Water scarcity is occurring due to the rapid increase in the global population, intense industrialization, stringent water quality standards, and negative climate change. Contamination with heavy metals (arsenic, iron, lead, mercury, chromium), microbes (Escherichia coli, Staphylococus), biological compounds, and other components (fluoride, cadmium) is widely encountered. Carbon based materials, including activated carbon, MWCNT. graphene, r-graphene, cabon dot and fullerene provides cost effective option for water purification. The specific surface area, strong sorption, fast kinetics and like, are properties of these carbon based materials responsible potential water treatment application.
- Carbon Nanoparticles
- Water Purification
- Water treatment
- Activated Carbon
- MWCNT
- Graphene Oxide
1. Introduction
Water is the most abundant and irreplaceable resource available globally, however despite its importance, water scarcity is occurring due to the rapid increase in the global population, intense industrialization, stringent water quality standards, and negative climate change[1] [1]. Contamination with heavy metals (arsenic, iron, lead, mercury, chromium), microbes (Escherichia coli, Staphylococus), biological compounds, and other components (fluoride, cadmium) is widely encountered as major challenge related to water purification technology[1][2][3] [1,2,3]. The rising challenge of pure water availability is reflected in UN models that project that 1.8 billion people will face water scarcity by 2025. This is why the problem of water scarcity is listed in the Millennium Development Goals (MDGs) [2][4][2,4].
Conventional water treatment includes absorption, membrane filtering, and distillation processes[2][3][4] [2,3,4]. Industrial effluent, agricultural waste water, sewage, and radioactive waste water are sources of contamination, as shown in Figure 1. Carbon nanoparticles can potentially be used for technological innovation in terms of improving water treatment due to being eco-friendly and cost-effective[5] [5] and because of their specific surface area, strong sorption, fast kinetics[1] [1], thermal stability, and conductivity[2][6][7] [2,6,7]. Single-walled and multiwalled carbon nanotubes (CNTs, MWCNTs), graphene, and fullerene are among the most popular examples of carbon nanoparticles[1] [1]. Graphene has been one of the most widely tested materials for water purification technology since its discovery in 2010[4] [4].
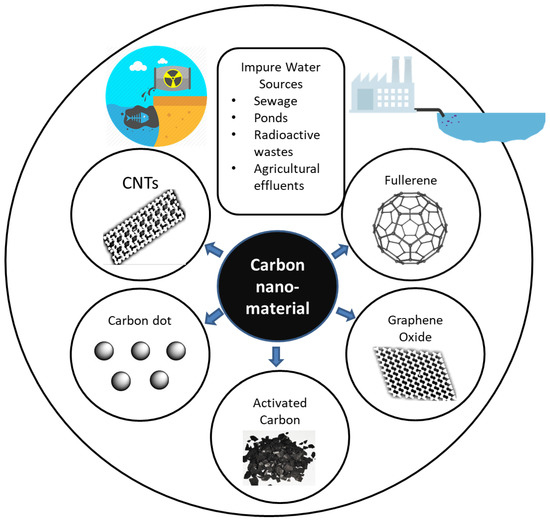
Figure 1. Use of carbon for water purification. CNTs, carbon nanotubes.
2. Properties of Carbon Materials
The structural properties of carbon nanomaterials such as CNTs, graphene oxide, carbon dots, fullerene, and activated carbon are largely responsible for their use in water purification technology. However, they also have some other additional characteristics that make them potential candidates. For example, the electrically conductive and selective ion transfer properties of CNTs could be important for desalination[8] [8]. Additionally, 2D graphene is a versatile absorbent due to its high affinity to target contaminants because its surface functional groups, which are present on single sheets of carbon, are utilized to absorb heavy metals[6] [6]. The formation of hierarchically structured layers allows absorption of heavy metals due to the formation of mesopores within the walls of the macropores[9] [9]. Liu et al.[10] [10] synthesized L-cysteine-functionalized MWCNTs for selective sorption of heavy metals and observed that the use of MWCNT–cysteine allows effective sorption of cadmium.
