Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Catherine Yang and Version 2 by Catherine Yang.
Zinc-ion batteries (ZIBs) have been attracting extensive attention due to their outstanding properties and the potential to be the solution for next-generation energy storage systems. However, the uncontrollable growth of zinc dendrites and water-splitting issues seriously restrict their further scalable application. Solid polymer electrolytes (SPEs) have been regarded as a promising alternative to address these challenges and facilitate the practical advancement of zinc batteries.
- solid polymer electrolytes
- zinc batteries
- nanocomposites
- zinc dendrites
1. Homogenous Polymer Matrix SPEs
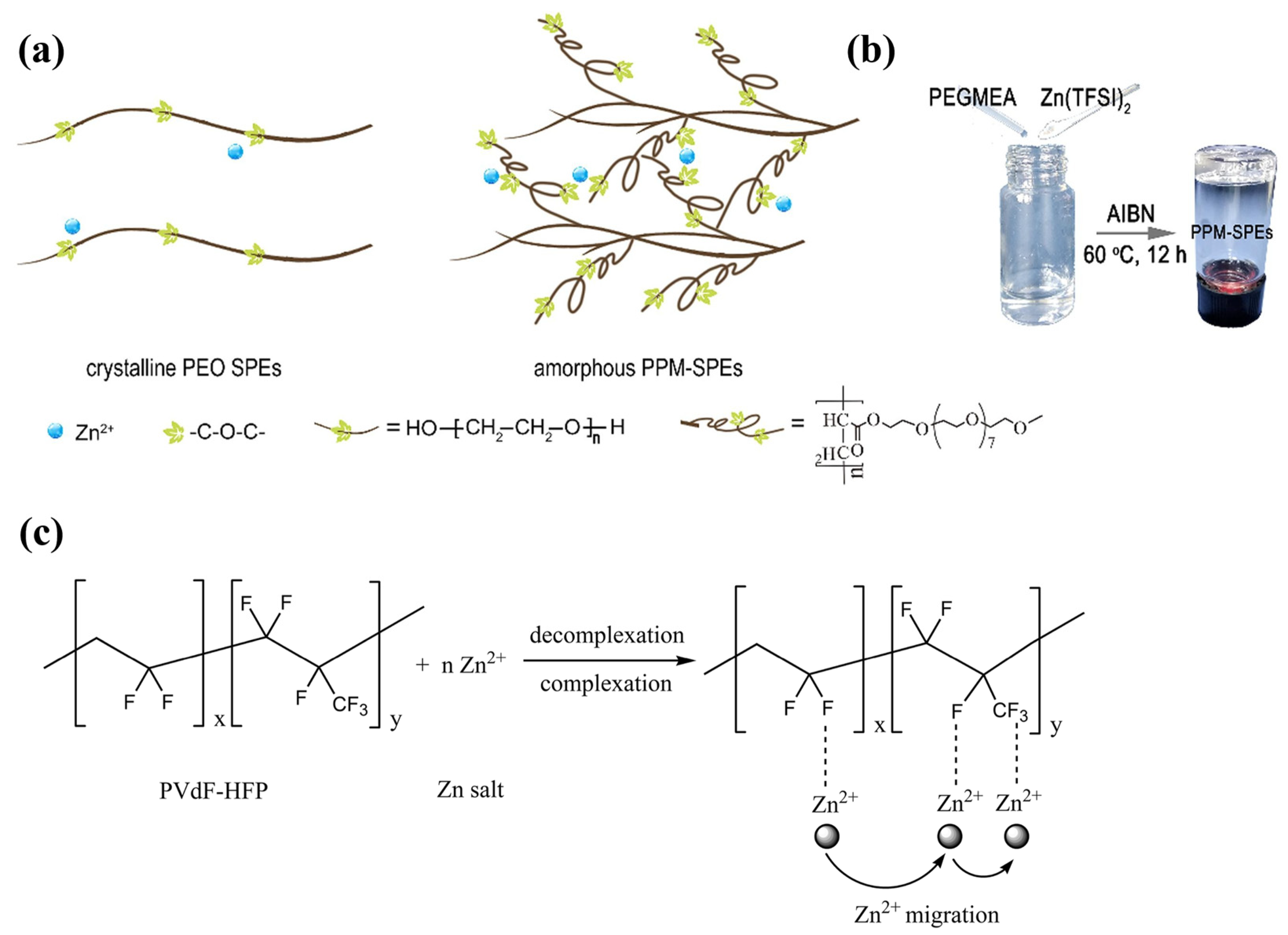
In the early SPE studies, organic polymer matrices such as polyethylene oxide (PEO) [1], polyvinylidene fluoride (PVDF) [2], polyacrylamide (PAM) [3], and polyvinyl alcohol (PVA) [4] were widely adopted with a low-molecular-weight plasticiser to enhance the ionic conductivity of the electrolytes. Nonetheless, these polymers were commonly reported with low ionic conductivity and poor interface compatibility. For instance, as one of the most common electrolyte materials for LIBs and ZIBs, the flexible chains of PEO have allowed Zn2+ ions to be transferred by segmental motion [5]. However, efficient Zn2+ conduction and zinc electrochemistry have been challenging to achieve owing to the high degree of crystallisation of PEO and its interfacial incompatibility with Zn metal. This leads to the poor ionic conductivity of PEO, reported as approximately 1.09 × 10−6 to 2.87 × 10−5 S cm−1 at room temperature. To enhance the ion transfer, Zhao et al. [6] utilised poly(ethylene glycol) methyl ether acrylate (PEGMEA) to synthesise in situ-polymerised PEO-based SPEs. This approach formulated a cross-linking structure within the amorphous regime and improved the ionic conductivity to 2.87 × 10−5 S cm−1, as revealed in Figure 1a. Except for overcoming the poor interfacial ion transport, the in situ strategy also improves the reversibility of Zn electrochemistry in symmetrical cells. Except for PEO, Poly(vinylidene fluoride) (PVdF) is one of the common electrolytes in ZIB development owing to its porous semicrystalline structures, where the strong electron-withdrawing effect can be observed at the CF2 functional groups. This accelerates the isolation of the metal salts, increasing the number of charges that contributes to the ionic conductivity [7]. Song et al. [8] utilised solution casting methods to synthesise PVdF-HFP (poly(vinylidene fluoride-hexafluoropropylene))-based SPEs, where an ionic conductivity of 2.44 × 10–5 S cm−1 was achieved at room temperature, with a mass ratio of 0.4 between Zn(Tf)2 and PVdF-HFP. Furthermore, the intrinsic structure of PVdF-HFP also allows the flow of charge through complexation and decomplexation within the polymer segments, as depicted in Figure 1b. Nevertheless, PVDF possesses similar drawbacks to PEO, where low ionic conductivity, high interfacial resistance, rapid degradation, and poor mechanical strength can be observed in early ZIB studies [9].
