T.; Jegen, M.; Johannssen, H.; Hösli, L.; Haenraets, K.; Ghanem, A.; et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 2015, 85, 1289–1304.
- Picanço, E.D.A.; Lopes-Paulo, F.; Marques, R.G.; Diestel, C.F.; Caetano, C.E.R.; de Souza, M.V.M.; Moscoso, G.M.; Pazos, H.M.F. L-arginine and glycine supplementation in the repair of the irradiated colonic wall of rats. Int. J. Color. Dis. 2011, 26, 561–568. Gonzalez-Piña, R.; Nuño-Licona, A. Effects of glycine on motor performance in rats after traumatic spinal cord injury. Proc. West. Pharmacol. Soc. 2007, 50, 131–133.
- Gameiro, A.; Reimann, F.; Habib, A.M.; O’malley, D.; Williams, L.; Simpson, A.K.; Gribble, F.M. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J. Physiol. 2005, 569, 761–772. Luka, Z.; Cerone, R.; Phillips, J.A.; Mudd, H.S.; Wagner, C. Mutations in human glycine N-methyltransferase give insights into its role in methionine metabolism. Hum. Genet. 2002, 110, 68–74.
- Gonzalez-Ortiz, M.; Medina-Santillan, R.; Martinez-Abundis, E.; von Drateln, C.R. Effect of glycine on insulin secretion and action in healthy first-degree relatives of type 2 diabetes mellitus patients. Horm. Metab. Res. 2001, 33, 358–360. Martínez-Chantar, M.L.; Vázquez-Chantada, M.; Ariz, U.; Martínez, N.; Varela, M.; Luka, Z.; Capdevila, A.; Rodríguez, J.; Aransay, A.M.; Matthiesen, R.; et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology 2008, 47, 1191–1199.
- Gannon, M.C.; Nuttall, J.A.; Nuttall, F.Q. The metabolic response to ingested glycine. Am. J. Clin. Nutr. 2002, 76, 1302–1307. Walrand, S.; Chiotelli, E.; Noirt, F.; Mwewa, S.; Lassel, T. Consumption of a functional fermented milk containing collagen hydrolysate improves the concentration of collagen-specific amino acids in plasma. J. Agric. Food Chem. 2008, 56, 7790–7795.
- Yan-Do, R.; Duong, E.; Fox, J.E.M.; Dai, X.; Suzuki, K.; Khan, S.; Bautista, A.; Ferdaoussi, M.; Lyon, J.; Wu, X.; et al. A glycine-insulin autocrine feedback loop enhances insulin secretion from human β-cells and is impaired in type 2 diabetes. Diabetes 2016, 65, 2311–2321. Picanço, E.D.A.; Lopes-Paulo, F.; Marques, R.G.; Diestel, C.F.; Caetano, C.E.R.; de Souza, M.V.M.; Moscoso, G.M.; Pazos, H.M.F. L-arginine and glycine supplementation in the repair of the irradiated colonic wall of rats. Int. J. Color. Dis. 2011, 26, 561–568.
- Gao, X.; Wang, Y.; Sun, G. High dietary choline and betaine intake is associated with low insulin resistance in the Newfoundland population. Nutrition 2017, 33, 28–34. Gameiro, A.; Reimann, F.; Habib, A.M.; O’malley, D.; Williams, L.; Simpson, A.K.; Gribble, F.M. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J. Physiol. 2005, 569, 761–772.
- Benveniste, M.; Mayer, M.L. Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. Biophys. J. 1991, 59, 560–573. Gonzalez-Ortiz, M.; Medina-Santillan, R.; Martinez-Abundis, E.; von Drateln, C.R. Effect of glycine on insulin secretion and action in healthy first-degree relatives of type 2 diabetes mellitus patients. Horm. Metab. Res. 2001, 33, 358–360.
- Clements, J.D.; Westbrook, G.L. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron 1991, 7, 605–613. Gannon, M.C.; Nuttall, J.A.; Nuttall, F.Q. The metabolic response to ingested glycine. Am. J. Clin. Nutr. 2002, 76, 1302–1307.
