Pluripotent stem cells (PSCs) are highly proliferative cells that can self-renew indefinitely in vitro. Upon receiving appropriate signals, PSCs undergo differentiation and can generate every cell type in the body. These unique properties of PSCs require specific gene expression patterns that define stem cell identity and dynamic regulation of intracellular metabolism to support cell growth and cell fate transitions. PSCs are prone to DNA damage due to elevated replicative and transcriptional stress. Therefore, mechanisms to prevent deleterious mutations in PSCs that compromise stem cell function or increase the risk of tumor formation from becoming amplified and propagated to progenitor cells are essential for embryonic development and for using PSCs including induced PSCs (iPSCs) as a cell source for regenerative medicine.
- pluripotent stem cells
- ATP-binding cassette
- transporters
- lipid
- cholesterol
- oxidative stress
1. Introduction
2. Lipid Transporters (ABCA1 and ABCC1)
Lipids are a diverse class of biomolecules. Glycerophospholipids, specifically phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylinositol (PI), as well as sphingolipids and cholesterol, serve as building blocks for membranes and organelles [51][35]. Some ATP-binding cassette (ABC) transporters (ABCC1 [52,53][36][37]) act as “floppases” by catalyzing the movement of specific phospholipid species from the cytosolic leaflet to the extracellular leaflet of the plasma membrane (PM) [54][38], while others (ABCA1 [55][39]) function as extracellular phospholipid translocases (Figure 1). Indeed, ABC transporters have been shown to contribute to the asymmetric distribution of different phospholipids in the lipid bilayer, with PC and sphingolipids such as sphingomyelin (SM) residing predominantly in the outer leaflet of the PM, whereas anionic lipids such as PE, PS, and PI accumulate in the inner leaflet [56,57][40][41]. Increasing evidence indicates that changes in the composition and distribution of these phospholipids in the lipid bilayer can regulate signal transduction pathways that are known to regulate PSC cell fates [58,59][42][43].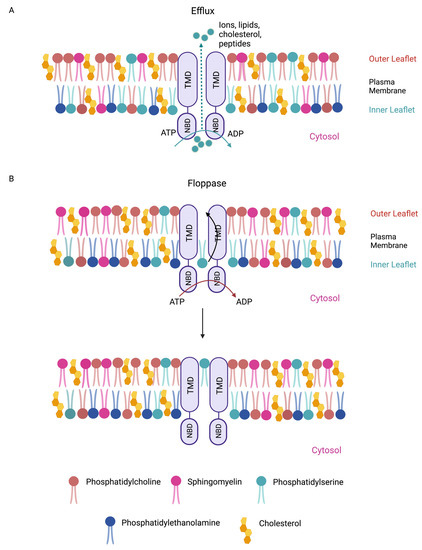
3. Cholesterol Transporters (ABCA1 and ABCG1)
Cholesterol is an important constituent of cell membranes. The bulk of cellular cholesterol (~90%) is localized at the PM [71][55]. Cholesterol homeostasis is determined by the biosynthesis, uptake, and efflux of cholesterol. ABCA1 and ABCG1 play crucial roles in the efflux of cellular cholesterol and thus are important regulators of membrane cholesterol level [72,73,74][56][57][58]. Cholesterol is a key modulator of membrane fluidity [75[59][60],76], which in turn regulates cell behaviors such as adhesion, proliferation, and migration [77][61]. However, recent evidence indicates that changes in PM stiffness may also regulate cell fate changes in PSCs [78][62]. It has been shown that the rigidification of the PM precedes or coincides with downregulation of gene expression programs that stabilize the pluripotent state in PSCs, suggesting that a decrease in membrane fluidity may prime PSCs to exit from pluripotency. Consistent with the notion that maintenance of membrane fluidity contributes to stem cell maintenance, enzymes in the cholesterol biosynthesis pathways have been shown to be expressed at higher levels in PSCs, thereby increasing membrane cholesterol content and fluidity [78,79][62][63]. Importantly, the inhibition of cholesterol production in PSCs accelerates their exit from pluripotency, as indicated by the rapid downregulation of stem cell marker alkaline phosphatase [78][62]. These observations underscore the importance of cholesterol homeostasis in stem cell maintenance. WResearchers propose that dissecting the mechanisms by which the expression and activities of ABCA1 and ABCG1 are controlled in PSCs will advance our understanding of the role of cholesterol efflux in regulating