Activated carbon is a widely used material in existing water purification systems[11][12][13][14][15][16] [11,12,13,14,15,16], due to its cost-effectiveness and availability. However, it has limited removal efficiency due to its low specific surface area, lower number of active sites, non-selectivity, and low absorption kinetics[17] [17]. Activated carbon is a highly porous material with a heterogeneous range of pores (measuring approximately <2 nm in diameter) that are present all around its internal structure[18] [18]. These pores increase the specific surface area to up to 2500 m2/g[19] [19]. Typically, activated carbon is made up of 80% carbon, with the remaining 20% composed of other elements such as oxygen and nitrogen[20][21] [20,21].
CNTs are promising materials, as they are able to generate size-exclusion membranes, and due to their nanoscale features are capable of blocking the transport of certain microorganisms across these membranes. The main advantage of this is that the membranes can effectively filter bacteria from aqueous solutions, where the amount of CNT loading also has an effect on the virus filtration capacity[22] [22]. An epoxy-entrapped, vertically aligned CNT material displayed similar antibiofouling properties, with physical damage and oxidative stress to microorganisms proposed as the mechanisms of action[23] [23]. A more direct approach for fabricating antibacterial CNT water purification membranes was achieved with the incorporation of the natural bactericide nisin through adsorption onto CNTs[24] [24]. The nisin-adsorbed CNTs were then coated onto a polycarbonate filter membrane, where bacteria become entrapped and are then neutralized. This approach provides a treatment method for nanometer-range bacteria.
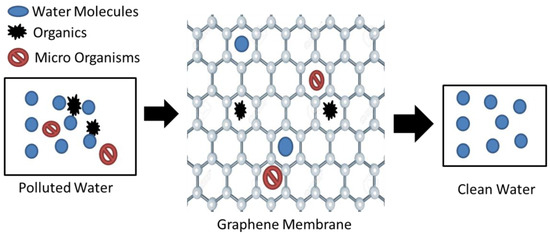
Graphene oxide (GO) and reduced graphene oxide (rGO) are the two main derivatives of graphene[25] [25]. Graphene oxide (GO) acts as a promising sorbent, having the ability to efficiently remove heavy metals and radionuclides. GO has attracted attention within the research community due to its extremely powerful separation ability. The presence of -OH, -COOH, -C=O, and other hydrophilic groups is the possible reason for this[26][27] [26,27]. The highly functionalized operative surfaces of GO give it the potential to remove pollutants from water, as displayed in Figure 2 [28]. It can be observed that the graphene structure is only permeable to water molecules, and hence filters out impurities.
The vast technological advancements in water purification methods and significant contributions of carbon materials motivated us to prepare a detailed review of the topic. In order to keep the paper focused, the most appropriate articles were selected from Google Scholar, PatSeer, Scopus, and Science Direct databases. Combinations of keywords such as “water purification” and “carbon nanoparticles” were used. The focus was on studies published in the last decade. The date of the last search was 8 November 2020. Figure 3 shows the flow chart for relevant research article identification and selection. The selection of relevant articles was performed in two stages, as below.
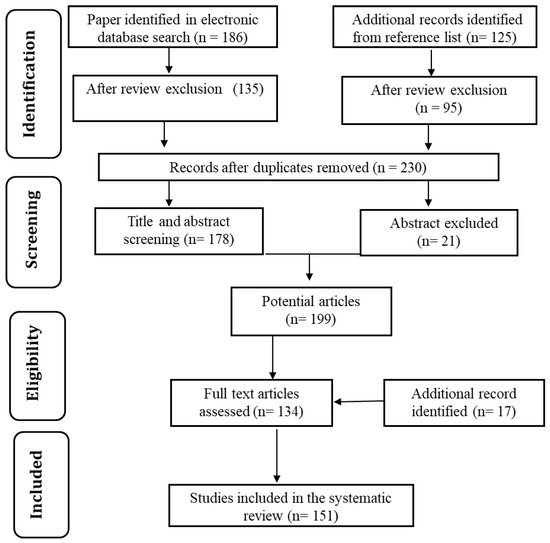
Figure 3. Flowchart of identification and selection strategy used for research articles.
