Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Vladimir Z Mordkovich.
Hydrocarbons obtained through FTS from a mixture of CO and H2, also known as synthesis gas, are one of the promising sources of hydrocarbon feedstocks for further use in chemical and petrochemical industries [1,2,3,4]. The composition of hydrocarbon mixtures depends on both the catalyst properties and the process parameters.
- Fischer-Tropsch synthesis
- bifunctional catalysts
- zeolite
1. Introduction
Hydrocarbons obtained through FTS from a mixture of CO and H2, also known as synthesis gas, are one of the promising sources of hydrocarbon feedstocks for further use in chemical and petrochemical industries [1,2,3,4][1][2][3][4]. The composition of hydrocarbon mixtures depends on both the catalyst properties and the process parameters (Figure 1). The products of FTS usually follow the molecular weight distribution (MWD) [1,2[1][2][3][4][5][6],3,4,5,6], which is very wide and nonselective for any products being determined by the competition between the processes of growth and hydrocarbon chain termination. For example, high molecular weight n-paraffins are selectively formed in the presence of the Co-based catalysts on alumina, silica or titania supports. Such a mixture of hydrocarbons should be further processed at high temperature and pressure in excess of hydrogen for future use.
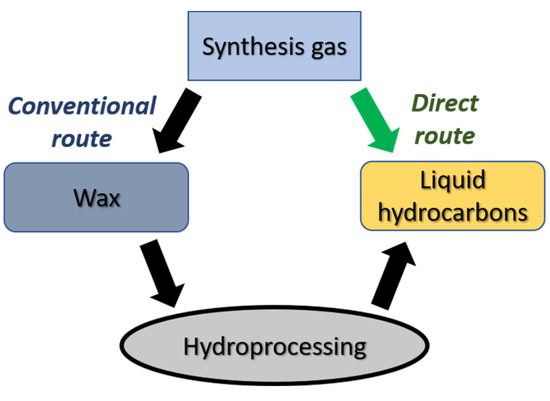
Figure 1.
Potential routes of syngas-to-liquid hydrocarbon process based on Fischer–Tropsch synthesis.
An alternative process is FTS aimed at the direct production of liquid hydrocarbons (synthetic oil) from CO and H2 [6,7,8,9][6][7][8][9]. The idea of combining metal sites active in FTS with acid sites of zeolite active in the cracking and isomerization of hydrocarbons was proposed in the middle of 1970s and continues to be of keen interest to the scientific community [6,7,8,9,10,11,12,13,14,15,16][6][7][8][9][10][11][12][13][14][15][16]. Such catalysts are routinely referred to as bifunctional or hybrid. The composition of the hydrocarbons obtained in their presence depends on the relative rates of reactions on acid and metal sites. The latter depend on concentrations of metal and zeolite sites, their location relative to each other and properties of the support pore system. Therefore, one has to consider the role of mass transfer while developing new bifunctional FTS catalysts [17]. In order to provide intensive mass transfer on the surface of the pellet, it is necessary to reduce diffusion limitations or average molecular weight of the formed hydrocarbons. In the first case, an extended system of macro- and meso-pores is necessary; in the second case, a source of atomic hydrogen or an additional function of the catalyst, —for example, provided by sites with Bronsted acidity—is necessary.
In order to obtain hydrocarbons with a narrower MWD, it is necessary to study both the mutual influence of the components of bifunctional catalysts and the location of metal/acid sites. The composition of FTS products can be controlled by changing the number or/and strength of acid sites that are determined by Si/Al ratio [13].
2. The Role of Cobalt in the Formation of Fischer–Tropsch Synthesis Products
Thermodynamic calculations show that hydrocarbons of any molecular weight, type and structure except acetylene can be formed from CO and H2 in the presence of Co-containing catalysts [1,2,3,4,18][1][2][3][4][18]. However, the FTS contains a lot of consecutive and parallel reactions and thermodynamic calculations based on the assumptions about concurrent equilibrium, so it only allows to evaluate the probability of products formation approximately. In addition, the rates of each reaction depend on the process parameters: for example, with an increase in temperature, the probability of forming unsaturated hydrocarbons and aldehydes increases, and the probability of forming saturated hydrocarbons decreases. With an increase in pressure, the content of heavy hydrocarbons increases. At low-temperature FTS in the presence of Co and Fe catalysts, the fraction of branched products up to C14 does not vary significantly with molecular mass and hydrogen saturation [19]. An increase in the hydrogen content in the synthesis gas favors the formation of saturated linear hydrocarbons. In the case of CO content, an increase favors the formation of olefins and aldehydes, while the degree of carbonization of the catalyst increases. As a result, the actual composition of FTS products differs significantly from thermodynamic calculations. The main FTS reactions correspond to the polymerization mechanism where the single fragment is CHx monomer formed by interaction of CO and H2 [1,2,3,4,18,20][1][2][3][4][18][20]:nCO + (2n + 1)H
2
→ C
n
H
2n+2
+ nH
2O
O
nCO + 2nH
2
→ C
n
H
2n
+ nH
2O
O
References
- Ronald, F.; Probstein, R.; Edwin, H. (Eds.) Synthetic Fuels; Courier Corporation: New York, NY, USA, 2006; 490p.