- Mizusawa, N.; Kimura, Y.; Ishii, A.; Yamanari, T.; Nakazawa, S.; Teramoto, H.; Ono, T.A. Impact of replacement of D1 C-terminal alanine with glycine on structure and function of photosynthetic oxygen-evolving complex. J. Biol. Chem. 2004, 279, 29622–29627. Yan-Do, R.; Duong, E.; Fox, J.E.M.; Dai, X.; Suzuki, K.; Khan, S.; Bautista, A.; Ferdaoussi, M.; Lyon, J.; Wu, X.; et al. A glycine-insulin autocrine feedback loop enhances insulin secretion from human β-cells and is impaired in type 2 diabetes. Diabetes 2016, 65, 2311–2321.
- Collingridge, G.L.; Volianskis, A.; Bannister, N.; France, G.; Hanna, L.; Mercier, M.; Tidball, P.; Fang, G.; Irvine, M.W.; Costa, B.M.; et al. The NMDA receptor as a target for cognitive enhancement. Neuropharmacology 2013, 64, 13–26. Gao, X.; Wang, Y.; Sun, G. High dietary choline and betaine intake is associated with low insulin resistance in the Newfoundland population. Nutrition 2017, 33, 28–34.
- Zafra, F.; Ibáñez, I.; Bartolomé-Martín, D.; Piniella, D.; Arribas-Blázquez, M.; Giménez, C. Glycine transporters and its coupling with NMDA receptors. Glial Amino Acid Transp. 2017, 16, 55–83. Benveniste, M.; Mayer, M.L. Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. Biophys. J. 1991, 59, 560–573.
- Fujita, H.; Sato, K.; Wen, T.C.; Peng, Y.; Sakanaka, M. Differential expressions of glycine transporter 1 and three glutamate transporter mRNA in the hippocampus of gerbils with transient forebrain ischemia. J. Cereb. Blood Flow Metab. 1999, 19, 604–615. Clements, J.D.; Westbrook, G.L. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron 1991, 7, 605–613.
- Zafra, F.; Aragón, C.; Giménez, C. Molecular biology of glycinergic neurotransmission. Mol. Neurobiol. 1997, 14, 117–142. Mizusawa, N.; Kimura, Y.; Ishii, A.; Yamanari, T.; Nakazawa, S.; Teramoto, H.; Ono, T.A. Impact of replacement of D1 C-terminal alanine with glycine on structure and function of photosynthetic oxygen-evolving complex. J. Biol. Chem. 2004, 279, 29622–29627.
- Al-Khrasani, M.; Mohammadzadeh, A.; Balogh, M.; Király, K.; Barsi, S.; Hajnal, B.; Köles, L.; Zádori, Z.S.; Harsing, L.G., Jr. Glycine transporter inhibitors: A new avenue for managing neuropathic pain. Brain Res. Bull. 2019, 152, 143–158. Collingridge, G.L.; Volianskis, A.; Bannister, N.; France, G.; Hanna, L.; Mercier, M.; Tidball, P.; Fang, G.; Irvine, M.W.; Costa, B.M.; et al. The NMDA receptor as a target for cognitive enhancement. Neuropharmacology 2013, 64, 13–26.
- Gomeza, J.; Hülsmann, S.; Ohno, K.; Eulenburg, V.; Szöke, K.; Richter, D.; Betz, H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron 2003, 40, 785–796. Zafra, F.; Ibáñez, I.; Bartolomé-Martín, D.; Piniella, D.; Arribas-Blázquez, M.; Giménez, C. Glycine transporters and its coupling with NMDA receptors. Glial Amino Acid Transp. 2017, 16, 55–83.
- Tsai, G.; Ralph-Williams, R.J.; Martina, M.; Bergeron, R.; Berger-Sweeney, J.; Dunham, K.S.; Jiang, Z.; Caine, S.B.; Coyle, J.T. Gene knockout of glycine transporter 1: Characterization of the behavioral phenotype. Proc. Natl. Acad. Sci. USA 2004, 101, 8485–8490. Fujita, H.; Sato, K.; Wen, T.C.; Peng, Y.; Sakanaka, M. Differential expressions of glycine transporter 1 and three glutamate transporter mRNA in the hippocampus of gerbils with transient forebrain ischemia. J. Cereb. Blood Flow Metab. 1999, 19, 604–615.
- Oeckl, P.; Hengerer, B.; Ferger, B. G-protein coupled receptor 6 deficiency alters striatal dopamine and cAMP concentrations and reduces dyskinesia in a mouse model of Parkinson’s disease. Exp. Neurol. 2014, 257, 1–9. Zafra, F.; Aragón, C.; Giménez, C. Molecular biology of glycinergic neurotransmission. Mol. Neurobiol. 1997, 14, 117–142.