-
In the initial stage, the bibliographies were searched according to the article title and abstract. Moreover, relevant patents were identified based on the title and claim. Articles were filtered to meet the following three basic topic criteria: (a) water purification; (b) use of carbon nanoparticles; (c) subject related to cost-effective and eco-friendly technology. Additional papers were also searched from articles’ reference lists. After review and exclusion of the database sources, 230 studies remained.
-
In the second stage, the studies were read in full to identify the most relevant studies. Patents were also examined to identify the most appropriate ones. This process led to a final number of 151 potential studies.
Figure 4a,b shows the chronological distribution of the recent publications and the geographical locations of the contributing authors for articles relating to water purification involving carbon-based nanoparticles. It can be observed that activated carbon, CNTs, and graphene are the most used carbon materials. This reflects the increase in attention from the research community towards testing carbon-based nanoparticles in water purification systems. Additionally, it can be observed that the researchers in this field are spread globally.
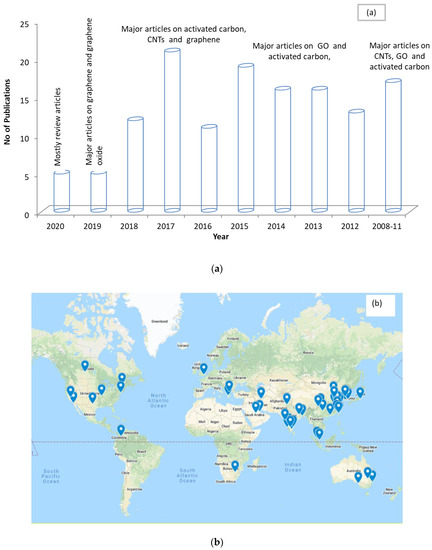
Figure 4. Representation of publications by year (a) and geographical location (b) from 2010–2020 for articles on water purification using carbon nanoparticles.
Carbon-based materials are gaining popularity due to their superior structural properties, ease of handling, eco-friendly nature, and cost-effectiveness. They are widely recommended for adsorption-based water purification methods due to their renewable nature[29] [29]. The innovative use of carbon materials for water purification technologies is one of the focus areas within the research community, which can be observed from the 71 patents included in the present review. Carbon materials are used both alone and in composite structures, with fiber (basalt fiber) often used to improve the effectiveness [30][31][30,31]. Han et al. [32] prepared CNT-based photothermal nanocomposites and observed improved water purification via solar steam generation. Li et al.[33] [33] prepared super-aligned carbon-nanotube-activated carbon composite electrodes and observed improvements in the water distillation capability. Among the articles, physical absorption, photodegradation, and solar-driven evaporation processes are widely studied[34] [34]. The recent review on carbon-based nanoparticles for water purification provides insights on heavy metal removal[35] [35], osmosis membranes[36] [36], and carbon material properties[37] [37]. The present review covers recent articles and patents related to water purification using carbon materials.
The present study reviews the recent developments in water purification technology using carbon materials. The initial section discusses carbon nanomaterial fabrication. The next section covers patents related to water purification using carbon materials from the last decade. It provides insights into recently developed water purification technologies. Further, recent research articles on water purification using activated carbon, CNTs, graphene, graphene oxide, and other carbon materials are discussed.
3. Recent Patents for Water Treatment Using Carbon Material
This section explores the recent patents for water treatments using carbon materials. Activated carbon, GO, rGO, CNTs, fullerene, carbon dots, and carbon composites are widely used to develop and improve water purification technologies. This is reflected by the number of patents that integrate processes and technologies for use in small- and large-scale water purification.
Patent CN109502896A[38] [49] involves a water purifier that use graphene in membrane form along with polypropylene cotton, which can successfully remove impurities ranging from iron rust to dust. Patent CN107265530A[39] [50] is a multiple-effect water purifier that utilizes acrylamide-oligomer-grafted oxidized graphene for purification, using processes such as flocculation and filtration to remove phenyl compounds and heavy metal ions. Patent CN108002366A[40] [51] uses graphene in a completely different form, whereby a new kind of graphene water cleaning foam was made using solar energy, which can evaporate the moisture present in sewage using solar radiation, with a very high ion removal rate (>99.5%) and high bacteria removal rate (>99.9%), meeting drinking water standards.