membrane fluidity and stem cell pluripotency. In addition to regulating membrane fluidity, cholesterol, together with SM, has been shown to assemble dynamic, cholesterol-rich microdomains in the outer leaflet of the PM [80][64]. These compartmentalized domains, known as lipid rafts, have been shown to enrich specific receptors and their effectors to promote receptor–effector interactions, thereby lowering activation barriers. The ability of lipid rafts to partition and concentrate select signaling machineries depends on the intrinsic affinity of these signaling proteins to lipid rafts, which has been shown to be influenced by amino acid sequences in the TM domains of membrane receptors and protein palmitoylation [81,82][65][66]. Oligomerization of receptors has also been reported to increase their affinity to lipid rafts and residence time in these lipid subdomains [83][67], hinting at a potential mechanism by which lipid rafts amplify signaling. WResearchers suggest that a small change in the concentration of signaling components in lipid rafts may be sufficient, through amplification, to initiate signaling cascades. Therefore, lipid rafts may play an important role in increasing the responsiveness of signal transduction machineries to cellular stimuli. It has been shown that ABCA1 and ABCG1 deficiency in macrophages leads to an increase in the number of lipid rafts and enhanced signaling responses [84][68]. This is likely due to the propensity of lipid rafts to cluster, resulting in the amplification of signals [85,86][69][70]. These observations suggest an inhibitory function of ABCA1 and ABCG1 in lipid raft formation, via the mobilization of cholesterol from lipid rafts to non-raft domains. It will be of interest to determine the mechanisms by which ABC transporters are recruited to lipid rafts. This is because the active efflux of membrane cholesterol by ABC transporters could facilitate the fine-tuning and dissolution of signal transduction hubs in lipid rafts and signal termination. Lipid rafts are also detected in PSCs, but their roles in stem cell maintenance are less well-understood [87][71]. The self-renewal of mouse PSCs requires leukemia inhibitory factor (LIF) signaling [88][72]. It has been shown that depletion of membrane cholesterol in mouse PSCs by methyl-β-cyclodextrin (Mβ-CD), which has been shown to disrupt lipid rafts, compromises the recruitment of LIF receptor and its co-receptor gp130 to rafts and blunts LIF receptor-JAK-STAT3 signaling [87][71]. The observed reduction in expression levels of key pluripotency-associated transcription factors OCT4 and SOX2 in Mβ-CD-treated PSCs indicates a destabilized pluripotent state when lipid raft formation is impaired. These observations are consistent with the role of lipid rafts in enriching specific receptors and facilitating their activation. Lipid rafts have also been implicated in other signaling pathways that are known to promote stem cell self-renewal and pluripotency, such as EGFR [70][54] and RAS [89][73], and those that destabilize the stem cell state, including insulin receptor [90][74] and hedgehog [91][75]. An outstanding question is how ABC transporters may control lipid raft formation and dynamics to partition competing signaling in PSCs to favor self-renewal over differentiation.4. Redox Regulation and Oxidative Stress (ABCC1 and ABCC4)
