Triple negative breast cancer (TNBC) has a negative expression of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptors (HER2). The survival rate for TNBC is generally worse than other breast cancer subtypes. TNBC treatment has made significant advances, but certain limitations remain. Treatment for TNBC can be challenging since the disease has various molecular subtypes. A variety of treatment options are available, such as chemotherapy, immunotherapy, radiotherapy, and surgery. Chemotherapy is the most common of these options. TNBC is generally treated with systemic chemotherapy using drugs such as anthracyclines and taxanes in neoadjuvant or adjuvant settings. Developing resistance to anticancer drugs and off-target toxicity are the primary hindrances to chemotherapeutic solutions for cancer.
1. Introduction
Cancer continues to be a growing menace to society; in one year, a mortality rate of 8.2 million was recorded worldwide
[1]. Over 13.1 million people are expected to die from cancer globally by 2030, making it the leading cause of death after cardiovascular diseases
[1][2][1,2]. Cancer can affect any part of the body, occurring in all genders
[2].
Breast cancer is the most commonly diagnosed cancer among women and has surpassed lung cancer, with about 2.3 million cases yearly
[3][4][3,4]. This situation calls for a comprehensive understanding of the disease and the development of improved therapeutics. Breast cancer is a multifactorial disease involving environmental, hormonal, genetic, and various lifestyle and nutritional factors. Hence, breast cancer patients experience various clinical, pathological, and molecular peculiarities
[5]. The molecular subtypes of breast cancer are classified by the expression profile of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2).
Triple negative breast cancer (TNBC) has low or no expression of estrogen receptors (ER), progesterone receptors (PR), and the human epidermal growth factor receptors (HER2; consequently, it does not respond to drugs that target these receptors
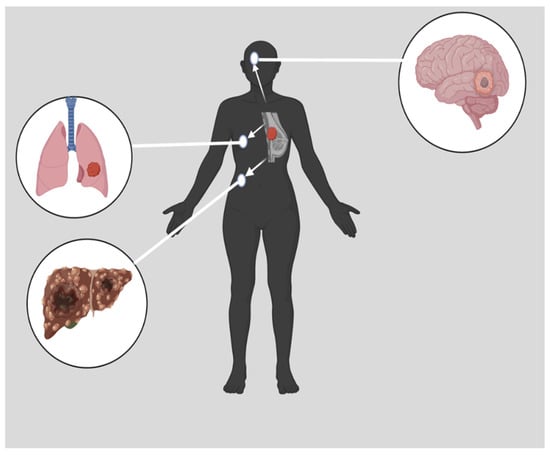
[6][7][8][9][6,7,8,9] and there is currently no standardized TNBC treatment regimen. TNBC is characterized by a high level of cell invasiveness and visceral metastasis to organs, usually the brain, lungs, and liver (
Figure 1), with an average survival time of 18 months
[10][11][10,11]. TNBC is more likely to metastasize to the central nervous system and internal organs such as the liver, bones, and lungs. Patients with TNBC have a much shorter survival period once distant metastases have occurred
[12]. TNBC is usually detected late in the body at the advanced stage
[11][13][11,13]. It is more likely that TNBC is related to hereditary conditions than other breast cancer subtypes. In the American Cancer Society’s study of newly diagnosed breast cancer, 10% of patients had breast cancer gene 1 (BRCA1) and breast cancer gene 2 (BRCA2) mutations, whereas 35% of patients with TNBC carried a BRCA1 mutation, and 8% carried a BRCA2 mutation. There is a greater than one-third chance of developing TNBC among BRCA1 mutation carriers
[14]. Typically, TNBC is diagnosed via imaging and immunohistochemistry (IHC), which are operator-dependent and time-consuming procedures
[15]. To enhance the diagnostic efficiency of TNBC, rapid and advanced technologies such as immuno positron emission tomography (PET), nanobiosensor, circulating tumor nucleic acids (ctNAs), and blood-based liquid biopsy are essential
[16]. Compared to other breast cancer subtypes, TNBC is the most immunogenic subtype with a limited treatment option
[17].
Figure 1. Metastasized TNBC. Many distinct characteristics are associated with TNBC. These characteristics include a high level of cell invasiveness and metastasis to other organs, such as the brain, the lungs, or the liver
[10]. Created with
Biorender.Com, accessed on 14 June 2023.
TNBC is the most aggressive and heterogeneous of all breast cancer subtypes; hence it has been described as fatal
[10][18][19][10,18,19]. Literature evidence indicates that it is reportedly the greatest cause of mortality in women
[20][21][20,21]. TNBC accounts for 15–20% of all breast cancer. Population-based studies confirm that TNBC is prevalent in young pre-menopausal women under 40 years, African American, and Hispanic women of lower socioeconomic backgrounds
[11][22][23][24][11,22,23,24]. Non-Hispanic African American women have a lower breast cancer incidence than non-Hispanic White women
[25]. However, African American women have doubled triple-negative breast cancer incidence
[26]. Possibly, this is due to the relatively low number of African American women receiving treatment recommended by guidelines. In addition, there is a difference in treatment outcomes between African American women and white women with TNBC. According to a population-based cohort study of 23,213 patients with TNBC, African American women were significantly more likely to die from breast cancer than white women
[27]. The authors suggested that disparities in surgery and chemotherapy may contribute to this phenomenon.