To overcome the addressed drawbacks of conventional SPEs, especially low ionic conductivity and high interfacial resistance, Ma et al. [10] reported an amorphous SPE via in situ ring-opening polymerisation of a precursor of 1,3-dioxolane (DOL) within an electrochemical cell, as shown in Figure 2a. This synthesis method led to the incorporation of a substantial concentration of zinc salts into the SPE matrix. The in situ-formed SPEs demonstrated a high ion conductivity of 1.96 × 10−2 S cm−1 at room temperature and non-dry properties. Owing to the well-connected pathways and lesser interfacial defects, only 10% interfacial impedance was retained compared to a conventional battery, leading to a higher columbic efficiency. Moreover, the developed battery successfully achieved charging/discharging cycles of 1800 h without dendrite growth. To further strengthen the ionic conductivity and mechanical properties, Liu et al. [11] synthesised dry polymer “PHP” by introducing a 2,6-bis((propylimino)methyl)-4-chlorophenol (Hbimcp) ligand into the poly(propylene oxide) (PPO) polymer chain, where the SPE film is obtained after being slowly volatilised in a polytetrafluoroethylene mould at room temperature. The PHP SPE was reported with a moderate ion conductivity of 10−5 S cm−1 and significant flexibility at 1000% elongation strain and a fast self-healing capability. Also, the ZIB exhibited a remarkable cycling stability (125% capacity retention after 300 cycles) and a high coulombic efficiency (94% after 300 cycles). These favourable properties can be attributed to the unique coordination system of PHP, where the strong coordination withstands stress during stretching while the fast ligand exchange between zinc ions and the PHP polymer chains endows ion conductivity. Moreover, the imine bonds in the polymer also enable acidic degradation of the electrolyte that improves its recyclability.
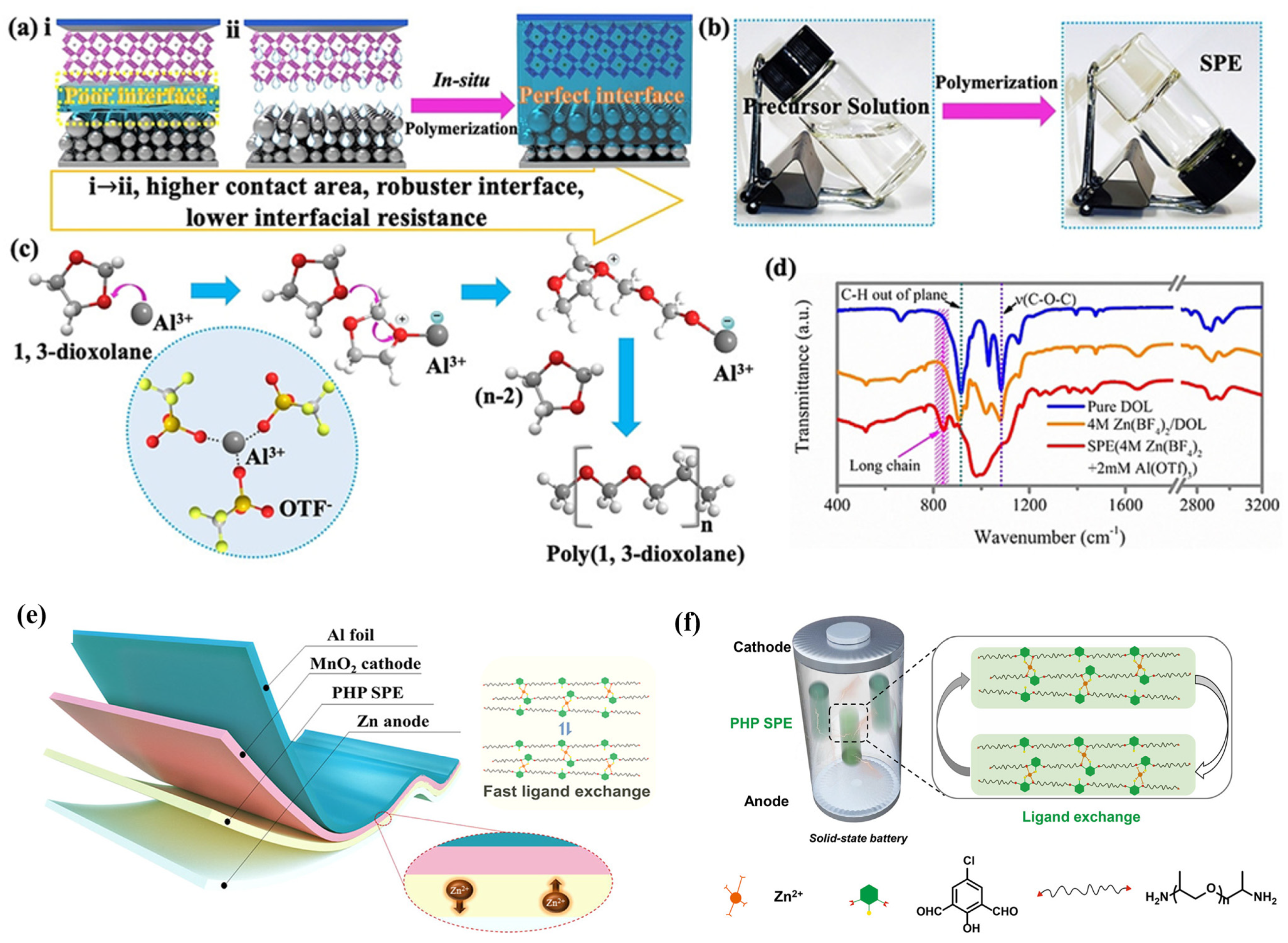
Figure 2. (a) Schematic illustration of in situ-formed SPEs for Zn batteries. (b) Photos of polymerisation process. (c) Illustration of the polymerisation mechanism of DOL. (d) The FT-IR spectrum of pure DOL, Zn(BF4)2/DOL solution, and SPE. Reproduced with permission from [10]. (e) Schematic of the fast ligand exchange within the PHP-based ZIB. (f) The zinc ion conduction mechanism within the PHP-SPE-based all-solid-state battery. Reproduced with permission from [11].
Except for electromechanical and cost efficiency, environmentally friendly and recyclable energy storage devices are also vital to establish a positive transition towards sustainable energy storage. Brige et al. [12] fabricated bio-based SPEs utilising hydroxyethylcellulose (HEC), an amorphous homogenous biopolymer. HEC contains side chains composed of ethylene oxide groups that are grafted onto the cellulose backbone. As a result, despite HEC possessing favourable film-forming characteristics, comparable to PEO, the low capability of salt-dissociation of HEC caused a poor ion conductivity of 10−6 S cm−1. In order to enhance the electromechanical performance of bio-based SPEs, Huang et al. [13][14] developed bio-based SPEs by introducing kappa-carrageenan and guar gum for flexible Zn-MnO2 batteries, which are eco-friendly, low-cost, and highly conductive. Figure 3a,b presents the molecular formula of kappa-carrageenan and the photograph of kappa-carrageenan electrolyte. By mixing the biomass with a ZnSO4 and MnSO4 mixture solution, the kappa-carrageenan electrolytes exhibited an excellent ion conductivity of 3.32 × 10−2 S cm−1, fast charging and discharging capability (120.0 mAh g−1 at 6.0 A g−1), impressive cycling stability (80% remaining after 450 cycles), and moderate bending durability (95% after 300 cycles). Furthermore, the mechanical stiffness of the SPE was also reinforced by employing rice paper as a scaffold. While the guar-gum-electrolyte-based flexible quasi-solid-state (QSS) ZIB (Figure 3c) delivered an enhanced flexibility and conductivity compared to kappa-carrageenan, the eco-friendly electrolyte had an astonishing ion conductivity of 1.07 × 10−2 S cm−1 at ambient, which is greater than conventional quasi-solid-state polymer electrolytes. The QSS ZIB also delivered a fast charging and discharging capability (131.6 mAh g−1 at 6.0 A g−1) and effective zinc dendrites suppression during cycling, as revealed in Figure 3d. Especially, the ZIB achieved an outstanding cycling stability, retaining 100% of its capacity after 1900 cycles and 85% after 2000 cycles, for a Zn stripping/plating testing time more than 300 hours as shown in Figure 3e. The ZIB also exhibited remarkable durability when subjected to bending, maintaining 81.3% of its capacity after undergoing 180° bending for 1000 cycles. The biopolymer electrolytes synthesised by Huang et al. exhibited significant potential for a bio-based electrolyte of high-performance ZIBs and achieved an outstanding ion conductivity and cycling performance for SPE-based ZIBs.
