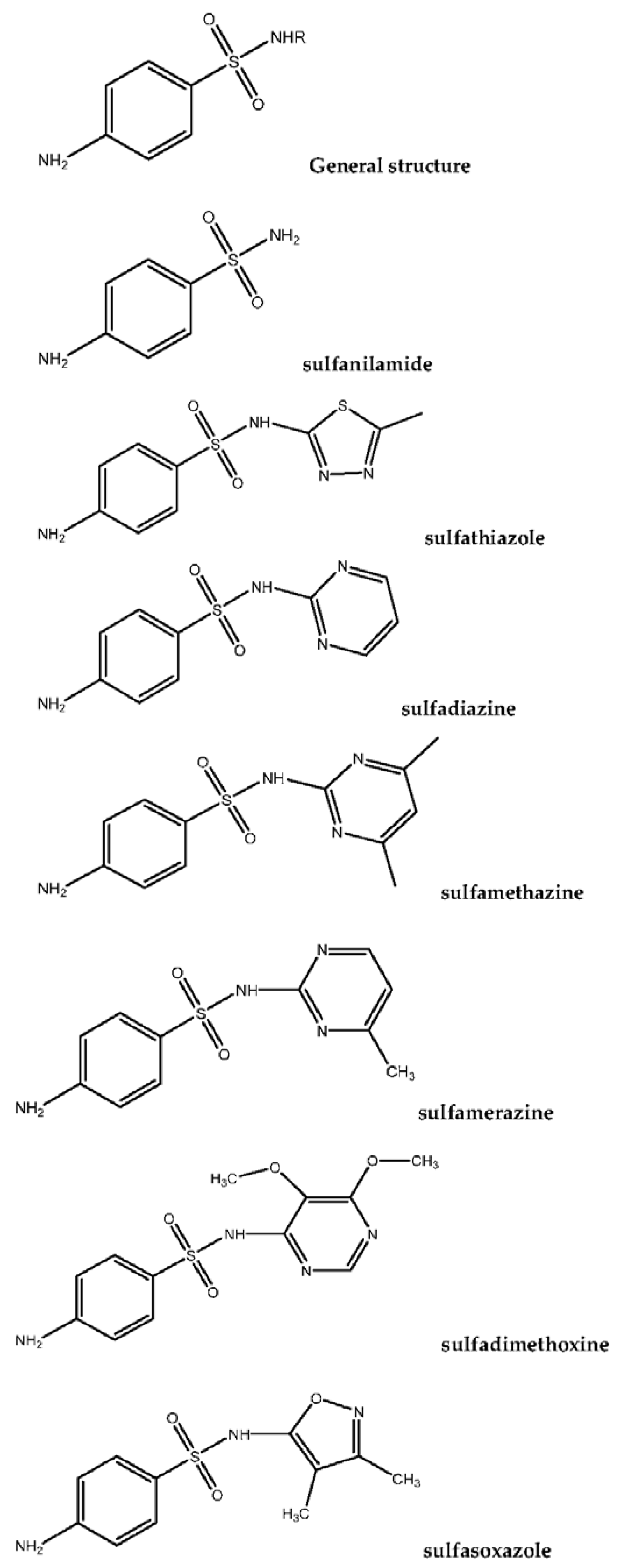
Sulfonamides (SAs) represent a significant category of pharmaceutical compounds due to their effective antimicrobial characteristics. SAs were the first antibiotics to be used in clinical medicine to treat a majority of diseases, since the 1900s. In the dairy farming industry, sulfa drugs are administered to prevent infection, in several countries. This increases the possibility that residual drugs could pass through milk consumption even at low levels. These traces of SAs will be detected and quantified in milk.
- sulfonamides
- determination
- extraction
- microextraction
- milk
1. Introduction
-
their maximum operating temperatures are in the range between 240–280 °C
-
they are not stable with the organic solvents due to swelling
-
they break easily
-
the possibility of stripping of coatings due to analyst’s handling errors.
