Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ruben Mas-Ballesté and Version 2 by Sirius Huang.
Organocatalysis, the use of chiral organic molecules as catalysts, has emerged as a highly efficient alternative to traditional asymmetric catalysis methods. Chiral porous organic frameworks have emerged as candidates for heterogeneous asymmetric organocatalysis.
- covalent organic frameworks
- covalent triazine frameworks
- conjugated microporous polymers
- asymmetric organocatalysis
- chirality
1. Introduction
Chirality has important implications for many biological processes and plays a crucial role in the development of new drugs and materials [1][2][1,2]. For instance, more than half of the commercial pharmaceuticals are chiral. Thus, in the recent years, a growing interest in enantiomerically pure compounds has emerged in medicinal chemistry because of the possible toxicity of inactive enantiomers [3][4][3,4]. In addition, chirality is relevant for a wide variety of other fields, such as crop protection, flavors, fragrancies, and synthetic chemistry [5][6][7][8][5,6,7,8]. Therefore, asymmetric catalysis, the use of chiral catalysts to selectively produce a desired chiral product, is an important research field that includes several strategies, such as the use of metal complexes or enzymes.
Organocatalysis, the use of chiral organic molecules as catalysts, has emerged as a highly efficient alternative to traditional asymmetric catalysis methods. Chiral organocatalysts correspond to different kinds of molecules such as amines or amino acids, Brønsted acids, phosphoric acids, or imidazolidinones [9]. Asymmetric organocatalysis offers several advantages over traditional strategies. For instance, chiral organic molecules are generally less toxic and more environmentally friendly than metal catalysts [10]. Furthermore, chiral organic molecules can be readily synthesized and are often less expensive. Asymmetric organocatalysis also offers high functional group compatibility, as the reactions can be carried out under mild conditions, reducing the risk of unwanted side reactions. However, organocatalysis suffers from some general drawbacks, such as the need for high catalyst loading, low catalyst stability, and the difficulty in catalyst recovery [11].
Owing to the inherent advantages of asymmetric organocatalysis, the research on systems that allow to overcome their common drawbacks, described above, is a major goal in modern chemistry. To this end, the incorporation of organocatalytic fragments into porous materials is a strategy that has recently started to blossom [12]. A particularly appealing family of porous frameworks with a great potential are those constructed by the covalent assembly of exclusively organic building blocks. Such materials, covalent organic frameworks and their amorphous analogs, offer several advantages over other types of porous materials [13][14][13,14]. First, porous organic materials can present high stability under a wide range of conditions, including high temperatures and acidic or basic environments. Moreover, they possess an extraordinarily tailorable structures and, therefore, their properties can be precisely tuned by modifying their molecular precursors, which can include chiral moieties of different nature [15]. Thus, in the literature a growing number of examples of covalent organic frameworks containing chiral fragments have started to appear, as well as their non-crystalline counterparts.
2. Critical Considerations on the Classification of Porous Organic Frameworks
Porous organic frameworks are materials designed according to the reticular strategy by connecting predetermined building blocks to generate predictable structures and topologies. The term reticular refers to the structure of the materials obtained, which consists of a network of nodes and linkers that form a three-dimensional framework, and can arise from the expansion of a 3D geometry or from the ordered staking of layered structures [16]. Strictly, the definition reticular chemistry implies the isolation of crystalline materials, which is not always the case for the extended structures included herein. In fact, researchers have included catalytic applications of crystalline and amorphous structures. The goal of reticular design is to create materials with tailored properties, such as specific pore sizes, shapes, and pore surface functionalities, which can be used for a variety of applications, including catalysis. As a result of their potential advanced catalytic applications, these materials could address some of the most urgent global social challenges, such as energy and environmental sustainability, and are, therefore, the subject of intense research worldwide [17]. A particular research playground is the synthetic design and application of porous organic frameworks. One of the most attractive features of these materials is the fact that they are constructed from interconnected networks of organic molecules and, as a consequence, possess a high degree of tunability. Therefore, it is possible to design and synthesize extended organic materials with a wide range of different properties, making them suitable for use in a variety of different contexts. The assembly of organic materials following reticular design has resulted in a plethora of materials that correspond to different denominations. The first and most popular family of reticular organic materials are the Covalent Organic Frameworks (COFs), which are defined as follows: “Class of materials that form two- or three-dimensional structures through reactions between organic precursors resulting in strong, covalent bonds to afford porous, stable, and crystalline materials” [18]. Depending on the geometry of the selected building blocks, the extended structures can adopt several laminar or 3D topologies. This structural design gives rise to engineered pores of predictable sizes and shapes, as shown in Figure 1. The most common approaches for synthesizing COFs include solvothermal, solid-state, microwave-assisted methods, and condensation reactions performed at room temperature [19]. In all cases, the reactivity between the functional groups on the organic building blocks leads to the formation of strong covalent bonds as links of the extended organic framework. The types of linkages that have been used for the preparation of COFs include, among others, boronic esters, boroxine, imine, hydrazone, phenazine, azine, imide, and triazine moieties (Scheme 1) [20][21][20,21].