ROS are natural byproducts of cellular metabolism. ROS can cause damage to the basic building blocks of cells including DNA, protein, and lipids. Therefore, ROS pose significant threats to the ability of PSCs to maintain genome and proteome integrity as they self-renew. In addition to cellular damages inflicted by ROS build-up, an imbalance in ROS levels can also lead to the misregulation of redox sensor molecules via the oxidation of cysteine residues. Some of these redox sensors are key signaling effectors such as AKT and MAPK [92,93][76][77]. Therefore, it is conceivable that an increase in ROS concentration destabilizes the pluripotent cell state in part by interfering with signaling pathways essential for stem cell maintenance [94][78]. ROS levels in cells are determined by the rate of ROS generation and the rate of ROS scavenging by antioxidants. PSCs are able to maintain relatively low ROS levels compared to those of differentiated cells, in part due to their reliance on glycolysis rather than oxidative phosphorylation for energy production, which is known to generate less ROS [95,96][79][80]. Nevertheless, the neutralization of ROS species by antioxidants remains a critical mechanism in regulating ROS homeostasis in PSCs as it is essential for stem cell maintenance [97,98][81][82]. Glutathione (GSH) is a major antioxidant in cells [99,100][83][84]. GSH levels are balanced by its synthesis, transport, efflux, and degradation. Studies have shown that ABCC1 is a major GSH exporter and can regulate intracellular GSH levels. The overexpression of ABCC1 reduces intracellular GSH levels, while ABCC1 deficiency increases GSH concentrations [101,102][85][86]. Importantly, ABCC1 can export both GSH and various oxidized glutathione derivatives (e.g., glutathione disulfide (GSSG)), although with distinct substrate affinity [103,104,105][87][88][89]. Therefore, in addition to cellular enzymes that can degrade GSH (e.g., CHAC1 [106,107][90][91]) or regenerate GSH from GSSG (e.g., GSH reductase [108][92]), ABCC1 likely plays an integral role in maintaining the redox equilibrium in PSCs. It has been shown that oxidative stress downregulates key PSC-specific transcription factors OCT4 and SOX2, and compromises AKT signaling [97][81]. While the precise mechanism is unclear, the destabilization of OCT4 proteins and inactivation of AKT via the oxidation of critical cysteines residues could compromise the gene transcription and cellular signaling required for stem cell maintenance [92,109,110][76][93][94]. Like oxidative stress, reductive stress induced by excessive levels of GSH can also impair PSC functions. Physiological levels of ROS have been shown to promote PSC proliferation and accurate DNA synthesis [111][95]. High concentrations of antioxidants interfere with cell cycle progression and lead to the accumulation of DNA breaks [112][96], likely due to the toxic effects of high antioxidant levels on the stability of cell cycle regulators and proteins involved in the DNA damage response and DNA repair [111][95]. The balance between ROS and antioxidants must be optimal, as both extremes, oxidative and reductive stress, are damaging to PSCs. Functional studies on the role of ABCC1 and ABCC4 in PSCs will address the precise role of GSH/GSSG efflux in establishing a cellular redox state favorable for stem cell self-renewal and genome maintenance.References
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156.
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638.
- Diamante, L.; Martello, G. Metabolic regulation in pluripotent stem cells. Curr. Opin. Genet. Dev. 2022, 75, 101923.
- Wu, J.; Ocampo, A.; Belmonte, J.C.I. Cellular Metabolism and Induced Pluripotency. Cell 2016, 166, 1371–1385.
- Gu, W.; Gaeta, X.; Sahakyan, A.; Chan, A.B.; Hong, C.S.; Kim, R.; Braas, D.; Plath, K.; Lowry, W.E.; Christofk, H.R. Glycolytic Metabolism Plays a Functional Role in Regulating Human Pluripotent Stem Cell State. Cell Stem Cell 2016, 19, 476–490.
- Chen, H.; Guo, R.; Zhang, Q.; Guo, H.; Yang, M.; Wu, Z.; Gao, S.; Liu, L.; Chen, L. Erk signaling is indispensable for genomic stability and self-renewal of mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 2015, 112, E5936–E5943.
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G.; et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005, 122, 947–956.
- Hainer, S.J.; Bošković, A.; McCannell, K.N.; Rando, O.J.; Fazzio, T.G. Profiling of Pluripotency Factors in Single Cells and Early Embryos. Cell 2019, 177, 1319–1329.e11.