2. The Demand of Microextraction Techniques
As already mentioned, sample preparation is the most demanding step in any analytical workflow. The main purpose of sample preparation is to transfer the analytes of interest from a complex matrix to a compatible medium for further determination by an analytical technique. In addition, sample preparation often includes procedures such as clean up, analyte enrichment and derivatization. So, it is clear that this step is time consuming and usually requires the use of large organic solvent volumes and the waste of reagents and consumables. For these reasons, the trend is the introduction of more “green” and micro-techniques in sample preparation. The idea of sustainable ecological development was introduced in 1987 in a report of the World Commission on Environment and Development. The term green chemistry was mentioned by P. Anastas in 1991 at the US Environmental Protection Agency (EPA). As a result, in 1993, the comprehensive US Green Chemistry Program was established, which included cooperation among many governmental agencies and research institutions. While Anastas and co-workers were elaborating the ideas of green chemistry, the first paradigms of green analytical chemistry were introduced. GAC, introduced in 1999, became a whole part of chemical nomenclature, and numerous reviews and original studies have been published in this topic. The principles of green chemistry and by extension of GAC are presented in Table 1 [14].|
No. |
Principle of GAC |
|---|---|
|
1 |
Direct analytical approaches should be preferred in order to avoid sample preparation |
-
Although there is a wide range of chemistries, many choices for manipulating solvent and pH conditions, optimization is time consuming. In many cases, several steps are required.
-
The cost per sample is higher than that of simple liquid–liquid extraction (LLE).
3. Microextraction Techniques for the Determination of SAs in Milk
The reported techniques are summarized in Table 2.|
Analyte |
Extraction Type |
Determination |
LOD |
Recovery |
Reference |
|
|---|---|---|---|---|---|---|
|
sulfachloropyridazine, sulfadiazine, sulfadimethoxine, sulfamethazine, sulfamethoxazole, sulfamethoxypyridazine, sulfaquinoxaline, sulfathiazole, sulfisoxazole |
MSPE |
HPLC-DAD |
7–14 μg/L |
81.88%–114.9% |
[16] | |
|
2 |
Minimum sample size and minimum number of samples are the main goals of this principle |
|||||
|
sulfadiazine, sulfamerazine, sulfamethazine sulfamethizole, sulfamethoxazole, sulfadimethoxine |
SBSE |
HPLC-MS/MS |
0.9–10.5 μg/L |
68%–115% |
[17] |
3 |
|
In situ measurements are considered necessary |
||||||
sulfamerazine, sulfamethizole, sulfadoxine, sulfamethoxazole, sulfisoxazole |
MSPE |
HPLC-UV |
1.16–1.59 μg/L |
62.0%–104.3% |
[19] |
|
9 |
The use of energy should be reduced |
|||||
|
10 |
Reagents ensured from renewable sources should be preferred |
|||||
|
11 |
-
elimination or reduction of the use of chemical substances(such as solvents, reagents, preservatives, additives for pH adjustment)
Toxic reagents should be minimized or replaced |
12 |
- elimination of energy consumption
4 | |||||||
|
sulfadiazine, sulfamethazine, sulfamonomethoxine, sulfamethoxazole, sulfaquinoxaline |
Energy saving and reagent reduction is achieved by process integration |
||||||
SBSE |
HPLC-DAD |
4.29–26.3 μg/L |
54.8%–126% |
[20] |
5 |
Automated and miniaturized methods should be developed and applied |