- Oeckl, P.; Ferger, B. Increased susceptibility of G-protein coupled receptor 6 deficient mice to MPTP neurotoxicity. Neuroscience 2016, 337, 218–223. Al-Khrasani, M.; Mohammadzadeh, A.; Balogh, M.; Király, K.; Barsi, S.; Hajnal, B.; Köles, L.; Zádori, Z.S.; Harsing, L.G., Jr. Glycine transporter inhibitors: A new avenue for managing neuropathic pain. Brain Res. Bull. 2019, 152, 143–158.
- Chen, Z.; Hu, B.; Wang, F.; Du, L.; Huang, B.; Li, L.; Qi, J.; Wang, X. Glycine bidirectionally regulates ischemic tolerance via different mechanisms including NR2A-dependent CREB phosphorylation. J. Neurochem. 2015, 133, 397–408. Gomeza, J.; Hülsmann, S.; Ohno, K.; Eulenburg, V.; Szöke, K.; Richter, D.; Betz, H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron 2003, 40, 785–796.
- Liu, R.; Liao, X.Y.; Pan, M.X.; Tang, J.C.; Chen, S.F.; Zhang, Y.; Lu, P.X.; Lu, L.J.; Zou, Y.Y.; Qin, X.P.; et al. Glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of M1 microglial polarization via the NF-κB p65/Hif-1α signaling pathway. J. Immunol. 2019, 202, 1704–1714. Tsai, G.; Ralph-Williams, R.J.; Martina, M.; Bergeron, R.; Berger-Sweeney, J.; Dunham, K.S.; Jiang, Z.; Caine, S.B.; Coyle, J.T. Gene knockout of glycine transporter 1: Characterization of the behavioral phenotype. Proc. Natl. Acad. Sci. USA 2004, 101, 8485–8490.
- Gusev, E.I.; Skvortsova, V.I.; Dambinova, S.; Raevskiy, K.S.; Alekseev, A.A.; Bashkatova, V.G.; Kovalenko, A.V.; Kudrin, V.S.; Yakovleva, E.V. Neuroprotective effects of glycine for therapy of acute ischaemic stroke. Cerebrovasc. Dis. 2000, 10, 49–60. Oeckl, P.; Hengerer, B.; Ferger, B. G-protein coupled receptor 6 deficiency alters striatal dopamine and cAMP concentrations and reduces dyskinesia in a mouse model of Parkinson’s disease. Exp. Neurol. 2014, 257, 1–9.
- Ikejima, K.E.N.I.C.H.I.; Iimuro, Y.U.J.I.; Forman, D.T.; Thurman, R.G. A diet containing glycine improves survival in endotoxin shock in the rat. Am. J. Physiol.-Gastrointest. Liver Physiol. 1996, 271, G97–G103. Oeckl, P.; Ferger, B. Increased susceptibility of G-protein coupled receptor 6 deficient mice to MPTP neurotoxicity. Neuroscience 2016, 337, 218–223.
- Senthilkumar, R.; Viswanathan, P.; Nalini, N. Glycine modulates hepatic lipid accumulation in alcohol-induced liver injury. Pol. J. Pharmacol. 2003, 55, 603–611. Chen, Z.; Hu, B.; Wang, F.; Du, L.; Huang, B.; Li, L.; Qi, J.; Wang, X. Glycine bidirectionally regulates ischemic tolerance via different mechanisms including NR2A-dependent CREB phosphorylation. J. Neurochem. 2015, 133, 397–408.
- Zeb, A.; Rahman, S.U. Protective effects of dietary glycine and glutamic acid toward the toxic effects of oxidized mustard oil in rabbits. Food Funct. 2017, 8, 429–436. Liu, R.; Liao, X.Y.; Pan, M.X.; Tang, J.C.; Chen, S.F.; Zhang, Y.; Lu, P.X.; Lu, L.J.; Zou, Y.Y.; Qin, X.P.; et al. Glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of M1 microglial polarization via the NF-κB p65/Hif-1α signaling pathway. J. Immunol. 2019, 202, 1704–1714.