There have not been many new treatment methods developed for TNBC compared to other types of breast cancer due to the aggressive nature of this type of breast cancer
[28]. Chemotherapy takes advantage of the high growth rate of tumor tissues. Angiogenesis inhibitors cut the blood flow to tumor tissues, and interference in the ribonucleic acid (RNA) messenger pathway causes apoptosis in the already impaired deoxyribonucleic acid (DNA)
[11][29][11,29]. There is a tendency for TNBC tissues to express more epithelial growth factor receptor (EGFR) protein than other breast cancer subtypes, which makes them more resistant to conventional treatment
[7]. Several inflammatory molecules, including tumor-associated macrophages (TAM), tumor-infiltrating lymphocytes (TILs), and cytokines, reduce the immune response to TNBC during chemotherapy, increasing the possibility of complications related to TNBC. Controlling these inflammatory molecules may improve patients’ overall and disease-free survival rates with TNBC. The manipulation of microRNAs (miRNA) in TNBC can reduce the risk of early relapse and metastasis by influencing cancer cell survival, proliferation, invasion, and metastasis
[28]. TNBC is associated with chemoresistance due to the poor prognosis, relapse, and distal metastasis of TNBC
[30].
2. Triple Negative Breast BCancer
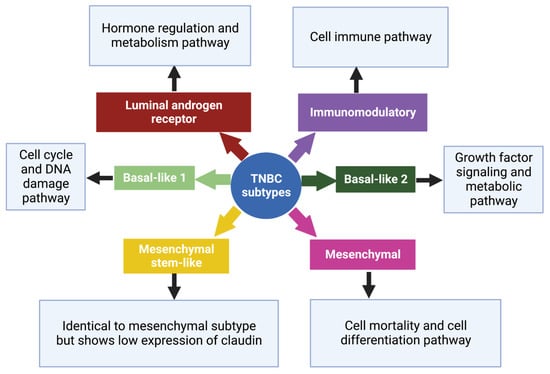
TNBCs typically resemble basal-like breast cancer (BLBC) with overlapping gene expression characteristics
[11]. Based on gene expression profiles, six TNBC subtypes, each with a different gene expression profile and ontology, were identified which are basal-like 1 (BL-1), basal-like 2 (BL-2), an immunomodulatory subtype (IM), a mesenchymal subtype (M), a mesenchymal stem-like subtype (MSL), and a luminal androgen receptor subtype (LAR)
[28][31][28,31], (
Figure 2). Despite this, most triple-negative breast cancers (80%) express markers of basal-associated cancer, including basal cytokeratin, vimentin, EGFR, and mutated BRCA1/2
[22]. The genetic variations among these subtypes of TNBC suggest individualized therapy rather than a generalized approach
[32].
Figure 2. Subtypes of TNBC with their characteristic pathways. On the basis of gene expression profiles, six TNBC subtypes have been classified, each with a specific gene expression profile and ontology
[28]. Created with
Biorender.com, accessed on 15 June 2023.
2.1. Basal-like 1 and 2 Subtypes
Approximately 75% of TNBCs are basal-like subtypes, so TNBC makes up the majority of these subtypes. A high level of DNA damage response is associated with the basal-like 1 subtype. According to studies, basal-like immune-suppressed subtypes of TNBC exhibit lower levels of B cell, T cell, and natural killer cells, resulting in worse prognoses. Generally, all BRCA1/2 mutations are associated with basal-like gene patterns. Anti-ER and HER2 therapies would not be effective on basal-like breast cancers since neither of these proteins is typically expressed in this type of cancer
[14][33][14,33]. This subtype of TNBC is marked by high expression of cell cycle-related genes, DNA damage response (DDR) genes, and chemotherapy sensitivity
[11][32][11,32]. According to studies, basal-like subtypes expressing higher levels of cell cycle genes and DNA damage response genes were sensitive to platinum drugs (cisplatin), and the BL-1 subtype has shown sensitivity to polyadenosine diphosphate (ADP)-ribose) polymerase (PARP) inhibitors
[34][35][36][37][34,35,36,37].
2.2. Luminal Androgen Receptor Subtype
The luminal androgen receptors regulate the synthesis of steroids and porphyrin, as well as the metabolism of androgens and estrogens. In most TNBCs, androgen receptor (AR) expression is associated with a high survival rate. AR is, therefore, a prognostic indicator. This subtype has a 10-fold higher androgen receptor expression than other subtypes. The findings of recent studies suggest that AR inhibitors could be beneficial to patients with TNBC who are AR-positive
[14][38][14,38].
2.3. Mesenchymal and Mesenchymal Stem-like Subtypes
In addition to the mesenchymal subtypes featuring pathways involved in cell motility and differentiation, the mesenchymal stem-like subtype has components interfering with the EGFR, calcium signaling, and G-protein receptors. It is characterized by elevated expression of signal transducer and activator of transcription (STAT) genes responsible for developing T-cells, B-cells, and natural killer cells. Various changes are observed in the M subtype, influencing extracellular matrix (ECM) receptor interaction. Epithelial–mesenchymal transformation (EMT) and cancer stem cells are characteristic of the M subtype
[39]. Phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) inhibition and non-receptor tyrosine kinase (Src) inhibition were effective in suppressing the epithelial–mesenchymal transition (EMT) and growth factor pathways in mesenchymal and MSL cell lines
[34].
2.4. Immunomodulatory Subtype
This subtype shows similarity to the basal-like subtype. The prognosis in this subtype is favorable, but the histological grade is high. These subtypes are immune-evading due to a saturation of immune cell signaling. These tumors likely achieve immune escape by recruiting immune suppressive cells or activating immune checkpoint molecules, which provides a potential reason to use an immune checkpoint blockade therapeutically
[32].