|
|
sulfapyridine, sulfadiazine, sulfachloropyridazine, sulfadoxin, sulfamethoxazole, sulfadimethoxin, sulfamethizol, sulfameter, sulfamethazine |
DLLME |
HPLC-FL |
0.60–1.21 μg/L |
90.8%–104.7% |
6 |
Derivatization is not preferable |
|
[ | ] | ||||||
|
(same analytes with the above) |
QuEChERS |
HPLC-FL |
1.15–2.73 μg/L |
83.6%–104.8% |
[21] |
7 |
Production of a large volume of analytical waste should be avoided and proper management of analytical waste should be provided |
|
sulfamethazine |
MIP-silica column |
HPLC-UV |
7.9 μg/L |
79.3%–87.4% |
[22] |
8 |
Multi-analyte or multi-parameter methods are preferred versus methods using single-analyte |
|
sulfamethazine, sulfamethoxazole, sulfadizaine, sulfaquinoxaline, sulfametoxydiazine, sulfadimethoxine, sulfamethizole |
RA-MIP |
HPLC-UV |
0.8–2.7 μg/L |
93%–107% |
[23] |
The operator should work under safe conditions |
- proper management of analytical waste
-
increased safety for the operator
sulfamethazine, sulfisoxazole, sulfadimethoxine | |||||
FPSE | |||||
HPLC-UV |
- |
22.98%–49.5% |
[24] |
||
|
sulfadimidine, sulfachloropyridazine, sulfamonomethoxine, sulfachloropyrazine |
M-G-PTE |
LC-UV |
0.004–0.012 μg/L |
90.1%–113.5% |
[25] |
|
sulfamethazine , sulfamethoxypyridazine, sulfamethoxydiazine, sulfamethoxazole, sulfadimethoxine, sulfaphenazole |
IL-based MADLLME |
HPLC-FL |
0.018–0.031 μg/L |
97.3%–107.9% |
[26] |
References
- Martins, T.A.; Melo, J.; Barreto, F.; Hoff, R.B.; Jank, L.; Bittencourt, M.S.; Arsand, J.B.; Schapoval, E.E.S. A simple, fast and cheap non-SPE screening method for antibacterial residue analysis in milk and liver using liquid chromatography–tandem mass spectrometry. Talanta 2014, 129, 374–383.
- Dmitrienko, G.S.; Kochuk, V.E.; Apyari, V.V.; Tolmacheva, V.V.; Zolotov, Y.A. Recent advances in sample preparation techniques and methods of sulfonamides detection—A review. Anal. Chim. Acta 2014, 850, 6–25.
- Dmitrienko, G.S.; Kochuk, V.E.; Tolmacheva, V.V.; Apyari, V.V.; Zolotov, Y.A. Determination of the total content of some sulfonamides in milk using solid-phase extraction coupled with off-line derivatization and spectrophotometric detection. Food Chem. 2015, 188, 51–56.
- European Commission Decision 2002/657/EC. Available online: http://www.fao.org/fao-who-codexalimentarius/en/ (accessed on 20 November 2016).
- Shao, M.; Zhang, X.; Li, N.; Shi, J.; Zhang, H.; Wang, Z.; Zhang, H.; Yu, A.; Yu, Y. Ionic liquid-based aqueous two-phase system extraction of sulfonamides in milk. J. Chromatogr. B 2014, 961, 5–12.
- Spietelum, A.; Marcinkowski, L.; Guardi, M.; Namieśnik, J. Recent developments and future trends in solid phase microextraction techniques towards green analytical chemistry. J. Chromatogr. A 2013, 1321, 1–13.
- Płotka-Wasylka, J.; Szczepanska, N.; Guardia, M.; Namiesnik, J. Miniaturized solid-phase extraction techniques. Trends Anal. Chem. 2015, 73, 19–38.
- Ouyang, G.; Pawlizyn, J. Recent developments in SPME for on-site analysis and monitoring. Trends Anal. Chem. 2006, 25, 692–703.
- Merkle, S.; Kleeberg, K.K.; Fritsche, J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography 2015, 2, 293–381.
- Baltussen, E.; Sandrs, P. Stir Bar Sorptive Extraction SBSE (SBSE), a Novel Extraction Technique for Aqueous Samples: Theory and Principles. J. Microcolumn Sep. 1999, 11, 693–749.
- Sarafraz-Yazdi, A.; Amiri, A. Liquid-phase microextraction. Trends Anal. Chem. 2010, 20, 1–14.
