Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Maximilian Daniel Lyon and Version 1 by Maximilian Daniel Lyon.
Sperm cells must undergo a complex maturation process after ejaculation to be able to fertilize an egg. One component of this maturation is hyperpolarization of the membrane potential to a more negative value. The ion channel responsible for this hyperpolarization, SLO3, was first cloned in 1998, and since then much progress has been made to determine how the channel is regulated and how its function intertwines with various signaling pathways involved in sperm maturation.
- membrane hyperpolarization
- SLO3
- contraception
- potassium channels
- sperm
- acrosomal exocytosis
- capacitation
- hyperactivated motility
1. Introduction
Sperm have a long and complex maturation process that completes after they are expelled from the body in which they are produced. This post-ejaculatory process gives sperm the capacity to fertilize an oocyte and thus is termed capacitation [1,2]. Capacitation occurs in the female genital tract and involves many molecular changes including increases in cyclic AMP, protein tyrosine phosphorylation [3], intracellular pH [4,5,6,7], potassium ion (K+) conductance [8], and intracellular calcium (Ca2+) concentration [7,9,10,11,12]. Additionally, the plasma membrane hyperpolarizes to a more negative potential [8,13,14,15,16,17]. These changes culminate in two major physiological changes. The sperm become hyperactive, characterized by an asymmetry of flagellar beating and change in the forces generated [18,19]. This facilitates sperm release from the oviductal reservoir and helps them penetrate through the cumulus and extracellular matrix surrounding the egg (zona pellucida) [20,21]. Additionally, they undergo acrosomal exocytosis, which helps them penetrate the zona pellucida [22,23,24] and exposes binding sites that allow the sperm membrane to fuse with the membrane of the oocyte [25]. Each step of capacitation is required for normal sperm function, but how each step is regulated and regulates other steps has not been fully determined.
A key component of sperm capacitation in many species, from marine invertebrates to mammals, is changes in membrane potential [26,27,28]. Membrane potential is the electrical potential difference (voltage) across a cell’s plasma membrane and is determined by the differences in ion concentrations across the membrane and the selective permeability of the membrane to said ions. One of the most prominent ions for controlling membrane potential in sperm is K+. In 1987, K+-dependent transient membrane hyperpolarization was first reported in sea urchin sperm in response to a signal from the egg jelly [28]. This hyperpolarization was later shown to also occur in murine and bovine sperm and to be associated with capacitation [13]. Like their mammalian counterparts, human sperm undergo a capacitation-associated hyperpolarization from approximately −40 mV [29] to approximately −58 mV [30].
One candidate for the driver of sperm membrane hyperpolarization in mice was SLO3, a potassium channel expressed exclusively in sperm discovered in 1998 [36]. In 2007 a potassium current was identified in mouse sperm that shared several key features with this channel [37]. This current, dubbed IKSper, was found to be activated by intracellular pH. The magnitude of the current meant that it was capable of driving large changes in membrane potential [8,37]. These traits matched those of SLO3, and it was confirmed that SLO3 was responsible for IKSper when a SLO3 knockout mouse was generated [37,38,39,40]. Deletion of SLO3 completely abolished the IKSper current. Additionally, sperm from SLO3 knockout mice lack the hyperpolarization that occurs during capacitation. These sperm also lack the resulting Ca2+ influx through CatSper, the primary Ca2+ channel in sperm that is also necessary for fertility. [38,39]. As a result, SLO3 knockout mice are completely male-infertile.
Several lines of evidence suggest that defects in hyperpolarization can result in human infertility as well. For example, failure to hyperpolarize was correlated with a failure to undergo acrosomal exocytosis in mice [13,27], indicating that sperm membrane hyperpolarization is a key event in sperm capacitation. In humans, electrophysiological studies of patients undergoing in vitro fertilization (IVF) or intra-cytoplasmic sperm injection (ICSI) revealed that ~10% of patients with subfertility have depolarized membrane potentials caused by K+ conductance abnormalities [31]. Sperm isolated from men with idiopathic infertility or asthenozoospermia had a significantly more depolarized membrane potential than those from men with normal fertility [32]. Furthermore, capacitated sperm are more hyperpolarized than non-capacitated sperm [26,33]. In 2020, two groups independently used flow cytometry to quantitate membrane potential in sperm from normozoospermic donors and showed that the ability of sperm to hyperpolarize in capacitating conditions correlated with hyperactivation of motility, acrosomal exocytosis, and success in IVF [34,35].
Given the importance of sperm membrane potential in capacitation and fertilization, many researchers have focused on identifying the responsible K+ channels. Two recent papers provide genetic evidence supporting the role of SLO3 in human fertility. Lv et al. reported that a missense mutation and a splice variant of human SLO3 channels are associated with male infertility [96]. However, it should be noted that the male patient in the study presented with asthenozoospermia, a condition characterized by reduced or absent motile sperm [113]. This disorder is not known to be associated with SLO3-deficient mice, as these mice exhibit normal sperm count and motility [38]. Therefore, the presence of this condition suggests that other sperm functional defects unrelated to SLO3 function may have contributed to the infertility observed [96].