- Zhong, Z.; Wheeler, M.D.; Li, X.; Froh, M.; Schemmer, P.; Yin, M.; Bunzendaul, H.; Bradford, B.; Lemasters, J.J. L-Glycine: A novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 229–240. Gusev, E.I.; Skvortsova, V.I.; Dambinova, S.; Raevskiy, K.S.; Alekseev, A.A.; Bashkatova, V.G.; Kovalenko, A.V.; Kudrin, V.S.; Yakovleva, E.V. Neuroprotective effects of glycine for therapy of acute ischaemic stroke. Cerebrovasc. Dis. 2000, 10, 49–60.
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxidative Med. Cell. Longev. 2017, 2017, 1716701. Ikejima, K.E.N.I.C.H.I.; Iimuro, Y.U.J.I.; Forman, D.T.; Thurman, R.G. A diet containing glycine improves survival in endotoxin shock in the rat. Am. J. Physiol.-Gastrointest. Liver Physiol. 1996, 271, G97–G103.
- Howard, A.; Tahir, I.; Javed, S.; Waring, S.M.; Ford, D.; Hirst, B.H. Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. J. Physiol. 2010, 588, 995–1009. Senthilkumar, R.; Viswanathan, P.; Nalini, N. Glycine modulates hepatic lipid accumulation in alcohol-induced liver injury. Pol. J. Pharmacol. 2003, 55, 603–611.
- van Bergenhenegouwen, J.; Braber, S.; Loonstra, R.; Buurman, N.; Rutten, L.; Knipping, K.; Savelkoul, P.J.; Harthoorn, L.F.; Jahnsen, F.L.; Garssen, J.; et al. Oral exposure to the free amino acid glycine inhibits the acute allergic response in a model of cow’s milk allergy in mice. Nutr. Res. 2018, 58, 95–105. Zeb, A.; Rahman, S.U. Protective effects of dietary glycine and glutamic acid toward the toxic effects of oxidized mustard oil in rabbits. Food Funct. 2017, 8, 429–436.
- Ham, D.J.; Murphy, K.T.; Chee, A.; Lynch, G.S.; Koopman, R. Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clin. Nutr. 2014, 33, 448–458. Zhong, Z.; Wheeler, M.D.; Li, X.; Froh, M.; Schemmer, P.; Yin, M.; Bunzendaul, H.; Bradford, B.; Lemasters, J.J. L-Glycine: A novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 229–240.
- Ceyhan, G.; Timm, A.-K.; Bergmann, F.; Günther, A.; Aghdassi, A.; Demir, I.; Mayerle, J.; Kern, M.; Lerch, M.; Büchler, M.; et al. Prophylactic glycine administration attenuates pancreatic damage and inflammation in experimental acute pancreatitis. Pancreatology 2011, 11, 57–67. Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxidative Med. Cell. Longev. 2017, 2017, 1716701.
- Hartog, A.; Leenders, I.; van der Kraan, P.M.; Garssen, J. Anti-inflammatory effects of orally ingested lactoferrin and glycine in different zymosan-induced inflammation models: Evidence for synergistic activity. Int. Immunopharmacol. 2007, 7, 1784–1792. Howard, A.; Tahir, I.; Javed, S.; Waring, S.M.; Ford, D.; Hirst, B.H. Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. J. Physiol. 2010, 588, 995–1009.
- Wheeler, M.D.; Thurman, R.G. Production of superoxide and TNF-α from alveolar macrophages is blunted by glycine. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 277, L952–L959. van Bergenhenegouwen, J.; Braber, S.; Loonstra, R.; Buurman, N.; Rutten, L.; Knipping, K.; Savelkoul, P.J.; Harthoorn, L.F.; Jahnsen, F.L.; Garssen, J.; et al. Oral exposure to the free amino acid glycine inhibits the acute allergic response in a model of cow’s milk allergy in mice. Nutr. Res. 2018, 58, 95–105.
- Froh, M.; Thurman, R.G.; Wheeler, M.D. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 283, G856–G863. Ham, D.J.; Murphy, K.T.; Chee, A.; Lynch, G.S.; Koopman, R. Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clin. Nutr. 2014, 33, 448–458.