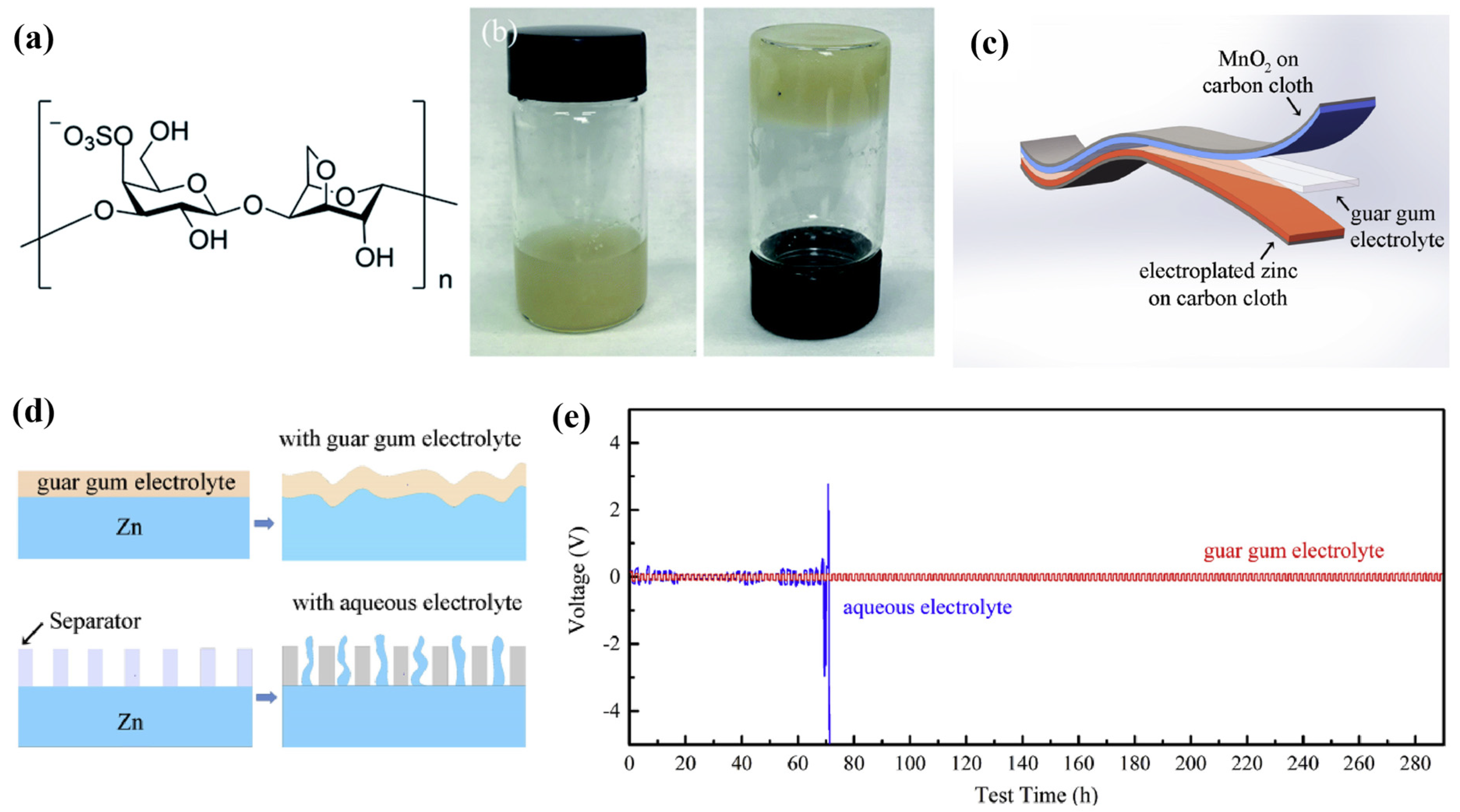
Figure 3. (a) The molecular formula of kappa-carrageenan. (b) Photograph of kappa-carrageenan electrolyte after adding 2 M ZnSO4 and 0.1 M MnSO4 aqueous solutions. Reproduced with permission from [13]. (c) Schematic illustration of the structure of the solid-state Zn–MnO2 battery. (d) Schematic diagram of the morphology change of the zinc foil in guar gum electrolyte and aqueous electrolyte after cycling. (e) Zn stripping/plating from Zn/Zn symmetrical cells at 0.24 mA cm −2 in guar gum electrolyte and aqueous electrolyte. Reproduced with permission from [14].
2. Hybrid Polymer Matrix SPEs
The engineering of high-performance SPEs is complex and challenging as the electrolyte has to be conductive with a high charging/discharging capability and capacity retention, as well as mechanically stretchable or stiff to be flexible according to applications. However, it is rare to discover homogeneous materials with all the aforementioned properties. Hence, SPEs composed of hybrid polymer matrices have gained significant research interest, where the polymers are often synthesised through crosslinking, self-assembly, or copolymerisation. With various combinations of polymer matrices, this could grant SPEs with favourable properties, such as efficient ion transportation in amorphous structures, crosslinking reinforcement in mechanical robustness, and a flame-retardant mechanism.
Some of the pioneering works can be found in the SPE study conducted by Ye and Xu [15], where PVDF-HFP was combined with poly(ethylene glycol) dimethyl ethers (PEGDMEs) via the solution casting method. The polymer/polymer matrix revealed that PEGDME possesses significant salt solvation, while the blending of PVDF-HFP provides mechanical support, resulting in an ionic conductivity at 1.7 × 10−2 S cm−1 in ZIB composed of 0.5 M Zn(TFSI)2/PEGDME/PVDF-HFP. However, drawbacks such as cyclability and large weight loss leading to an ion conductivity decrement had yet to be investigated in the early work. Rathika et al. [16] employed a similar blending approach to synthesise a 90 wt% PEO/10 wt% PVdF blended polymer electrolyte with 15 wt% zinc triflate salt (Zn(CF3SO3)2), as illustrated in Figure 4a. The blending of the polymer/polymer interface led to an amorphous phase, reducing the crystallinity of the host polymer blend matrix. This amorphous structure, as depicted in Figure 4b, facilitates the establishment of ion–polymer interactions between the polymer matrix and zinc triflate salt. The presence of this unique amorphous structure reduces the cohesive forces acting upon the polymer blend chains, allowing for increased segmental mobility. This enhanced mobility of segments within the polymer blend electrolyte system promotes improved charge mobility, thereby facilitating the movement of charges through the electrolyte.

Figure 4. (a) The illustration of the formation mechanism of PEO/PVDF-blended SPEs. (b) Schematic of crosslinking and ion-polymer interactions. Reproduced with permission from [16].
Except for polymer blending, Lu et al. [17] proposed the synthesis approach of manipulating the heteroleptic coordination that integrates polyacrylamide (PAAM) ligands with acetamide coligands for zinc ion centres. As illustrated in Figure 5, the in situ catalytic polymerisation initiated by Lewis-acidic Deep eutectic solvents (DESs) significantly enhanced the mobility of Zn2+ and the polymer by enabling the formation of eutectic ion channels with both labile Zn2+−polymer bonding. The heteroleptic coordination polymer electrolytes (HCPEs) exhibited an ionic conductivity of 4.7 × 10−3 S cm−1 and a Zn2+ transference number of 0.44 at room temperature, attributed to the ligand exchange process to accelerate long-range Zn2+ transport. The increment in available distinct ligands in the limited metal coordination sphere not only established a well-lubricated ion channel to reduce the ionic migration barrier, but it also produced a flexible coordination sphere to enable fast ligand exchange. The solid-state ZIB also performed reversibility of the Zn plating/stripping process (1200 h), with a cyclability of 350 cycles with Mo6S8 cathodes and a coulombic efficiency of ∼99%. Furthermore, the HCPEs exhibited remarkable flexibility and high stretchability, functioning as elastomers rather than mechanically rigid structures. Notably, these HCPEs demonstrate an impressive maximum stretchability exceeding 800% when subjected to a stretching speed of 20 mm/min. This exceptional stretchability can be attributed to the enhanced dynamics of ligand exchange within the HCPEs.