Patent US3612279A[41] [52] involves a device containing four large perforated tubes wrapped with filter cloth coated with a mixture of activated powdered carbon, diatomaceous earth, and fibers. The four tubes are inserted into a tank fed at the bottom with impure water, which passes through the filter cloth, entering the tubes through perforations. Each of these tubes is connected on top, then finally a union pipe serves as an outlet for the filtered water. In this process, materials such as rust, sediment, chlorine, and other undesirable substances are left outside the tank. Patent US2014166591A1[42] [53] involves a water filter that utilizes granulated active carbon, along with two other technologies—ultraviolet “C” (UVC) and photocatalytic oxidation (PCO). In the beginning, the granulated active carbon particles are coated with titanium dioxide, which turns all of the surfaces into powerful semiconductors, triggering a powerful PCO process when UVC photons come into contact with them. The carbon particles absorb the organic compounds, while the other technologies finish the oxidation process. Additionally, this process can regenerate the carbon particles endlessly. Patent W02010144175A1[43] [54] involves a design in which activated carbon is used in the filtration mesh. The main objective of this design is to decrease the content of leachable arsenic present in water, which can be toxic if consumed. To achieve this, an adsorbent for leachable arsenic is introduced within the carbon particles at a concentration of less than 5%. Tianye[44] [55] developed an active carbon filter using active carbon from bamboo, along with other chemicals such as sodium hydroxide, potassium hydroxide, octadecyl acrylate, bentonite, and silicon dioxide. It contained a natural antibacterial agent that was added into the active carbon material. The filter process was economical, had a large absorption capacity, and did not create any secondary pollution.
Patent CN106830474A[45] [56] involves a water purification system that can be used for healthy wine production using various filtration methods and processes. It contains an ultraviolet sterilizer, ultrafiltration membrane, precision filter, and an active carbon filter. All of these steps and processes are connected and the water is filtered through these systems. Patent CN109081477A[46] [57] involves another filter that can be used for rural water supply and purification systems using a heavy metal ion treatment system, a water softening system, an activated carbon filter system, and adsorption layers that utilize graphene oxide, KDF55 alloy, and polyacrylic acid. It is claimed that the filter can purify toxic organisms and compounds and is easy to operate.
Patent CN10304386AJ[47] [58] involves a three-stage filter that is used to tackle the problem of water loss and weak acid water production, which were the problems created by the filters currently available in the market. Therefore, the first stage involves microfiltration, as well as a composite molecular sieve; the second phase involves spherical preparation using active carbon and spontaneous medium-distance infrared rays; the last stage involves a nanofiltration membrane. Due to the requirement of low pressure, the absence of a pump saves energy. This design also manages to preserve water resources as intended. Patent CN209361991U[48] [59] is a design for a household filter with an easy-to-replace activated carbon filter element. The design aims to eliminate the inconvenience faced by consumers by using a handy filter. Patent CN107298506A[49] [60] involves an active carbon filter, wherein the purifier body is divided into a filtering cavity and an absorbing cavity, with the stirrers located in the filtering cavity. In the adsorbing cavity, numerous active carbon spheres are placed. The aim is that stirrers will speed up the water purification process.