- Stoffels, B.; Türler, A.; Schmidt, J.; Nazir, A.; Tsukamoto, T.; Moore, B.A.; Schnurr, C.; Kalff, J.C.; Bauer, A.J. Anti-inflammatory role of glycine in reducing rodent postoperative inflammatory ileus. Neurogastroenterol. Motil. 2011, 23, 76-e8. Ceyhan, G.; Timm, A.-K.; Bergmann, F.; Günther, A.; Aghdassi, A.; Demir, I.; Mayerle, J.; Kern, M.; Lerch, M.; Büchler, M.; et al. Prophylactic glycine administration attenuates pancreatic damage and inflammation in experimental acute pancreatitis. Pancreatology 2011, 11, 57–67.
- Weinberg, J.M.; Bienholz, A.; Venkatachalam, M.A. The role of glycine in regulated cell death. Cell. Mol. Life Sci. 2016, 73, 2285–2308. Hartog, A.; Leenders, I.; van der Kraan, P.M.; Garssen, J. Anti-inflammatory effects of orally ingested lactoferrin and glycine in different zymosan-induced inflammation models: Evidence for synergistic activity. Int. Immunopharmacol. 2007, 7, 1784–1792.
- Yamashina, S.; Ikejima, K.; Enomoto, N.; Takei, Y.; Sato, N. Glycine as a Therapeutic Immuno-Nutrient for Alcoholic Liver Disease. Alcohol. Clin. Exp. Res. 2005, 29, 162S–165S. Wheeler, M.D.; Thurman, R.G. Production of superoxide and TNF-α from alveolar macrophages is blunted by glycine. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 277, L952–L959.
- AlShakweer, W.; Alwelaie, Y.; Mankung, A.M.; Graeber, M.B. Bone marrow-derived microglia in pilocytic astrocytoma. Front. Biosci. 2011, 3, 371–379. Froh, M.; Thurman, R.G.; Wheeler, M.D. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 283, G856–G863.
- Xia, C.Y.; Zhang, S.; Gao, Y.; Wang, Z.Z.; Chen, N.H. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int. Immunopharmacol. 2015, 25, 377–382. Stoffels, B.; Türler, A.; Schmidt, J.; Nazir, A.; Tsukamoto, T.; Moore, B.A.; Schnurr, C.; Kalff, J.C.; Bauer, A.J. Anti-inflammatory role of glycine in reducing rodent postoperative inflammatory ileus. Neurogastroenterol. Motil. 2011, 23, 76-e8.
- Zhu, T.; Miller, A.G.; Deliyanti, D.; Berka, D.R.; Agrotis, A.; Campbell, D.J.; Wilkinson-Berka, J.L. Prorenin stimulates a pro-angiogenic and proinflammatory response in retinal endothelial cells and an M1 phenotype in retinal microglia. Clin. Exp. Pharmacol. Physiol. 2015, 42, 537–548. Weinberg, J.M.; Bienholz, A.; Venkatachalam, M.A. The role of glycine in regulated cell death. Cell. Mol. Life Sci. 2016, 73, 2285–2308.
- Xu, Y.; Xu, Y.; Wang, Y.; Wang, Y.; He, L.; Jiang, Z.; Huang, Z.; Liao, H.; Li, J.; Saavedra, J.M.; et al. Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKb-dependent AMPK activation. Brain Behav. Immun. 2015, 50, 298–313. Yamashina, S.; Ikejima, K.; Enomoto, N.; Takei, Y.; Sato, N. Glycine as a Therapeutic Immuno-Nutrient for Alcoholic Liver Disease. Alcohol. Clin. Exp. Res. 2005, 29, 162S–165S.
- Rose, M.L.; Cattley, R.C.; Dunn, C.; Wong, V.; Li, X.; Thurman, R.G. Dietary glycine prevents the development of liver tumors caused by the peroxisome proliferator WY-14,643. Carcinogenesis 1999, 20, 2075–2081. AlShakweer, W.; Alwelaie, Y.; Mankung, A.M.; Graeber, M.B. Bone marrow-derived microglia in pilocytic astrocytoma. Front. Biosci. 2011, 3, 371–379.
- Spittler, A.; Reissner, C.M.; Oehler, R.; Gornikiewicz, A.; Gruenberger, T.; Manhart, N.; Brodowicz, T.; Mittlboeck, M.; Boltz-Nitulescu, G.; Roth, E. Immunomodulatory effects of glycine on LPS-treated monocytes: Reduced TNF-alpha production and accelerated IL-10 expression. FASEB J. 1999, 13, 563–571. Xia, C.Y.; Zhang, S.; Gao, Y.; Wang, Z.Z.; Chen, N.H. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int. Immunopharmacol. 2015, 25, 377–382.