Figure 5. Schematic diagram of the concept of ZLPE and HCPEs. Reproduced with permission from [17].
Other than hybrid polymer composition, the inclusion of a bio-based polymer into the hybrid polymer matrix was often adopted in SPE research, as the biopolymer offers mechanical support, biocompatibility, and environmental friendliness. Li et al. [18] introduced a novel approach for developing a hierarchical polymer electrolyte (HPE) using gelatin and polyacrylamide (PAM) as the base materials, coupled with an a-MnO2 nanorod and carbon nanotube (CNT) cathode. Figure 6 illustrates the synthesis route of the HPE, which was synthesised by grafting PAM onto gelatin chains that are filled in the network of a polyacrylonitrile (PAN) electrospun fibre membrane. The grafting process is achieved through a free radical polymerisation method, enabling the fabrication of the HPE. The porous hierarchical structure and significant water retention capability of the polymeric network offered an impressive ionic conductivity of 1.76 × 10−2 S cm−1, while the superior interfacial contact between the electrodes and HPE endowed enhanced ion diffusion and higher reaction kinetics for long-term cycling (capacity retention of 97% after 1000 cycles at 2772 mA g−1). Moreover, the grafting of PAM onto a gelatin hydrogel (gelatin-g-PAM) offered favourable mechanical strength and excellent capacity retention (averaging above 90%) after undergoing several destructive conditions, including being cut, bent, hammered, punctured, burnt, sewed using a commercial sewing machine, and even rinsed without any packaging.
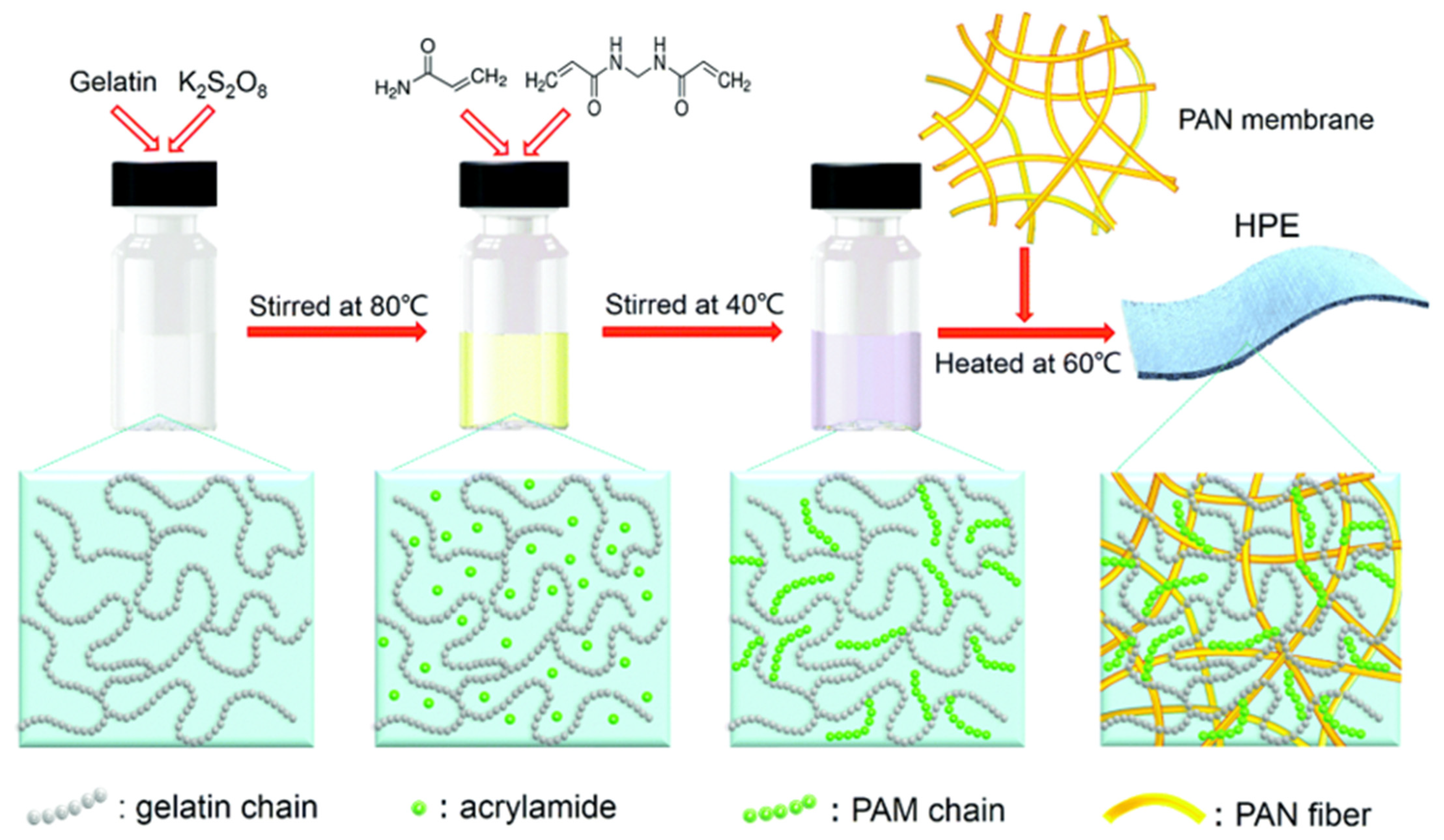
Figure 6. The synthesis route of the HPE. Reproduced with permission from [18].
The polymeric architecture of SPE is essential to engineer as it constructs the pathway of ion transportation and also polymer mobility that provides mechanical stability. Qiu et al. [19] developed a zwitterionic triple-network structure hydrogel electrolyte incorporating PAM, gelatin, and [(2-methylacryloxy)ethyl]dimethyl-(3-sulfonic acid propyl)ammonium hydroxide (DMAPS) for a flexible ZIB. The SPE was fabricated using a facile approach, as revealed in Figure 7a, where the mixture was polymerised at 60 °C and refrigerated. In terms of electromechanical efficiency, the addition of DMAPS on PAM/gelatin ZIBs caused an ionic conductivity of 3.51× 10−2 S cm−1 to be achieved. This can be attributed to the addition of ion transportation pathways offered by the intrinsic zwitterionic groups on polymer chains, thus effectively accelerating the rate of ion transport. The PAM/gelatin/DMAPS electrolyte also revealed a self-healing ability owing to the hydrogen bond interaction and delivered an excellent coulombic efficiency of over 99%. Additionally, the mechanical tests in Figure 7c,d suggested that the inclusion of gelatin improves the elasticity and the toughness of the electrolyte, while DMAPS reduces the elasticity and toughness owing to its poly-zwitterion characteristic.

Figure 7. (a) Demonstrations of the synthesis process of the hydrogel. (b) The ionic conductivity of hydrogels with different compositions. (c) The tensile-stress–strain curves of the hydrogels. (d) The cycling loading–unloading curves of the hydrogel. Reproduced with permission from [19].