4. Recent Research Papers on Water Purification Using Carbon Material
Nasrabadi and Foroutan[4] observed that CNTs have the potential to act as nanoelectrodes for separation of Na
+
−
+ move towards negatively charged CNTs and vice versa, leading to desalination. This study displays the potential applications of CNTs as efficient desalination nanomaterials. Ntim and Mitra[50] proposed a multiwalled carbon nanotube–zirconia (MWCNT-ZrO
2
2
Beobide et al. [51] stated that industrial waste water contains highly biodegradable compounds. Due to these highly biodegradable compounds, it is not effective to use a membrane bioreactor (MBR) alone, so it is used in combination with nanofiltration or reverse osmosis. Surface-enhanced Raman spectroscopy (SERS) is used to measure the level of methyl blue (MB). MB is used in the textile industry as an aromatic dye. At a concentration of 500 ng/mL it affects the human nervous system. By using CNTs, there was a clear reduction in MB at 1622cm
−1. Bakajin et al.[52] used CNTs in their initial experimentation stage by considered the three important factors i.e. capital, energy and operational cost. CNTs have a uniform pore size, which eliminates the need for multistage pretreatment efforts. The membrane surfaces of CNTs are hydrophilic, making cleaning easy using backwashing or rinsing. Das et al.[53]stated that due to a lack of fresh water for daily usage purposes, CNTs were used for filtration, as they can remove the pollutants and salt from water. The usage of CNTs in water is becomes more effective than other conventional options due to the low energy consumption and antifouling and self-cleaning properties (CNT membranes have a self-cleaning capacity).
Masinga et al.[54] discussed the synthesis of nanocomposites of β cyclodextrin polymers and nitrogen-doped carbon nanotubes under microwave irradiation. The polymers synthesized in the microwave showed better results in the removal of PnP from water than the conventional polymers. Zhang et al.[55] outlined a construction strategy for porous TiO
2
Nguyen-Phan et al.[56] prepared reduced graphene oxide–titanate (rGO-Ti) hybrids by including spherical TiO
2 nanoparticles with graphene. The RGO sheets were used as a platform for the deposition of titanate, which showed much better results compared to pure materials. Sreeprasad et al.[5] showed a simple synthetic process for rGO–metal oxide composites and outlined their water purification applications. The primary cause for composite formation is the reaction between the rGO and metal precursor. The composites are used in water purification and can also be used in engineering and science fields, such as in catalysis and for fuel cells.
Peng et al.[57] worked on the removal of arsenate from water. The authors synthesized GO-FeOOH composites, showing excellent absorption of arsenate from water. This experimental procedure showed the three-dimensional matrix method is less efficient than using two-dimensional GO sheets. Sun et al.[58] fabricated graphene oxide–silver nanoparticles (GO-AgNPs) onto cellulose acetate (CA). In the filtration process, GO-AgNPs have higher standards of infiltration than the GO and silver membrane. The presence of GO-AgNPs provides strong antibacterial activity in the membrane. This study shows the good potential for antibiofouling membrane development for membrane separation.
Manafi et al.[59] observed that the insolvent phase polyacrylamide (PAM) –graphene-based nanocomposites were synthesized to allow better dispersion of graphene nanoplatelets in the matrix. This process was carried out using acid in the case of functionalized graphene nanoplatelets (FGNp) to achieve the fine dispersion found in the PAM matrix. In addition to GO, both the efficiency and supernatant turbidity decreased. There was a concentration change of GO and the water was cleaned efficiently. Moreover, Lompe et al.[60] showed that a composite material can be formed by combining powdered activated carbon (PAC) with the magnetic properties of iron oxide nanoparticles (NPs). The elimination of ammonia did not influence the iron oxide NPs on the PAC in a bioreactor. The PAC showed good performance in terms of both being adsorbent and removing biological growth in drinking water. Kim et al.[61]studied graphene-based nanofiltration membranes, which were used for water purification. By altering the level of arc discharge, the degree of oxidation increased from 28.1% at 1 A to 53.9% at 4 A. Filters made of graphene sheets have excellent ion rejection capabilities compared to polyamide membranes. The high-carbon nanochannels show a tendency towards high water flux and show rejection in the case of hydrophobic interactions.