- Maitlis, P.M.; Klerk, A. (Eds.) Greener Fischer–Tropsch Processes; Wiley-VCH: Weinheim, Germany, 2013; 372p.
- Sineva, L.V.; Mordkovich, V.Z. Trends in gas chemistry catalysis: Cobalt catalysts for Fischer–Topsch synthesis. Part 1. Sci. J. Russ. Gas Soc. 2019, 20, 42–57.
- Sineva, L.V.; Mordkovich, V.Z. Trends in gas chemistry catalysis: Cobalt catalysts for Fischer–Topsch synthesis. Part 2. Sci. J. Russ. Gas Soc. 2019, 21, 56–68.
- Steynberg, A.P.; Dry, M.E. (Eds.) Fisher–Tropsch Technology, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2004; p. 722.
- Henrici-Olive, G.; Olive, S. The Chemistry of the Catalyzed Hydrogenation of Carbon Monoxide; Springer: Berlin/Heidelberg, Germany, 1984; 232p.
- Lapidus, A.L.; Krylova, A.Y. Catalytic synthesis of isoalkanes and aromatic hydrocarbons from CO and H2. Russ. Chem. Rev. 1998, 67, 941.
- Sartipi, S.; Parashar, K.; Valero-Romero, M.J.; Santos, V.P.; Linden, B.; Makkee, M.; Kapteijn, F.; Gascon, J. Hierarchical H-ZSM-5-supported cobalt for the direct synthesis of gasoline-range hydrocarbons from syngas: Advantages, limitations, and mechanistic insight. J. Catal. 2013, 305, 179–190.
- Sineva, L.V.; Asalieva, E.Y.; Mordkovich, V.Z. The role of zeolite in the Fischer–Tropsch synthesis over cobalt–zeolite catalysts. Russ. Chem. Rev. 2015, 84, 1176.
- Murzin, D.Y. Mesolevel Bifunctional Catalysis. Kinet. Catal. 2020, 61, 80–92.
- Li, Y.; Wang, T.; Wu, C.; Li, H.; Qin, X.; Tsubaki, N. Gasoline-range hydrocarbon synthesis over Co/SiO2/HZSM-5 catalyst with CO2-containing syngas. Fuel Process. Technol. 2010, 91, 388–393.
- Chang, C.D.; Lang, W.H.; Silvestri, A.J. Synthesis gas conversion to aromatic hydrocarbons. J. Catal. 1979, 56, 268–273.
- Oukaci, R.; Wu, J.C.S.; Goodwin, J.R., Jr. Effect of SiAl ratio on secondary reactions during CO hydrogenation on zeolite-supported metal catalysts. J. Catal. 1988, 110, 47–57.
- Jong, S.-J.; Cheng, S. Reduction behavior and catalytic properties of cobalt containing ZSM-5 zeolites. Appl. Catal. A Gen. 1995, 126, 51–66.
- Botes, F.G.; Böhringer, W. The addition of HZSM-5 to the Fischer–Tropsch process for improved gasoline production. Appl. Catal. A Gen. 2004, 267, 217–225.
- Martinez, A.; Prieto, G. The Application of Zeolites and Periodic Mesoporous Silicas in the Catalytic Conversion of Synthesis Gas. Top. Catal. 2009, 52, 75–90.
- Sineva, L.V.; Gorokhova, E.O.; Gryaznov, K.O.; Ermolaev, I.S.; Mordkovich, V.Z. Zeolites as a tool for intensification of mass transfer on the surface of a cobalt Fischer–Tropsch synthesis catalyst. Catal. Today 2021, 378, 140–148.
- Falbe, J. Chemical Feedstocks from Coal, Reprint ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1982; 662p.
- Shi, B.; Keogh, R.A.; Davis, B.H. Fischer–Tropsch synthesis: The formation of branched hydrocarbons in the Fe and Co catalyzed reaction. J. Mol. Catal. A Chem. 2005, 234, 85–97.
- Cheng, K.; Kang, J.; King, D.L.; Subramanian, V.; Zhou, C.; Zhang, Q.; Wang, Y. Chapter Three—Advances in Catalysis for Syngas Conversion to Hydrocarbons. Adv. Catal. 2017, 60, 125–208.
- Kuipers, E.W.; Scheper, C.; Wilson, J.H.; Vinkenburg, I.H.; Oosterbeek, H. Non-ASF Product Distributions Due to Secondary Reactions during Fischer–Tropsch Synthesis. J. Catal. 1996, 158, 288–300.
- Jam, S.; Ahangary, M.; Tavasoli, A.; Sadaghiani, K.; Pour, A.N. Enhancement of distillate selectivity in Fischer-Tropsch synthesis by using iron and cobalt catalysts in a novel dual-bed reactor. React. Kinet. Catal. Lett. 2006, 89, 71–79.
- Shi, B.; Wu, L.; Liao, Y.; Jin, C.; Montavon, A. Explanations of the Formation of Branched Hydrocarbons during Fischer–Tropsch Synthesis by Alkylidene Mechanism. Top. Catal. 2014, 57, 451–459.
- Shi, B.; Liao, Y.; Naumovitz, J.L. Formation of 2-alkenes as secondary products during Fischer–Tropsch synthesis. Appl. Catal. A: Gen. 2015, 490, 201–206.
More