- Dunn, S.-J.; Martello, G.; Yordanov, B.; Emmott, S.; Smith, A.G. Defining an essential transcription factor program for naïve pluripotency. Science 2014, 344, 1156–1160.
- Chen, X.; Xu, H.; Yuan, P.; Fang, F.; Huss, M.; Vega, V.B.; Wong, E.; Orlov, Y.L.; Zhang, W.; Jiang, J.; et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 2008, 133, 1106–1117.
- Young, R.A. Control of the embryonic stem cell state. Cell 2011, 144, 940–954.
- Li, X.; Hui, S.; Mirek, E.T.; Jonsson, W.O.; Anthony, T.G.; Lee, W.D.; Zeng, X.; Jang, C.; Rabinowitz, J.D. Circulating metabolite homeostasis achieved through mass action. Nat. Metab. 2022, 4, 141–152.
- Efroni, S.; Duttagupta, R.; Cheng, J.; Dehghani, H.; Hoeppner, D.J.; Dash, C.; Bazett-Jones, D.P.; Le Grice, S.; McKay, R.D.G.; Buetow, K.H.; et al. Global Transcription in Pluripotent Embryonic Stem Cells. Cell Stem Cell 2008, 2, 437–447.
- Fong, Y.W.; Cattoglio, C.; Tjian, R. The Intertwined Roles of Transcription and Repair Proteins. Mol. Cell 2013, 52, 291–302.
- Aguilera, A.; García-Muse, T. R loops: From transcription byproducts to threats to genome stability. Mol. Cell 2012, 46, 115–124.
- White, J.; Dalton, S. Cell cycle control of embryonic stem cells. Stem Cell Rev. 2005, 1, 131–138.
- Filion, T.M.; Qiao, M.; Ghule, P.N.; Mandeville, M.; van Wijnen, A.J.; Stein, J.L.; Lian, J.B.; Altieri, D.C.; Stein, G.S. Survival responses of human embryonic stem cells to DNA damage. J. Cell. Physiol. 2009, 220, 586–592.
- Suvorova, I.I.; Grigorash, B.B.; Chuykin, I.A.; Pospelova, T.V.; Pospelov, V.A. G1 checkpoint is compromised in mouse ESCs due to functional uncoupling of p53-p21Waf1 signaling. Cell Cycle 2016, 15, 52–63.
- Cervantes, R.B.; Stringer, J.R.; Shao, C.; Tischfield, J.A.; Stambrook, P.J. Embryonic stem cells and somatic cells differ in mutation frequency and type. Proc. Natl. Acad. Sci. USA 2002, 99, 3586–3590.
- Aladjem, M.I.; Spike, B.T.; Rodewald, L.W.; Hope, T.J.; Klemm, M.; Jaenisch, R.; Wahl, G.M. ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr. Biol. 1998, 8, 145–155.
- Heyer, B.S.; MacAuley, A.; Behrendtsen, O.; Werb, Z. Hypersensitivity to DNA damage leads to increased apoptosis during early mouse development. Genes Dev. 2000, 14, 2072–2084.
- Vitale, I.; Manic, G.; De Maria, R.; Kroemer, G.; Galluzzi, L. DNA Damage in Stem Cells. Mol. Cell 2017, 66, 306–319.
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920.
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676.
- Yamanaka, S. Induced Pluripotent Stem Cells: Past, Present, and Future. Cell Stem Cell 2012, 10, 678–684.
- Ma, H.; Morey, R.; O’Neil, R.C.; He, Y.; Daughtry, B.; Schultz, M.D.; Hariharan, M.; Nery, J.R.; Castanon, R.; Sabatini, K.; et al. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature 2014, 511, 177–183.
- Merkle, F.T.; Ghosh, S.; Kamitaki, N.; Mitchell, J.; Avior, Y.; Mello, C.; Kashin, S.; Mekhoubad, S.; Ilic, D.; Charlton, M.; et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature 2017, 545, 229–233.