Dueramae et al. [20] proposed an innovative polymer electrolyte from carboxymethyl cellulose (CMC) and poly(N-isopropylacrylamide) (PNiPAM) as an SPE for ZIBs, which was synthesised via the solution casting method. As a result, the blended CMC/PNiPAM SPEs revealed magnificent performance in tensile strength (37.9 MPa) and modulus (2.1 GPa). The observed increase in film stiffness upon the addition of PNiPAM can be ascribed to the enhanced rigidity resulting from the presence of both -NH and -OH groups within PNiPAM. These functional groups actively engage in robust intermolecular bonding and electrostatic interactions with the carboxyl and hydroxyl groups of CMC. The nature of these interactions encompasses hydrogen bonding, dipole–dipole interactions, as well as charge effects. Collectively, these interactions contribute to the overall reinforcement of the film structure, leading to increased stiffness. Moreover, the thermal stability of the CMC/PNiPAM was also examined, and the blended SPE was thermally more stable with a higher decomposition temperature compared to the pure CMC. The enhancement in thermal stability was suggested to be offered by the restricted chain motion between the respective functional groups of the CMC and PNiPAM molecules. The SPEs exhibited a moderate ionic conductivity of 1.68 × 10–4 S cm−1 and a high Zn2+ ion transference number of 0.56. This can be due to the porous structure of the SPE, which promotes zinc movement in the SPEs containing zinc triflate.
Among the reviews, bio-based polymers such as gelatin, cellulose, and gums have generally exhibited favourable electromechanical properties due to their intrinsic polymer structure. For instance, the linear chains with carboxymethyl substitution of CMC not only provide high mechanical flexibility and stability, but also create aligned charge transport paths. To further increase the biocompatibility of SPEs, Zhou et al. [21] developed a cellulose aerogel-gelatin (CAG) solid electrolyte for an implantable, biodegradable transient zinc-ion battery (TZIB). As shown in Figure 8, the gelatin electrolyte was grafted into a cellulose aerogel (CA) 3D porous framework via a super-assembly strategy. Subsequently, the 3D CA architecture was obtained via freeze-drying and pristine gelatin chain injection. The TZIB was then fabricated from a flexible silk protein film, in situ-evaporated Au film, screen-printed Zn film, and MnO2/rGO hybrid materials as the encapsulation layer, collector, cathode, and anode materials, respectively.
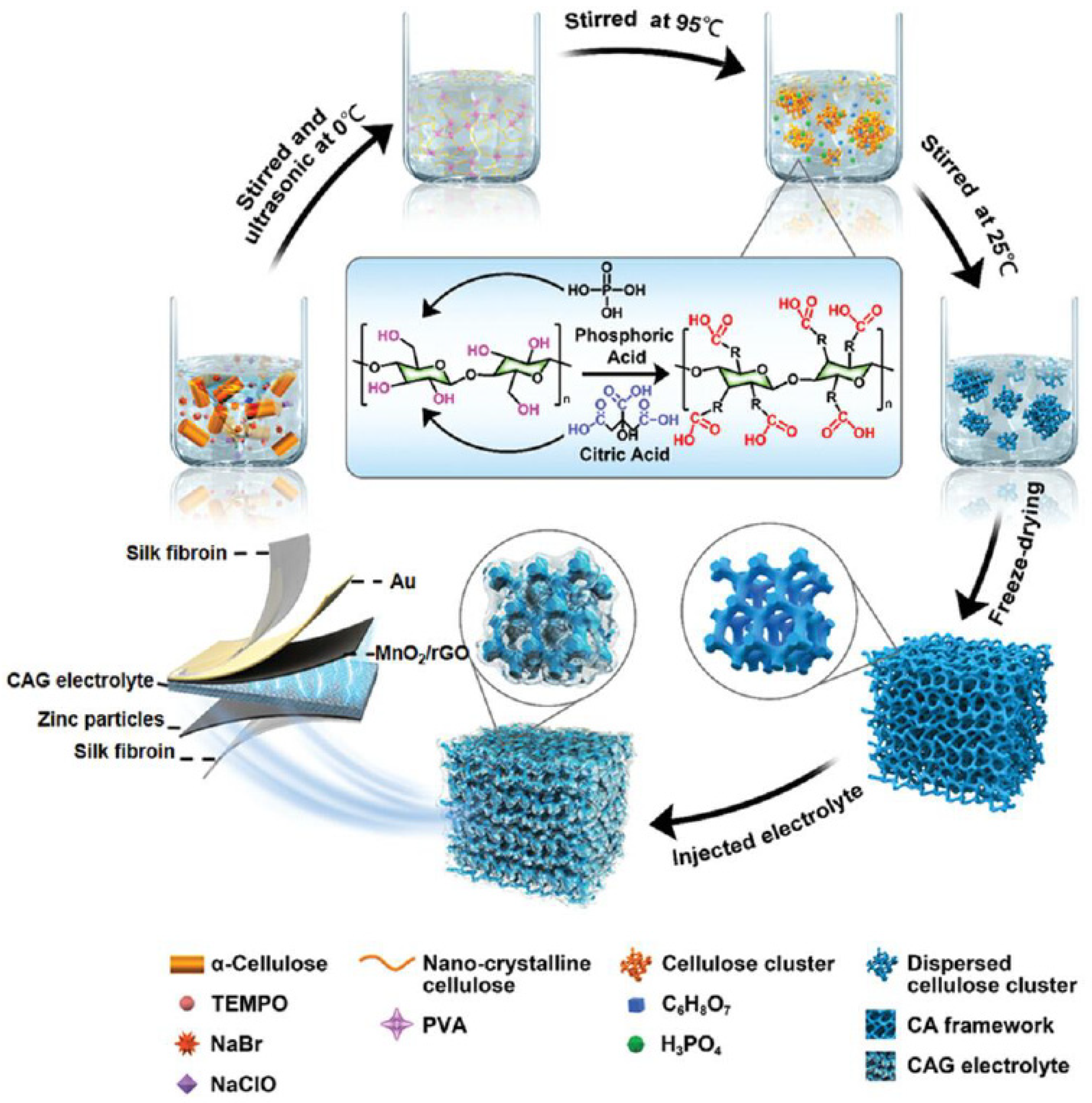
Figure 8. Demonstration of the super-assembly synthesis process. Reproduced with permission from [21].
As a result, the synthesised CAG film exhibited a highly porous three-dimensional (3D) structure, as illustrated in Figure 9a, possessing a significant liquid storage capacity. This unique structure endowed an extremely high ionic conductivity of 1.23 × 10−2 S cm−1 at ambient temperature while retaining great mechanical robustness (i.e., bending angle > 120°) and biodegradability. The porous 3D framework plays a crucial role in enhancing electrolyte absorption and reinforcing the TZIB body. Furthermore, the presence of micropores facilitates the migration of electrolyte ions. The TZIB based on the CAG film exhibited favourable cyclability performance, ensuring both implantability and biodegradability. Even after 15 cycles, the capacity retention remained at 96.4%, and it maintained 95.4% capacity after being subjected to bending or folding. Moreover, the TZIB can be effectively biodegraded by phosphate-buffered saline (PBS) solution within a 30-day period, as demonstrated in Figure 9f. Importantly, the degradation materials of TZIB do not contain any heavy metal ions, toxic polymers, or electrolytes that could adversely affect human health. This characteristic positions TZIB as a potential candidate for self-powered transient electronics or conventional self-powered implantable medical devices in future applications.