Figure 1.
Representative topologies commonly obtained in the assembly COFs.
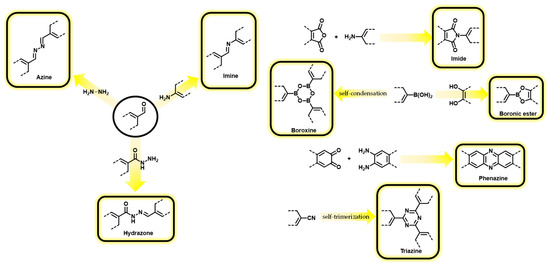
Scheme 1.
Reactions commonly used in the assembly of COFs.
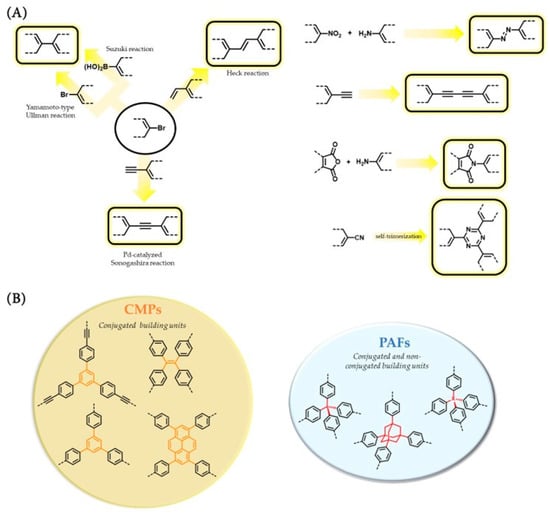
Scheme 2.
(
A
) Reactions commonly used in the assembly of CMPs and PAFs. (
B
) Representative basic structural motifs in CMPs and PAFs.

Figure 2.
Current classification of extended organic materials. The representative examples of amorphous materials are shown.
3. Introduction of Chirality into Porous Organic Frameworks
There are four main strategies to incorporate chirality into porous organic frameworks (Figure 3):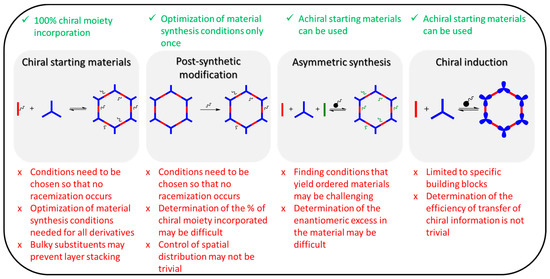
Figure 3. The reported approaches to incorporate chirality into reticular organic materials and related advantages and disadvantages (ticks denote advantages and X denotes disadvantages).
- (i)
-
Synthesis using chiral building blocks;
- (ii)
-
Post-synthetic modification;
- (iii)
-
Asymmetric synthesis;
- (iv)
-
External chiral induction.
4. Chiroptical Responses
Chiroptical responses, those employing right and left circularly polarized light, have been extensively used in the last half a century for the structural characterization of chiral systems [46][73]. These spectroscopies feature larger sensitivity to the geometry of the system under study compared to the non-chiral spectroscopy counterparts, i.e., electronic circular dichroism (ECD) vs. ultraviolet/visible spectroscopy (UV/Vis). While UV/Vis is only proportional to the transition electric dipole moment, ECD is proportional to the dot product of the transition electric dipole moment and transition magnetic dipole moment (Figure 4). In the illustrated example, while the electron density displacement along the three chromophores present in the system (black arrows) features an overall transition electric dipole moment perpendicular to the macrocycle (gray arrow), this circulation of electron density around the cycle generates a transition magnetic dipole moment perpendicular to the cycle (orange arrow). However, the antiparallel or antiparallel alignment of transition electric and magnetic dipole moments depends on the chirality of the system (antiparallel/parallel in the illustrated (M,M,M)/(P,P,P)-enantiomer) [47][74]. This particularity enables not only the determination of the absolute configuration [48][75], the handedness of a system, but also the determination of the conformation [49][76] and even the characterization of host–guest complexes [50][77] and self-assembled systems [51][78].
Figure 4. Representation of transition electric dipole moment (gray arrows: total transition electric dipole moment; black arrows: the contribution from the different chromophores in the molecule) and transition magnetic dipole moment (red arrows) of the lowest electronic transition of a (M,M,M)-configured (left) and (P,P,P)-configured (right) cyclic spirobifluorene oligomer [47][74].