Yin et al.[62] demonstrated how graphene nanosheets are used in an in situ interfacial polymerization process. GO nanosheets have a multilayer structure. The spacing between layers was 0.83 nm and the GO nanosheets were dispersed in the polyamide, which improved the salt rejection in water. The GO nanosheets also rejected NaCl and Na
2
4 from water. GO sheets serve as water channels. The addition of different nanoparticle in carbon material also attempted to improve magnetic and other desired properties[63]. Pawar et al.[64] showed that the hydrothermal method can be used for the synthesis of MWCNTs and GO, where the nanostructure is based on single-crystal hematite (Fe
2
3). The samples were analyzed by performing different tests, such as X-ray diffraction, field emission scanning electron microscopy, and high-resolution electron microscopy tests. This technique involves lower fabrication costs for material formation and is also suitable for photocatalysis, having a high success rate for water purification. Sharma et al.[1] used graphene–carbon nanotube–iron oxide composites formed via the synthesis of carbon and iron-based nanomaterials for water purification. Due to the large surface area of graphene and graphene oxide, the absorption rate of impurities is higher. Magnetic-based nanomaterials are used to remove impurities and the extraction of nanomaterials from treated water is achieved by using external magnets. Tuan et al.[65] showed that capacitive deionization can be used for water purification to reduce the harshness of water by using an electrochemical double layer. There is extensive ongoing research on the use of reduced graphene oxide as a catalyst as compared to all other carbon-based materials. The efficiency of CDI was observed to be 3.54 mg by using purified rGO as an electrode.
5. Conclusions
2
3
4 nanoparticle composites) and through structural modifications
Reference (vwertically aligned CNTs). These are new approaches that can improve the effectiveness and efficiency.
The recent patents over the last decade related to water purification technolo'll rearrangies using carbon materials were discussed. Activated carbon is the most widely used carbon material in either original or combined form. The recent research articles on water purification using carbon materials were also covered. The hydrophilic surfaces of CNTs enable backwashing and rinsing. The use of carbon materials in combined form, such as MWCNT-ZrO2, rGO–Ti, and GO-Ag, are recen the references after you submit attempts at improving the effectiveness of water purification. The usage of carbon nanomaterials as nanoelectrodes adds one more reason for their application as water purification materials.
In conclusion, carbon-based materials can be used for small- and large-scale water purification technologies. They are cost-effective and eco-friendly options, which have experienced a steady increase in attention from the research community.
d it)
- Virender, K.S.; McDonald, T.J.; Kim, H.; Garg, V.K. Magnetic graphene-carbon nanotube iron nanocomposites as adsorbents and antibacterial agents for water purification. Adv. Colloid Interface Sci. 2015, 225, 229–240.
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2009, 110, 132–145.
- Kar, S.; Bindal, R.C.; Tewari, P.K. Carbon nanotube membranes for desalination and water purification: Challenges and opportunities. Nano Today 2012, 7, 385–389.
- Nasrabadi, A.T.; Foroutan, M. Ion-separation and water-purification using single-walled carbon nanotube electrodes. Desalination 2011, 277, 236–243.
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles—Formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423.
- Chang, C.-F.; Truong, Q.D.; Chen, J.-R. Graphene sheets synthesized by ionic-liquid-assisted electrolysis for application in water purification. Appl. Surf. Sci. 2013, 264, 329–334.
- Sreeprasad, T.; Maliyekkal, S.M.; Lisha, K.; Pradeep, T. Reduced graphene oxide–metal/metal oxide composites: Facile synthesis and application in water purification. J. Hazard. Mater. 2011, 186, 921–931.
- Goh, P.S.; Ismail, A.F.; Ng, B.C. Carbon nanotubes for desalination: Performance evaluation and current hurdles. Desalination 2013, 308, 2–14.
- Hu, J.-S.; Zhong, L.-S.; Song, W.-G.; Wan, L.-J. Synthesis of hierarchically structured metal oxides and their application in heavy metal ion removal. Adv. Mater. 2008, 20, 29772008.
- Liu, Y.; Li, Y.; Yan, X.-P. Preparation, characterization, and application of L.-cysteine functionalized multiwalled carbon nanotubes as a selective sorbent for separation and preconcentration of heavy metals. Adv. Funct. Mater. 2008, 18, 1536–1543.
- Chengfeng, R.; Rongrong, R. Get Rid of Chlorine Residue Active Carbon Water Purification Filter Core. U.S. Patent CN08791367U, 26 April 2019.