In a more compelling case implicating human SLO3 in infertility, a man carrying a missense mutation of the Slo3 gene (c.1237A>T: Ile413Phe) exhibited sperm that failed to hyperpolarize, undergo acrosome reaction, and achieve successful fertilization in in vitro fertilization (IVF) procedures [97]. However, in intracytoplasmic sperm injection (ICSI), where sperm capacitation is not required, fertilization was successful, as expected for a mutation in the Slo3 gene. To further confirm the role of SLO3 in this phenotype, the authors generated a mouse line in which the endogenous Slo3 gene carried the same missense mutation found in the affected men. These mice also exhibited infertility. These findings provide clear evidence that SLO3 is necessary for fertility in both humans and mice and suggest its conserved role in acrosomal exocytosis [97].
A new inhibitor, VU0546110, was recently described which is more than 40-fold selective for human SLO3 over SLO1 [92]. This inhibitor completely inhibited hKSper, confirming that SLO1 channels do not meaningfully contribute to the current. This inhibition also had physiological effects, significantly inhibiting hyperpolarization, hyperactivation, and the acrosome reaction in human sperm. These downstream effects provide further evidence that human SLO3 is necessary for sperm hyperpolarization and fertility.
2. Structure and Gating of the SLO3 Pore-Forming Subunits
The pore-forming components of SLO channels are formed by homo-tetramers of α-subunits. Generally, SLO family α-subunits resemble those of voltage-gated K+ channels in having transmembrane domains symmetrically arranged around a water-filled, K+ selective pore. However, SLO2.1 and SLO2.2 channels have six transmembrane domains (S1–S6) and thus have intracellular N- and C-termini, as is common with members of the voltage-gated K+ channel family (Figure 1) [51,52,69]. In contrast, SLO1 and SLO3 have seven transmembrane domains (S0–S6) and thus have extracellular N-termini. The cytosolic domains of SLO family channels contain two regulators of K+ conductance (RCK) domains, RCK1 and RCK2. These domains sense several intracellular signals and confer each subfamily with distinctive properties [53,54,55,56,57,58]. For example, in SLO1, both RCK1 and RCK2 contain Ca2+ sensors [58,59,60]. The “calcium bowl” in RCK2 is composed of a highly conserved string of aspartate residues, which are negatively charged [61]. SLO2.1 and SLO2.2 are also modulated by Na+, Cl−, and activation of G-protein-coupled receptors [51,52,62,63,64,65]. The cytosolic domain may also be a point of interaction between monomers of different SLO family channels. The gating of SLO3 is similar to that of SLO1, as the opening of both channels is allosterically regulated by movement of a voltage sensor. This movement is driven by transmembrane potential and conformational change of the cytosolic gating ring induced by intracellular ligand binding. However, there are two important differences between the sensitivities of SLO1 and SLO3 channels to ligands. First, SLO1 is activated by acidification, whereas SLO3 is activated by alkalization [36,66]. Second, SLO1 has several Ca2+ binding sites and is activated by a broad range of Ca2+ concentrations [58,61,66]. Because of this, SLO1 can function over a broad range of voltages. In contrast, mouse SLO3 is insensitive to calcium and human SLO3 is several orders of magnitude less sensitive to Ca2+ than SLO1 [67] and functions in a narrow voltage range near the sperm resting potential. SLO1 and SLO3 are both sensitive to pH. SLO1 has two histidine residues in the gating ring, which may act as proton sensors and open the channel in response to low intracellular pH [66]. The mechanism of pH modulation of SLO3 is unknown. The half-activation point of SLO3 by pH is estimated to be around 7.7 [68], which is close to the pKa of histidine. With the recently solved structures of the human SLO3 gating ring [69] and complete SLO1 channel [70,71,72], the key residues governing SLO3 regulation by protons should be revealed soon.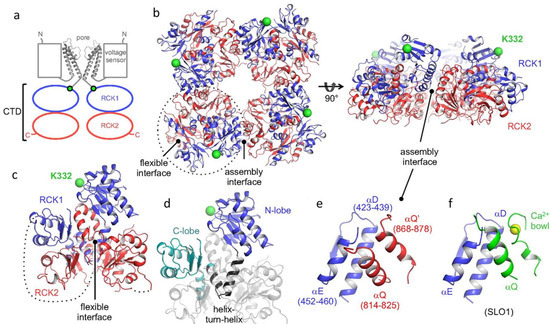
Figure 1. Human SLO3 gating ring structure determined by X-ray crystallography. (a) Cartoon of domain topology of two opposing SLO3 α-subunits. (b) Crystal structure of the gating ring of a hSLO3 tetramer with RCK1 and RCK2 domains colored in blue and red, respectively. (c) A single subunit of the hSLO3 channel and (d) highlight of RCK1. (e) A closeup of the hSLO3 assembly interface and (f) the corresponding region of SLO1 bound to Ca2+. The RCK1 N-terminal residue that connects to the transmembrane pore is shown as a green sphere. Ca2+ ion is shown as a yellow sphere. Reprinted/adapted with permission from [69].