- Kyriakides, O.; Halliwell, J.A.; Andrews, P.W. Acquired Genetic and Epigenetic Variation in Human Pluripotent Stem Cells. Adv. Biochem. Eng. Biotechnol. 2018, 163, 187–206.
- Assou, S.; Bouckenheimer, J.; De Vos, J. Concise Review: Assessing the Genome Integrity of Human Induced Pluripotent Stem Cells: What Quality Control Metrics? Stem Cells 2018, 36, 814–821.
- Yamamoto, T.; Sato, Y.; Yasuda, S.; Shikamura, M.; Tamura, T.; Takenaka, C.; Takasu, N.; Nomura, M.; Dohi, H.; Takahashi, M.; et al. Correlation Between Genetic Abnormalities in Induced Pluripotent Stem Cell-Derivatives and Abnormal Tissue Formation in Tumorigenicity Tests. Stem Cells Transl. Med. 2022, 11, 527–538.
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004.
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531.
- Martin, R.M.; Fowler, J.L.; Cromer, M.K.; Lesch, B.J.; Ponce, E.; Uchida, N.; Nishimura, T.; Porteus, M.H.; Loh, K.M. Improving the safety of human pluripotent stem cell therapies using genome-edited orthogonal safeguards. Nat. Commun. 2020, 11, 2713.
- Colter, J.; Murari, K.; Biernaskie, J.; Kallos, M.S. Induced pluripotency in the context of stem cell expansion bioprocess development, optimization, and manufacturing: A roadmap to the clinic. NPJ Regen. Med. 2021, 6, 72.
- van Meer, G.; de Kroon, A.I.P.M. Lipid map of the mammalian cell. J. Cell Sci. 2011, 124, 5–8.
- Kamp, D.; Haest, C.W. Evidence for a role of the multidrug resistance protein (MRP) in the outward translocation of NBD-phospholipids in the erythrocyte membrane. Biochim. Biophys. Acta 1998, 1372, 91–101.
- Dekkers, D.W.; Comfurius, P.; Schroit, A.J.; Bevers, E.M.; Zwaal, R.F. Transbilayer movement of NBD-labeled phospholipids in red blood cell membranes: Outward-directed transport by the multidrug resistance protein 1 (MRP1). Biochemistry 1998, 37, 14833–14837.
- Daleke, D.L. Phospholipid flippases. J. Biol. Chem. 2007, 282, 821–825.
- Segrest, J.P.; Tang, C.; Song, H.D.; Jones, M.K.; Davidson, W.S.; Aller, S.G.; Heinecke, J.W. ABCA1 is an extracellular phospholipid translocase. Nat. Commun. 2022, 13, 4812.
- Fadeel, B.; Xue, D. The ins and outs of phospholipid asymmetry in the plasma membrane: Roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 264–277.
- Oude Elferink, R.P.J.; Paulusma, C.C. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein). Pflugers Arch. 2007, 453, 601–610.
- Grecco, H.E.; Schmick, M.; Bastiaens, P.I.H. Signaling from the living plasma membrane. Cell 2011, 144, 897–909.
- Castanieto, A.; Johnston, M.J.; Nystul, T.G. EGFR signaling promotes self-renewal through the establishment of cell polarity in Drosophila follicle stem cells. Elife 2014, 3, e04437.
- Lanner, F.; Rossant, J. The role of FGF/Erk signaling in pluripotent cells. Development 2010, 137, 3351–3360.
- Levenstein, M.E.; Ludwig, T.E.; Xu, R.-H.; Llanas, R.A.; Van Den Heuvel-Kramer, K.; Manning, D.; Thomson, J.A. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells 2006, 24, 568–574.
- Haghighi, F.; Dahlmann, J.; Nakhaei-Rad, S.; Lang, A.; Kutschka, I.; Zenker, M.; Kensah, G.; Piekorz, R.P.; Ahmadian, M.R. bFGF-mediated pluripotency maintenance in human induced pluripotent stem cells is associated with NRAS-MAPK signaling. Cell Commun. Signal. 2018, 16, 96.