- Song, B.; Jian, L.; Honghu, P.; Shunpeng, Z.; Zhonghua, Z.; Jianqi, Z.; Guocheng, Z. Corncob Active Carbon Water Purification Filter Core. U.S. Patent CN204918207U, 2 October 2015.
- Du, H. Active Carbon Water Purification Device with Automatic Flushing and Pollutant Discharge Functions. U.S. Patent CN201678532U, 26 March 2010.
- Jie, L.; Min, L.; Jing, X. Active Carbon Water Purification Unit. U.S. Patent CN207478059U, 12 June 2018.
- Triestam, A. Hot Water Heater Has Water Container with Electrical Heating Body, Replaceable Active Carbon Water Filter(s) and/or UV Radiation Source(s); Water Flows Through Filter and/or Past UV Source. U.S. Patent DE19946064A1, 16 December 1999.
- Archer, V.L. Activated Carbon Water Filter. U.S. Patent CN209397001U, 17 September 2019.
- Gehrke, I.; Geiser, A.; Somborn-Schulz, A. Innovations in nanotechnology for water treatment. Nanotechnol. Sci. Appl. 2015, 8, 1–17.
- Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Souza, L.S.; Martins, A.C.; Silva, T.L.; Júnior, O.O.S.; Visentainer, J.V.; Almeida, V.C. NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies. Chem. Eng. J. 2015, 288, 778–788.
- Dąbrowski, A.; Podkościelny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon—A critical review. Chemosphere 2005, 58, 1049–1070.
- Cheng, W.; Dastgheib, S.A.; Karanfil, T. Adsorption of dissolved natural organic matter by modified activated carbons. Water Res. 2005, 39, 2281–2290.
- Brady-Estévez, A.S.; Kang, S.; Elimelech, M. A Single-Walled-Carbon-Nanotube Filter for Removal of Viral and Bacterial Pathogens. Small 2008, 4, 481–484.
- Baek, Y.; Kim, C.; Seo, D.K.; Kim, T.; Lee, J.S.; Kim, Y.H.; Ahn, K.H.; Bae, S.S.; Lee, S.C.; Lim, J.; et al. High performance and antifouling vertically aligned carbon nanotube membrane for water purification. J. Membr. Sci. 2014, 460, 171–177.
- Dong, X.; Yang, L. Dual functional nisin-multi-walled carbon nanotubes coated filters for bacterial capture and inactivation. J. Biol. Eng. 2015, 9, 1–10.
- Ain, Q.-U.-; Farooq, M.U.; Jalees, M.I. Application of magnetic graphene oxide for water purification: Heavy metals removal and disinfection. J. Water Process. Eng. 2019, 33, 101044.
- Lujanienė, G.; Šemčuk, S.; Lečinskytė, A.; Kulakauskaitė, I.; Mažeika, K.; Valiulis, D.; Pakštas, V.; Skapas, M.; Tumėnas, S. Magnetic graphene oxide based nano-composites for removal of radionuclides and metals from contaminated solutions. J. Environ. Radioact. 2016, 166, 166–174.
- Wang, J.; Chen, B. Adsorption and coadsorption of organic pollutants and a heavy metal by graphene oxide and reduced graphene materials. Chem. Eng. J. 2015, 281, 379–388.
- Zhang, Y.; Yan, L.; Xu, W.; Guo, X.; Cui, L.; Gao, L.; Wei, Q.; Du, B. Adsorption of Pb(II) and Hg(II) from aqueous solution using magnetic CoFe2O4-reduced graphene oxide. J. Mol. Liq. 2013, 191, 177–182.
- Do, M.H.; Phan, N.H.; Nguyen, T.K.P.; Pham, T.T.S.; Nguyen, V.K.; Vu, T.T.T. Activated carbon/Fe3O4 nanoparticle composite: Fabrication, methyl orange removal and regeneration by hydrogen peroxide. Chemosphere 2011, 85, 1269–1276.