- Li, X.; Bradford, B.U.; Wheeler, M.D.; Stimpson, S.A.; Pink, H.M.; Brodie, T.A.; Schwab, J.H.; Thurman, R.G. Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: Role for glycine-gated chloride channel. Infect. Immun. 2001, 69, 5883–5891. Zhu, T.; Miller, A.G.; Deliyanti, D.; Berka, D.R.; Agrotis, A.; Campbell, D.J.; Wilkinson-Berka, J.L. Prorenin stimulates a pro-angiogenic and proinflammatory response in retinal endothelial cells and an M1 phenotype in retinal microglia. Clin. Exp. Pharmacol. Physiol. 2015, 42, 537–548.
- Okekunle, A.P.; Li, Y.; Liu, L.; Du, S.; Wu, X.; Chen, Y.; Li, Y.; Qi, J.; Sun, C.; Feng, R. Abnormal circulating amino acid profiles in multiple metabolic disorders. Diabetes Res. Clin. Pract. 2017, 132, 45–58. Xu, Y.; Xu, Y.; Wang, Y.; Wang, Y.; He, L.; Jiang, Z.; Huang, Z.; Liao, H.; Li, J.; Saavedra, J.M.; et al. Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKb-dependent AMPK activation. Brain Behav. Immun. 2015, 50, 298–313.
- Gosmanov, N.R.; Gosmanov, A.R.; Gerich, J.E. Glucagon Physiology. In Endotext ; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Rose, M.L.; Cattley, R.C.; Dunn, C.; Wong, V.; Li, X.; Thurman, R.G. Dietary glycine prevents the development of liver tumors caused by the peroxisome proliferator WY-14,643. Carcinogenesis 1999, 20, 2075–2081.
- Yan-Do, R.; MacDonald, P.E. Impaired “glycine”-mia in type 2 diabetes and potential mechanisms contributing to glucose homeostasis. Endocrinology 2017, 158, 1064–1073. Spittler, A.; Reissner, C.M.; Oehler, R.; Gornikiewicz, A.; Gruenberger, T.; Manhart, N.; Brodowicz, T.; Mittlboeck, M.; Boltz-Nitulescu, G.; Roth, E. Immunomodulatory effects of glycine on LPS-treated monocytes: Reduced TNF-alpha production and accelerated IL-10 expression. FASEB J. 1999, 13, 563–571.
- White, P.J.; Lapworth, A.L.; An, J.; Wang, L.; McGarrah, R.W.; Stevens, R.D.; Ilkayeva, O.; George, T.; Muehlbauer, M.J.; Bain, J.R.; et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 2016, 5, 538–551. Li, X.; Bradford, B.U.; Wheeler, M.D.; Stimpson, S.A.; Pink, H.M.; Brodie, T.A.; Schwab, J.H.; Thurman, R.G. Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: Role for glycine-gated chloride channel. Infect. Immun. 2001, 69, 5883–5891.
- Nguyen, D.; Hsu, J.W.; Jahoor, F.; Sekhar, R.V. Eect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J. Clin. Endocrinol. Metab. 2014, 99, 169–177. Okekunle, A.P.; Li, Y.; Liu, L.; Du, S.; Wu, X.; Chen, Y.; Li, Y.; Qi, J.; Sun, C.; Feng, R. Abnormal circulating amino acid profiles in multiple metabolic disorders. Diabetes Res. Clin. Pract. 2017, 132, 45–58.
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. Gosmanov, N.R.; Gosmanov, A.R.; Gerich, J.E. Glucagon Physiology. In Endotext ; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000.
- Magnusson, M.; Wang, T.J.; Clish, C.; Engström, G.; Nilsson, P.; Gerszten, R.E.; Melander, O. Dimethylglycine deficiency and the development of diabetes mellitus. Diabetes 2015, 64, 3010–3016. Yan-Do, R.; MacDonald, P.E. Impaired “glycine”-mia in type 2 diabetes and potential mechanisms contributing to glucose homeostasis. Endocrinology 2017, 158, 1064–1073.