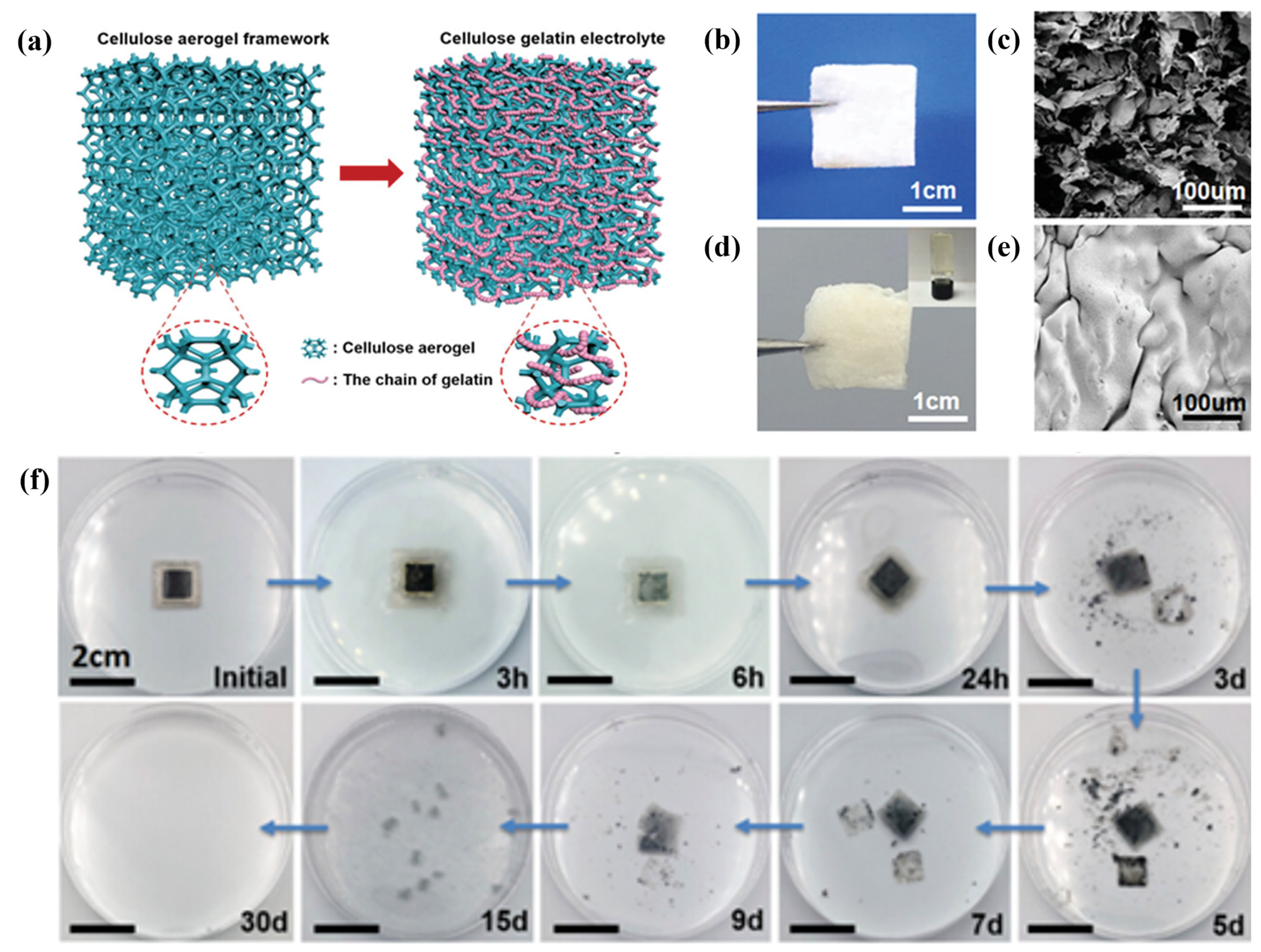
Figure 9. (a) Schematic diagrams of the structure and assembly of the CAG SPE. (b,c) The optical and SEM images of the CA framework. (d,e) Both optical and SEM photos of the fabricated CAG film. (f) Series of photos recording the biodegradation profile of the encapsulated battery in buffered protease solution at 37 °C. Reproduced with permission from [21].
Similar research also conducted by Zhou et al. [22] shows the fabrication of TZIB devices, including a plasticised gelatin-silk fibroin electrolyte film and a dual-yarn electrode structure. The gelatin-based electrolyte was synthesised as illustrated in Figure 10, where the gelatin solution was mixed with dissolved silk fibre. After ultrasonic treatment, the gelatin electrolyte was placed on the gelatin-silk film to obtain solidified film via rapid cooling to room temperature. Throughout the synthesis process, the polypeptide backbone of the silk protein underwent molecular conformational changes, dispersing among the doped gelatin chains in formic acid. Subsequently, as the acid volatilised, a plasticised silk protein film was formed. Over time, the gelatin molecule would gradually transit from a randomly coiled state to an ordered triple-helix structure. This structural arrangement facilitated the incorporation of β-sheets, endowed with robust mechanical properties, uniformly embedded within the gelatin–protein composite network, resulting in a homogeneous biomass composite framework. Consequently, the transient zinc-ion battery (TZIB) exhibited a high specific capacity of 311.7 mAh g−1 and excellent cycle stability with a capacity retention of 94.6% after 100 cycles. This performance is attributed to the notable ionic conductivity of 5.68 × 10−3 S cm−1 at room temperature. Regarding mechanical stability, the TZIB device demonstrated significant shape plasticity, retaining 82.5% of its capacity after undergoing 80 bends. Furthermore, it exhibited commendable biodegradability, fully degrading within 45 days under enzyme digestion.

Figure 10. Schematic diagram of fabrication and encapsulation of the fibre-shaped TZIB. TZIB, transient zinc-ion battery. Reproduced with permission from [22].
3. Nanocomposite Polymer Electrolytes
Except for incorporating various polymer matrices via cross-linking or polymerisation, including a small quantity of nanocomposites has also attracted research interests in enhancing the mechanical, thermal, electrical, and electrochemical properties of polymer electrolytes. This is owed to the distinctive microstructure with significant lateral dimensions and low thickness of the two-dimensional (2D) materials that endows a significant specific surface area and thermal/electrical conductivities. Sownthari and Austin Suthanthiraraj [23] employed organically modified montmorillonite (MMT) as an additive to enhance the electromechanical properties of a poly(ϵ-caprolactone) (PCL)-based electrolyte via the solution casting method. With 15 wt% of MMT, the modified MMT-PCL electrolyte acquired a moderate ionic conductivity of 9.5 × 10−5 S cm−1. This can be attributed to the enhanced dissociation of ion pairs and higher aggregates of dopant salt facilitated by the high dielectric constant of the nanoclay, where the number of free triflate ions increases the number of free Zn2+ for conduction. However, the MMT-PCL showed unsatisfied thermal stability, where an earlier decomposition temperature was observed due to the decrease in the electron density caused by the interaction of Zn2+ ions with the carbonyl oxygen. To investigate the feasibility of applying nanofillers into a hybrid polymer matrix, Prasanna and Suthanthiraraj [24] incorporated zirconia (ZrO2) nanofillers into a poly(vinyl chloride)(PVC)/poly(ethyl methacrylate) (PEMA)-based GPE also via the solution casting method. As a result, the 3 wt% of ZrO2 dispersed in PVC (30 wt%)/PEMA (70 wt%) GPE achieved an enhanced ionic conductivity at 3.63 × 10−4 S cm–1. The enhanced conductivity observed can be attributed to the Lewis-acid–base interaction between the electrolytic species and the OH/O sites present on the surface of the filler. This interaction creates additional hopping sites and conductive pathways for the migrating charged species, facilitating their movement within the system. Furthermore, the introduction of ZrO2 into the electrolyte has the effect of enhancing the flexibility of the polymer chains. This is achieved by impeding the reorganisation of the chains and stabilising the amorphous phase of the composite gel polymer electrolyte (GPE). The increased flexibility and stabilisation contribute to improved performance and overall characteristics of the GPE.