- Gusain, R.; Kumar, N.; Ray, S.S. Recent advances in carbon nanomaterial-based adsorbents for water purification. Co-ord. Chem. Rev. 2020, 405, 213111.
- Dimiev, A.M.; Alemany, L.B.; Tour, J.M. Graphene oxide. Origin of acidity, its instability in water, and a new dynamic structural model. ACS Nano 2013, 7, 576–588.
- Sun, L. Structure and synthesis of graphene oxide. Chin. J. Chem. Eng. 2019, 27, 2251–2260.
- Han, S.; Yang, J.; Li, X.; Li, W.; Zhang, X.; Koratkar, N.; Yu, Z. Flame synthesis of superhydrophilic carbon nanotubes/ni foam decorated with fe2onanoparticles for water purification via solar steam generation. ACS Appl. Mater. Interfaces 2020, 12, 13229–13238.
- Li, M.; Liang, S.; Wu, Y.; Yang, M.; Huang, X. Cross-stacked super-aligned carbon nanotube/activated carbon composite electrodes for efficient water purification via capacitive deionization enhanced ultrafiltration. Front. Environ. Sci. Eng. 2020, 14, 107.
- Libing, Q.; Yabei, T.; Yanqing, X.; Ning, L. Modified Basalt Fibre Applied to Water Quality Purification. CN107262042A, 20 October 2017.
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process. Eng. 2020, 37, 101339.
- Yadav, S.; Saleem, H.; Ibrar, I.; Naji, O.; Hawari, A.A.; AlAnezi, A.A.; Zaidi, S.J.; Altaee, A.; Zhou, J. Recent developments in forward osmosis membranes using carbon-based nanomaterials. Desalination 2020, 482, 114375.
- Sweetman, M.J.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, A.S.E.; Hayball, J.D. Activated carbon, carbon nanotubes and graphene: Materials and composites for advanced water purification. J. Carbon Res. 2017, 3, 18.
- Qianfeng, L. Graphene Water Purifier. U.S. Patent CN109502896A, 22 March 2019.
- Longjingling, H.; Information Tech Co. Ltd. A Kind of Multiple-Effect Water Treatment Agent and Preparation Method thereof and Method for Treating Water. U.S. Patent CN107265530A, 20 October 2017.
- Liangti, Q.; Cheng, H.; Zhang, P.P. Graphene Solar Energy Water Cleaning Foam as Well as Preparation Method and Application. U.S. Patent CN108002366A, 8 May 2018.
- Hostetter, W.E. Carbon Water Filter. U.S. Patent US3612279A, 12 October 1971.
- Tarifi, M.H. PCO/UVC/Carbon Water Filter. U.S. Patent US2014166591A1, 19 June 2014.
- Frank, A.; Brigano, J.; Schroeder, H.; Popovic, V. Activated Carbon Water Filter with Reduced Leachable Arsenic and Method for Making the Same. U.S. Patent WO2010144175A1, 16 December 2010.
- Chen, T. Active Carbon Water Treatment Agent. U.S. Patent CN107555518A, 9 January 2018.
- Yan, Z.; Li, M. Aqua Pure Extract System and Method Are Used in Health Preserving Wine Production. U.S. Patent CN106830474A, 13 June 2017.
- Zhao, W.; Jiang, Y.; Lin, X.; Wang, L.; Guo, S.; Shen, J.; Peng, P.; Wang, D. A Kind of Rural Area Sub-Prime of Energy-Saving and Emission-Reduction is for Water Purification Integral System. U.S. Patent CN109081477A, 25 December 2018.
- Iang, Y. Energy-Saving and Water-Saving Type Water Purifier. U.S. Patent CN103043836A, 17 April 2013.
- Wang, Y.; Wang, P.; Lin, Y.; Zhang, X.; Zhang, K.; Chen, T. A Kind of Active Carbon Filter Core that Household Water Filter is Conveniently Replaceable. U.S. Patent CN209361991U, 10 September 2019.
- Gao, X. Possesses the Activated Carbon Filter of Stirring Mixed Function. U.S. Patent CN107298506A, 27 October 2017.