- Fumes, H.B.; Silva, M.R.; Andrade, F.N.; Nazario, C.E.D.; Lanças, M.F. Recent advances and future trends in new materials for sample preparation. Trends Anal. Chem. 2015, 71, 9–25.
- Tobiszewski, M.; Mechlinska, A.; Zygmunt, B.; Namiesnik, J. Green analytical chemistry in sample preparation for determination of trace organic pollutants. Trends Anal. Chem. 2009, 28, 943–951.
- Galuszka, A.; Zdzislaw, M.; Namiesnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. Trends Anal. Chem. 2013, 50, 78–84.
- Chung, H.H.; Lee, J.B.; Chung, Y.H.; Lee, K.G. Analysis of sulfonamide and quinolone antibiotic residues in Korean milk using microbial assays and high performance liquid chromatography. Food Chem. 2009, 113, 297–301.
- Ibarra, I.; Miranda, J.M.; Rodriguez, J.A.; Nebot, C.; Cepeda, A. Magnetic solid phase extraction followed by high-performance liquid chromatography for the determination of sulphonamides in milksamples. Food Chem. 2014, 157, 511–517.
- Yu, C.; Bin, H. C18-coated stir bar sorptive extraction combined with high performance liquid chromatography-electrospray tandem mass spectrometry for the analysis of sulfonamides in milk and milk powder. Talanta 2012, 90, 77–84.
- Wen, Y.; Zhang, M.; Zhao, Q.; Feng, Y.Q. Monitoring of Five Sulfonamide Antibacterial Residues in Milk by In-Tube Solid-Phase Microextraction Coupled to High-Performance Liquid Chromatography. J. Agric. Food Chem. 2005, 53, 8468–8473.
- Li, Y.; Xu, W.; Li, Z.; Zhong, S.; Wang, W.; Wang, A.; Chen, J. Fabrication of CoFe2O4-grapheme nanocomposite and its application in the magnetic solid phase extraction of sulfonamides from milk samples. Talanta 2015, 144, 1279–1286.
- Huang, X.; Qiu, N.; Yuan, D. Simple and sensitive monitoring of sulfonamide veterinary residues in milk by stir bar sorptive extraction based on monolithic material and high performance liquid chromatography analysis. J. Chromatogr. A 2009, 1216, 8240–8245.
- Arroyo-Manzanares, N.; Gracia, G.L.; Garcia-Campana, M.A. Alternative sample treatments for the determination of sulfonamides in milk by HPLC with fluorescence detection. Food Chem. 2014, 143, 459–464.
- Su, S.; Zhang, M.; Li, B.; Zhang, H.; Dong, X. HPLC determination of sulfamethazine in milk using surface-imprinted silica synthesized with iniferter technique. Talanta 2008, 76, 1141–1146.
- Su, S.; Xu, W.; Jiang, P.; Wang, H.; Dong, X.; Zhang, M. Determination of sulfonamides in bovine milk with column-switching high performance liquid chromatography using surface imprinted silica with hydrophilic external layer as restricted access and selective extraction material. J. Chromatogr. A 2010, 1217, 7198–7207.
- Samanidou, V.; Kabir, A.; Furton, G.K.; Karageorgou, E.; Manousi, N. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436.
- Yan, H.; Sun, N.; Liu, S.; Row, H.K.; Song, Y. Miniaturized graphene-based pipette tip extraction coupled with liquid chromatography for the determination of sulfonamide residues in bovine milk. Food Chem. 2014, 158, 239–244.
- Wang, Z.; Xu, X.; Su, R.; Zhao, X.; Liu, Z.; Zhang, Y.; Li, D.; Li, X.; Zhang, H. Ionic liquid-based microwave-assisted dispersive liquid-liquid microextraction and derivatization of sulfonamides in river water, honey, milk, and animal plasma. Anal. Chim. Acta 2011, 707, 92–99.