- Kubara, K.; Yamazaki, K.; Ishihara, Y.; Naruto, T.; Lin, H.-T.; Nishimura, K.; Ohtaka, M.; Nakanishi, M.; Ito, M.; Tsukahara, K.; et al. Status of KRAS in iPSCs Impacts upon Self-Renewal and Differentiation Propensity. Stem Cell Rep. 2018, 11, 380–394.
- Chavan, T.S.; Muratcioglu, S.; Marszalek, R.; Jang, H.; Keskin, O.; Gursoy, A.; Nussinov, R.; Gaponenko, V. Plasma membrane regulates Ras signaling networks. Cell. Logist. 2015, 5, e1136374.
- Li, Z.-L.; Buck, M. Computational Modeling Reveals that Signaling Lipids Modulate the Orientation of K-Ras4A at the Membrane Reflecting Protein Topology. Structure 2017, 25, 679–689.e2.
- Gulshan, K.; Brubaker, G.; Conger, H.; Wang, S.; Zhang, R.; Hazen, S.L.; Smith, J.D. PI(4,5)P2 Is Translocated by ABCA1 to the Cell Surface Where It Mediates Apolipoprotein A1 Binding and Nascent HDL Assembly. Circ. Res. 2016, 119, 827–838.
- Hamon, Y.; Broccardo, C.; Chambenoit, O.; Luciani, M.F.; Toti, F.; Chaslin, S.; Freyssinet, J.M.; Devaux, P.F.; McNeish, J.; Marguet, D.; et al. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2000, 2, 399–406.
- Takahashi, K.; Kimura, Y.; Kioka, N.; Matsuo, M.; Ueda, K. Purification and ATPase activity of human ABCA1. J. Biol. Chem. 2006, 281, 10760–10768.
- Watabe, T.; Miyazono, K. Roles of TGF-β family signaling in stem cell renewal and differentiation. Cell Res. 2009, 19, 103–115.
- Yu, M.; Wei, Y.; Xu, K.; Liu, S.; Ma, L.; Pei, Y.; Hu, Y.; Liu, Z.; Zhang, X.; Wang, B.; et al. EGFR deficiency leads to impaired self-renewal and pluripotency of mouse embryonic stem cells. PeerJ 2019, 7, e6314.
- Lange, Y.; Ye, J.; Steck, T.L. How cholesterol homeostasis is regulated by plasma membrane cholesterol in excess of phospholipids. Proc. Natl. Acad. Sci. USA 2004, 101, 11664–11667.
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldán, A.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131.
- Duong, M.; Collins, H.L.; Jin, W.; Zanotti, I.; Favari, E.; Rothblat, G.H. Relative contributions of ABCA1 and SR-BI to cholesterol efflux to serum from fibroblasts and macrophages. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 541–547.
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 139–143.
- Szabo, G. Dual mechanism for the action of cholesterol on membrane permeability. Nature 1974, 252, 47–49.
- Subczynski, W.K.; Pasenkiewicz-Gierula, M.; Widomska, J.; Mainali, L.; Raguz, M. High Cholesterol/Low Cholesterol: Effects in Biological Membranes: A Review. Cell Biochem. Biophys. 2017, 75, 369–385.
- Reiss, K.; Cornelsen, I.; Husmann, M.; Gimpl, G.; Bhakdi, S. Unsaturated Fatty Acids Drive Disintegrin and Metalloproteinase (ADAM)-dependent Cell Adhesion, Proliferation, and Migration by Modulating Membrane Fluidity. J. Biol. Chem. 2011, 286, 26931–26942.