To further strengthen the electromechanical performance of the nanofiller-infused electrolyte, Chen et al. [25] successfully developed a SPE for high-performance all-solid-state zinc-ion batteries (ZIBs) by employing a blade casting method. The SPE is based on the poly(vinylidene fluoride-co-hexafluoropropylene) matrix, which is filled with poly(methyl acrylate)-grafted MXenes. The composite material, denoted as PVHF/MXene-g-PMA, demonstrates remarkable stability and reliability in ZIB applications. MXene, as a relatively recent addition to the family of two-dimensional (2D) materials, shows tremendous potential as an advanced inorganic filler. It is represented by the chemical formula Mn+1XnTx, where M represents a transition metal, X represents carbon or nitrogen, and T represents a terminating group (O, OH, or F). MXene stands out due to its expansive specific surface area and abundant surface functional groups, making it an attractive candidate for enhancing the material properties when incorporated as a filler. The fabrication process is shown in Figure 11a. Firstly, LiF and HCL solution were employed to obtain 2D MXene by removing aluminium alloys. The PMA was then grafted with MXene via an in situ polymerisation on MXene surfaces, where the hydroxyl groups provide active grafting sites for the PMA. On the other hand, F-H hydrogen bonds can also be associated between PVHF and the H atoms of PMA. The resulting SPE with a PMA content of 0.05 exhibited an excellent ion conductivity of 2.69 × 10−4 S cm−1 at room temperature, which is an ionic conductivity three orders of magnitude larger than that of the PVHF matrix owing to the high electrical conductivity of MXene. Importantly, the SPE is able to maintain significant ionic conductivities at different temperatures ranging from −25 °C to 85 °C, as revealed in Figure 11b. Mechanical-wise, Figure 11c suggests that the PVHF/MXene-g-PMA offers a significant enhancement in the elongation (60.1%), where the stress was mostly maintained at 13.2 MPa compared with the neat PVHF. Moreover, a significant coulombic efficiency of 98.9% was maintained for over 350 cycles in the cyclic stability tests. Dendrite-free Zn plating/stripping with high reversibility was achieved over 1000 and 200 h of cycling at 25 °C and 55 °C, where a remarkable capacity retention was maintained above 90% after 10,000 cycles at room temperature for more than 90 days.
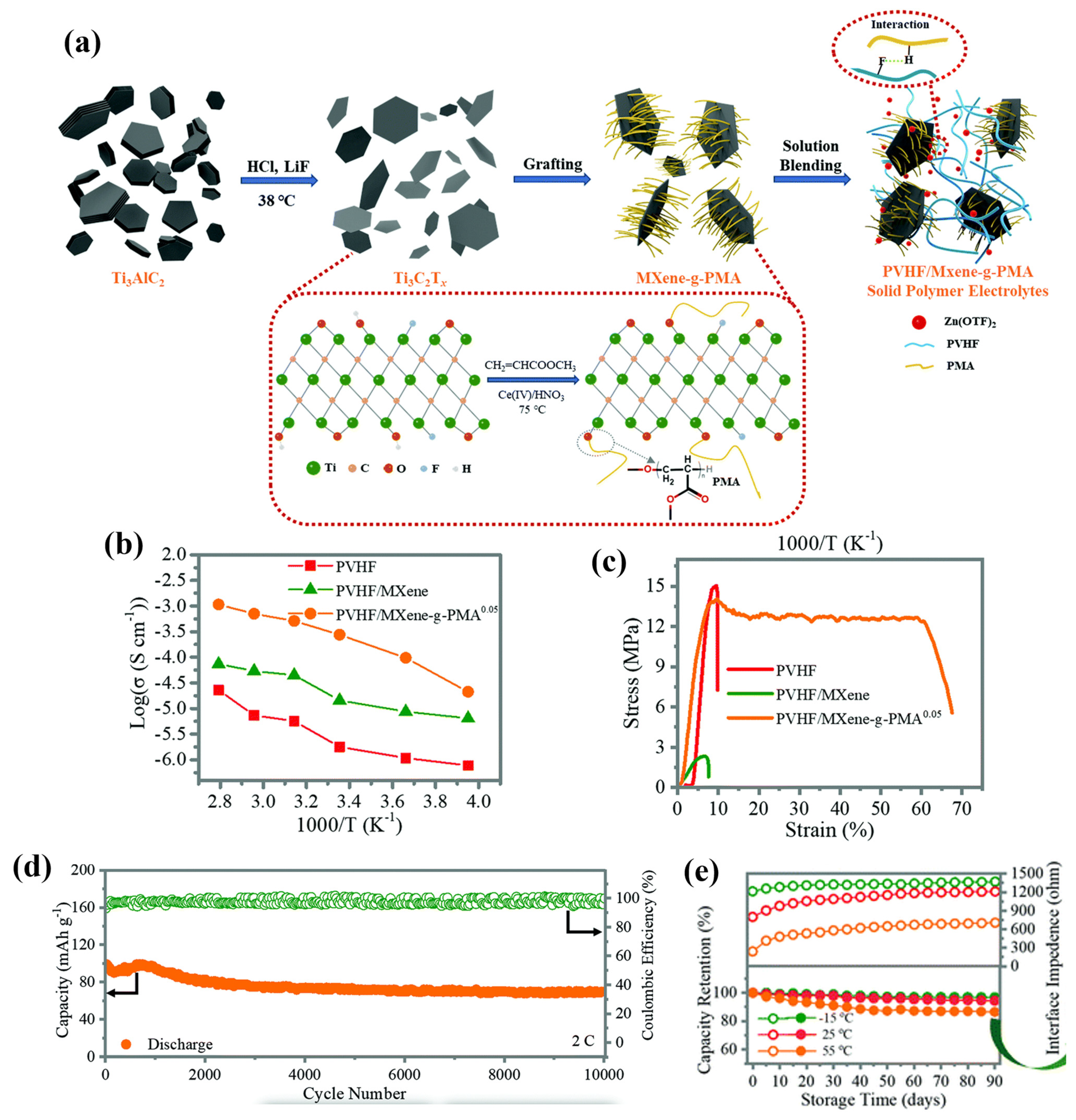
Figure 11. (a) The fabrication process of the PVHF/MXene-g-PMA SPEs. (b) Ionic conductivities at different temperatures. (c) Strain–stress curves. (d) Cycling performance and coulombic efficiency. (e) Capacity retention and interface impedance of the SPEs. Reproduced with permission from [25].
Liu et al. [26] reported a GPE for high-performance ZIBs employing MXene-derived TiO2 nanosheets as an additive, which was obtained via hydrothermal reaction. The nanosheet was mixed with Polyvinyl alcohol (PVA) and Zn(CF3SO3)2 in dissolved solution and frozen via the solution casting method, as revealed in Figure 12a. With 3 wt% of TiO2 additives, the resulting GPE (PZ3T) exhibited a moderate ionic conductivity of 1.243 × 10−5 S cm−1 and excellent mechanical reinforcement, where the tensile strength and elongation at break were significantly improved, as illustrated in Figure 12b. Importantly, the GPE also exhibited self-healing behaviour owing to the hydroxyl groups and hydrogen bonds between PVA chains and TiO2 nanoparticles. Moreover, an excellent coulombic efficiency of 99.8% and stable and reversible Zn plating/stripping for over 3000 h were observed, attributed to the dendrite suppression effect.