- Zhang, Y.; Lv, S.J.; Yan, H.; Wang, L.; Liang, G.P.; Wan, Q.X.; Peng, X. Effects of glycine supplementation on myocardial damage and cardiac function after severe burn. Burns 2013, 39, 729–735. White, P.J.; Lapworth, A.L.; An, J.; Wang, L.; McGarrah, R.W.; Stevens, R.D.; Ilkayeva, O.; George, T.; Muehlbauer, M.J.; Bain, J.R.; et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 2016, 5, 538–551.
- Ascher, E.; Hanson, J.N.; Cheng, W.; Hingorani, A.; Scheinman, M. Glycine preserves function and decreases necrosis in skeletal muscle undergoing ischemia and reperfusion injury. Surgery 2001, 129, 231–235. Nguyen, D.; Hsu, J.W.; Jahoor, F.; Sekhar, R.V. Eect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J. Clin. Endocrinol. Metab. 2014, 99, 169–177.
- Grotz, M.R.W.; Pape, H.C.; van Griensven, M.; Stalp, M.; Rohde, F.; Bock, D.; Krettek, C. Glycine reduces the inflammatory response and organ damage in a two-hit sepsis model in rats. Shock 2001, 16, 116–121. Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252.
- Yang, S.; Koo, D.J.; Chaudry, I.H.; Wang, P. Glycine attenuates hepatocellular depression during early sepsis and reduces sepsis-induced mortality. Crit. Care Med. 2001, 29, 1201–1206. Magnusson, M.; Wang, T.J.; Clish, C.; Engström, G.; Nilsson, P.; Gerszten, R.E.; Melander, O. Dimethylglycine deficiency and the development of diabetes mellitus. Diabetes 2015, 64, 3010–3016.
- Zhong, Z.; Enomoto, N.; Connor, H.D.; Moss, N.; Mason, R.P.; Thurman, R.G. Glycine improves survival after hemorrhagic shock in the rat. Shock 1999, 12, 54–62. Zhang, Y.; Lv, S.J.; Yan, H.; Wang, L.; Liang, G.P.; Wan, Q.X.; Peng, X. Effects of glycine supplementation on myocardial damage and cardiac function after severe burn. Burns 2013, 39, 729–735.
- Qu, W.; Ikejima, K.; Zhong, Z.; Waalkes, M.P.; Thurman, R.G. Glycine blocks the increase in intracellular free Ca2+ due to vasoactive mediators in hepatic parenchymal cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 283, G1249–G1256. Ascher, E.; Hanson, J.N.; Cheng, W.; Hingorani, A.; Scheinman, M. Glycine preserves function and decreases necrosis in skeletal muscle undergoing ischemia and reperfusion injury. Surgery 2001, 129, 231–235.
- Alvarado-Vásquez, N.; Zamudio, P.; Cerón, E.; Vanda, B.; Zenteno, E.; Carvajal-Sandoval, G. Effect of glycine in streptozotocin-induced diabetic rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 134, 521–527. Grotz, M.R.W.; Pape, H.C.; van Griensven, M.; Stalp, M.; Rohde, F.; Bock, D.; Krettek, C. Glycine reduces the inflammatory response and organ damage in a two-hit sepsis model in rats. Shock 2001, 16, 116–121.
- Benveniste, M.; Mayer, M.L. Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. . Biophys. J. 1991, 59, 560–573..Yang, S.; Koo, D.J.; Chaudry, I.H.; Wang, P. Glycine attenuates hepatocellular depression during early sepsis and reduces sepsis-induced mortality. Crit. Care Med. 2001, 29, 1201–1206.
- Zhong, Z.; Enomoto, N.; Connor, H.D.; Moss, N.; Mason, R.P.; Thurman, R.G. Glycine improves survival after hemorrhagic shock in the rat. Shock 1999, 12, 54–62.
- Qu, W.; Ikejima, K.; Zhong, Z.; Waalkes, M.P.; Thurman, R.G. Glycine blocks the increase in intracellular free Ca2+ due to vasoactive mediators in hepatic parenchymal cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 283, G1249–G1256.
- Alvarado-Vásquez, N.; Zamudio, P.; Cerón, E.; Vanda, B.; Zenteno, E.; Carvajal-Sandoval, G. Effect of glycine in streptozotocin-induced diabetic rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 134, 521–527.
- Benveniste, M.; Mayer, M.L. Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. . Biophys. J. 1991, 59, 560–573..