- Matsuzaki, T.; Matsumoto, S.; Kasai, T.; Yoshizawa, E.; Okamoto, S.; Yoshikawa, H.Y.; Taniguchi, H.; Takebe, T. Defining Lineage-Specific Membrane Fluidity Signatures that Regulate Adhesion Kinetics. Stem Cell Rep. 2018, 11, 852–860.
- Chen, W.-J.; Huang, W.-K.; Pather, S.R.; Chang, W.-F.; Sung, L.-Y.; Wu, H.-C.; Liao, M.-Y.; Lee, C.-C.; Wu, H.-H.; Wu, C.-Y.; et al. Podocalyxin-Like Protein 1 Regulates Pluripotency through the Cholesterol Biosynthesis Pathway. Adv. Sci. 2022, 10, e2205451.
- Jacobson, K.; Mouritsen, O.G.; Anderson, R.G.W. Lipid rafts: At a crossroad between cell biology and physics. Nat. Cell Biol. 2007, 9, 7–14.
- Scheiffele, P.; Roth, M.G.; Simons, K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997, 16, 5501–5508.
- Melkonian, K.A.; Ostermeyer, A.G.; Chen, J.Z.; Roth, M.G.; Brown, D.A. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 1999, 274, 3910–3917.
- Harder, T.; Scheiffele, P.; Verkade, P.; Simons, K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 1998, 141, 929–942.
- Yvan-Charvet, L.; Welch, C.; Pagler, T.A.; Ranalletta, M.; Lamkanfi, M.; Han, S.; Ishibashi, M.; Li, R.; Wang, N.; Tall, A.R. Increased inflammatory gene expression in ABC transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 2008, 118, 1837–1847.
- Janes, P.W.; Ley, S.C.; Magee, A.I.; Kabouridis, P.S. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol. 2000, 12, 23–34.
- Langlet, C.; Bernard, A.M.; Drevot, P.; He, H.T. Membrane rafts and signaling by the multichain immune recognition receptors. Curr. Opin. Immunol. 2000, 12, 250–255.
- Lee, M.Y.; Ryu, J.M.; Lee, S.H.; Park, J.H.; Han, H.J. Lipid rafts play an important role for maintenance of embryonic stem cell self-renewal. J. Lipid Res. 2010, 51, 2082–2089.
- Williams, R.L.; Hilton, D.J.; Pease, S.; Willson, T.A.; Stewart, C.L.; Gearing, D.P.; Wagner, E.F.; Metcalf, D.; Nicola, N.A.; Gough, N.M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 1988, 336, 684–687.
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39.
- Teo, A.K.K.; Nguyen, L.; Gupta, M.K.; Lau, H.H.; Loo, L.S.W.; Jackson, N.; Lim, C.S.; Mallard, W.; Gritsenko, M.A.; Rinn, J.L.; et al. Defective insulin receptor signaling in hPSCs skews pluripotency and negatively perturbs neural differentiation. J. Biol. Chem. 2021, 296, 100495.
- Li, Q.; Lex, R.K.; Chung, H.; Giovanetti, S.M.; Ji, Z.; Ji, H.; Person, M.D.; Kim, J.; Vokes, S.A. The Pluripotency Factor NANOG Binds to GLI Proteins and Represses Hedgehog-mediated Transcription. J. Biol. Chem. 2016, 291, 7171–7182.
- Murata, H.; Ihara, Y.; Nakamura, H.; Yodoi, J.; Sumikawa, K.; Kondo, T. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J. Biol. Chem. 2003, 278, 50226–50233.
- Ito, K.; Hirao, A.; Arai, F.; Takubo, K.; Matsuoka, S.; Miyamoto, K.; Ohmura, M.; Naka, K.; Hosokawa, K.; Ikeda, Y.; et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006, 12, 446–451.
- Ji, A.-R.; Ku, S.-Y.; Cho, M.S.; Kim, Y.Y.; Kim, Y.J.; Oh, S.K.; Kim, S.H.; Moon, S.Y.; Choi, Y.M. Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage. Exp. Mol. Med. 2010, 42, 175–186.