Figure 12. (a) The synthesis process of the TiO2-based GPE derived from MXene; (b) the percentage of elongation at break and tensile strength with contents of additives; (c) the cycling performance and coulombic efficiency of GPEs. Reproduced with permission from [26].
References
- Hiralal, P.; Imaizumi, S.; Unalan, H.E.; Matsumoto, H.; Minagawa, M.; Rouvala, M.; Tanioka, A.; Amaratunga, G.A.J. Nanomaterial-enhanced all-solid flexible zinc−carbon batteries. ACS Nano 2010, 4, 2730–2734.
- Tafur, J.P.; Abad, J.; Román, E.; Romero, A.J.F.J.E.C. Charge storage mechanism of MnO2 cathodes in Zn/MnO2 batteries using ionic liquid-based gel polymer electrolytes. ACS Nano 2015, 60, 190–194.
- Li, H.; Liu, Z.; Liang, G.; Huang, Y.; Huang, Y.; Zhu, M.; Pei, Z.; Xue, Q.; Tang, Z.; Wang, Y.J.A.n. Waterproof and tailorable elastic rechargeable yarn zinc ion batteries by a cross-linked polyacrylamide electrolyte. ACS Nano 2018, 12, 3140–3148.
- Kaltenbrunner, M.; Kettlgruber, G.; Siket, C.; Schwödiauer, R.; Bauer, S.J. Arrays of ultracompliant electrochemical dry gel cells for stretchable electronics. Adv. Mater. 2010, 22, 2065–2067.
- Yi, J.; Guo, S.; He, P.; Zhou, H.J.E. Status and prospects of polymer electrolytes for solid-state Li–O 2 (air) batteries. Energy Environ. Sci. 2017, 10, 860–884.
- Zhao, Z.; Wang, J.; Lv, Z.; Wang, Q.; Zhang, Y.; Lu, G.; Zhao, J.; Cui, G. In-situ formed all-amorphous poly (ethylene oxide)-based electrolytes enabling solid-state Zn electrochemistry. Chem. Eng. J. 2021, 417, 128096.
- Kumar, G. Electrochemical characterization of poly(vinylidenefluoride)-zinc triflate gel polymer electrolyte and its application in solid-state zinc batteries. Solid State Ion. 2003, 160, 289–300.
- Liu, J.; Khanam, Z.; Muchakayala, R.; Song, S. Fabrication and characterization of Zn-ion-conducting solid polymer electrolyte films based on PVdF-HFP/Zn (Tf) 2 complex system. J. Mater. Sci. Mater. Electron. 2020, 31, 6160–6173.
- Zhang, N.; Chen, X.; Yu, M.; Niu, Z.; Cheng, F.; Chen, J. Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev. 2020, 49, 4203–4219.
- Ma, L.; Chen, S.; Li, X.; Chen, A.; Dong, B.; Zhi, C. Liquid-Free All-Solid-State Zinc Batteries and Encapsulation-Free Flexible Batteries Enabled by In Situ Constructed Polymer Electrolyte. Angew. Chem. 2020, 132, 24044–24052.
- Liu, D.; Tang, Z.; Luo, L.; Yang, W.; Liu, Y.; Shen, Z.; Fan, X.-H. Self-healing solid polymer electrolyte with high ion conductivity and super stretchability for all-solid zinc-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 36320–36329.
- Brige, A.; Olsson, M.; Xiong, S.; Matic, A. A comparative study of hydroxyethylcellulose-based solid polymer electrolytes for solid state Zn batteries. Nano Sel. 2022, 4, 102–111.
- Huang, Y.; Liu, J.; Zhang, J.; Jin, S.; Jiang, Y.; Zhang, S.; Li, Z.; Zhi, C.; Du, G.; Zhou, H. Flexible quasi-solid-state zinc ion batteries enabled by highly conductive carrageenan bio-polymer electrolyte. RSC Adv. 2019, 9, 16313–16319.
- Huang, Y.; Zhang, J.; Liu, J.; Li, Z.; Jin, S.; Li, Z.; Zhang, S.; Zhou, H. Flexible and stable quasi-solid-state zinc ion battery with conductive guar gum electrolyte. Mater. Today Energy 2019, 14, 100349.
- Ye, H.; Xu, J.J. Zinc ion conducting polymer electrolytes based on oligomeric polyether/PVDF-HFP blends. J. Power Sources 2007, 165, 500–508.
- Rathika, R.; Padmaraj, O.; Suthanthiraraj, S.A. Electrical conductivity and dielectric relaxation behaviour of PEO/PVdF-based solid polymer blend electrolytes for zinc battery applications. Ionics 2017, 24, 243–255.
- Lu, G.; Qiu, H.; Du, X.; Sonigara, K.K.; Wang, J.; Zhang, Y.; Chen, Z.; Chen, L.; Ren, Y.; Zhao, Z.; et al. Heteroleptic Coordination Polymer Electrolytes Initiated by Lewis-Acidic Eutectics for Solid Zinc–Metal Batteries. Chem. Mater. 2022, 34, 8975–8986.
- Li, H.; Han, C.; Huang, Y.; Huang, Y.; Zhu, M.; Pei, Z.; Xue, Q.; Wang, Z.; Liu, Z.; Tang, Z.; et al. An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte. Energy Environ. Sci. 2018, 11, 941–951.
- Qiu, M.; Liu, H.; Tawiah, B.; Jia, H.; Fu, S. Zwitterionic triple-network hydrogel electrolyte for advanced flexible zinc ion batteries. Compos. Commun. 2021, 28, 100942.
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H.; Kita, R. Properties enhancement of carboxymethyl cellulose with thermo-responsive polymer as solid polymer electrolyte for zinc ion battery. Sci. Rep. 2020, 10, 12587.
- Zhou, J.; Zhang, R.; Xu, R.; Li, Y.; Tian, W.; Gao, M.; Wang, M.; Li, D.; Liang, X.; Xie, L.; et al. Super-Assembled Hierarchical Cellulose Aerogel-Gelatin Solid Electrolyte for Implantable and Biodegradable Zinc Ion Battery. Adv. Funct. Mater. 2022, 32, 2111406.
- Zhou, J.; Li, Y.; Xie, L.; Xu, R.; Zhang, R.; Gao, M.; Tian, W.; Li, D.; Qiao, L.; Wang, T.; et al. Humidity-sensitive, shape-controllable, and transient zinc-ion batteries based on plasticizing gelatin-silk protein electrolytes. Mater. Today Energy 2021, 21, 100712.
- Sownthari, K.; Suthanthiraraj, S.A. Preparation and properties of biodegradable polymer-layered silicate nanocomposite electrolytes for zinc based batteries. Electrochim. Acta 2015, 174, 885–892.
- Sai Prasanna, C.M.; Austin Suthanthiraraj, S. PVC/PEMA-based blended nanocomposite gel polymer electrolytes plasticized with room temperature ionic liquid and dispersed with nano-ZrO2 for zinc ion batteries. Polym. Compos. 2019, 40, 3402–3411.
- Chen, Z.; Li, X.; Wang, D.; Yang, Q.; Ma, L.; Huang, Z.; Liang, G.; Chen, A.; Guo, Y.; Dong, B.; et al. Grafted MXene/polymer electrolyte for high performance solid zinc batteries with enhanced shelf life at low/high temperatures. Energy Environ. Sci. 2021, 14, 3492–3501.
- Liu, C.; Tian, Y.; An, Y.; Yang, Q.; Xiong, S.; Feng, J.; Qian, Y. Robust and flexible polymer/MXene-derived two dimensional TiO2 hybrid gel electrolyte for dendrite-free solid-state zinc-ion batteries. Chem. Eng. J. 2022, 430, 132748.
More