- Varum, S.; Rodrigues, A.S.; Moura, M.B.; Momcilovic, O.; Easley, C.A., 4th; Ramalho-Santos, J.; Van Houten, B.; Schatten, G. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE 2011, 6, e20914.
- Zhang, C.; Skamagki, M.; Liu, Z.; Ananthanarayanan, A.; Zhao, R.; Li, H.; Kim, K. Biological Significance of the Suppression of Oxidative Phosphorylation in Induced Pluripotent Stem Cells. Cell Rep. 2017, 21, 2058–2065.
- Wang, C.-K.; Yang, S.-C.; Hsu, S.-C.; Chang, F.-P.; Lin, Y.-T.; Chen, S.-F.; Cheng, C.-L.; Hsiao, M.; Lu, F.L.; Lu, J. CHAC2 is essential for self-renewal and glutathione maintenance in human embryonic stem cells. Free Radic. Biol. Med. 2017, 113, 439–451.
- Guo, Y.-L.; Chakraborty, S.; Rajan, S.S.; Wang, R.; Huang, F. Effects of oxidative stress on mouse embryonic stem cell proliferation, apoptosis, senescence, and self-renewal. Stem Cells Dev. 2010, 19, 1321–1331.
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760.
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212.
- Marchan, R.; Hammond, C.L.; Ballatori, N. Multidrug resistance-associated protein 1 as a major mediator of basal and apoptotic glutathione release. Biochim. Biophys. Acta 2008, 1778, 2413–2420.
- Cole, S.P.C.; Deeley, R.G. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol. Sci. 2006, 27, 438–446.
- Diner, B.A.; Li, T.; Greco, T.M.; Crow, M.S.; Fuesler, J.A.; Wang, J.; Cristea, I.M. The functional interactome of PYHIN immune regulators reveals IFIX is a sensor of viral DNA. Mol. Syst. Biol. 2015, 11, 787.
- Mueller, C.F.H.; Widder, J.D.; McNally, J.S.; McCann, L.; Jones, D.P.; Harrison, D.G. The role of the multidrug resistance protein-1 in modulation of endothelial cell oxidative stress. Circ. Res. 2005, 97, 637–644.
- Ballatori, N.; Krance, S.M.; Marchan, R.; Hammond, C.L. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol. Aspects Med. 2009, 30, 13–28.
- Crawford, R.R.; Prescott, E.T.; Sylvester, C.F.; Higdon, A.N.; Shan, J.; Kilberg, M.S.; Mungrue, I.N. Human CHAC1 Protein Degrades Glutathione, and mRNA Induction Is Regulated by the Transcription Factors ATF4 and ATF3 and a Bipartite ATF/CRE Regulatory Element. J. Biol. Chem. 2015, 290, 15878–15891.
- Crawford, R.; Higdon, A.; Prescott, E.; Mungrue, I. CHAC1 degrades glutathione, sensitizing cells to oxidative injury (663.10). FASEB J. 2014, 28, 663-10.
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226.
- Marsboom, G.; Zhang, G.-F.; Pohl-Avila, N.; Zhang, Y.; Yuan, Y.; Kang, H.; Hao, B.; Brunengraber, H.; Malik, A.B.; Rehman, J. Glutamine Metabolism Regulates the Pluripotency Transcription Factor OCT4. Cell Rep. 2016, 16, 323–332.
- Watanabe, S.; Umehara, H.; Murayama, K.; Okabe, M.; Kimura, T.; Nakano, T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 2006, 25, 2697–2707.
- Ivanova, J.S.; Pugovkina, N.A.; Neganova, I.E.; Kozhukharova, I.V.; Nikolsky, N.N.; Lyublinskaya, O.G. Cell cycle-coupled changes in the level of reactive oxygen species support the proliferation of human pluripotent stem cells. Stem Cells 2021, 39, 1671–1687.
- Li, T.-S.; Marbán, E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells 2010, 28, 1178–1185.
