Patterning, stability, and dispersion of the semiconductor quantum dots (scQDs) are three issues strictly interconnected for successful device manufacturing. Recently, sSeveral authors adopted direct optical patterning (DOP) as a step forward in photolithography to position the scQDs in a selected area. However, the chemistry behind the stability, dispersion, and patterning has to be carefully integrated to obtain a functional commercial device.
- semiconductor quantum dots
- ligands
1. Introduction
1.2 The Semiconductor Quantum Dots
1.1 The Semiconductor Quantum Dots
1.3 The Quantum Size Effect and Its Role in the Modulation of the Electro-Optical Properties of the scQDs
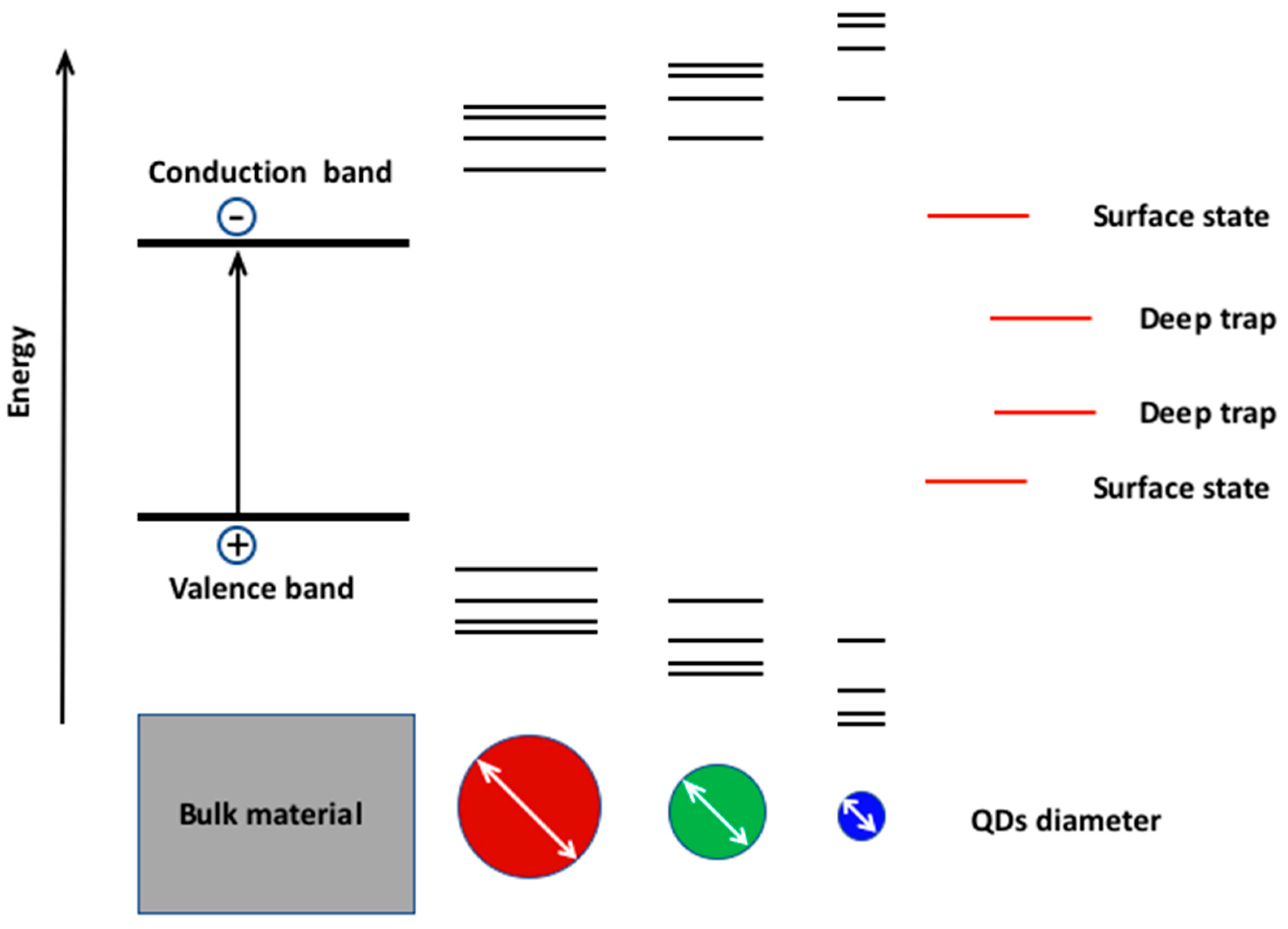
1.2. The Quantum Size Effect and Its Role in the Modulation of the Electro-Optical Properties of the scQDs
1.4 The Core@shell Systems
1.3. The Core@shell Systems
2. scQD Dispersion: tThe Ligands and the Surrounding Environment of the scQDs
3. QD Stability: tThe Effect of Oxygen and Moisture
Answering the question about the stability of the scQDs under ambient conditions in combination with light will help to adopt the necessary countermeasures to improve the life of any device equipped with this material.
Only recently, the group led by Peng clarified the role of oxygen[29] and water[30] by studying systematically their effect on a well-defined system, the CdSe/CdS core/shell scQDs, in defined experimental conditions in terms of atmosphere (only oxygen, only water, or their defined combination) and different phases at single scQD level or as an ensemble of scQDs in thin film and solution.
4. Stabilization of the QDs at Collective Level
The scQDs’ deterioration, i.e., the loss of the ligands and atoms from their structure, can be prevented by adopting two different strategies: (i) the encapsulation of each single scQD and (ii) the encapsulation of the scQDs embedding them within a matrix.
In the former, the main idea is to overcoat the single QD with a material that “freezes” its structure and hence its properties so that they can be incorporated into the final device. In the second approach, they are dispersed in a covalent network that does not protect the
single scQDs, but all of the scQDs.
4.1 The Thiol-Ene Network
4.1. The Thiol-Ene Network
A common way of collective encapsulation of the scQDs within a matrix is to use molecules forming a network through the thiol-ene chemistry [33][34].
4.2 The Polymers
4.2. The Polymers
The collective encapsulation with polymers was widely used [36]; however, the direct mixing of polymers and scQDs, even if it is relatively simple, suffers from the issue of scQD aggregation. When there is a chemical interaction between the scQDs and the polymer, the encapsulation strategy is more effective. Lesnyak’s group gave an interesting example of how these two obstacles, dispersion and stability, can be circumvented by using polymers [37].
4.3 The Siloxanes
4.3. The Siloxanes
The siloxane organic–inorganic hybrid materials [38] (SHMs) are compounds that have the tetravalent silicon bonded with oxygen and one or more bonds replaced by a covalent linkage with an organic substituent [39]. With this kind of chemistry, the siloxane polymers bear ceramic-like properties joined with the ones of the organic materials. From the functional point of view, this fact means that these materials display both the ceramic “character”, i.e., high-temperature stability, hardness, chemical resistance, and optical transparency, combined with the organic “character”, i.e., low temperature and solution processing, modulation of matrix porosity, and flexibility. Here will be described the capacity of siloxanes to act as a collective encapsulating agent with high chemical thermal and mechanical stability, as recently presented by Bae’s group for the stabilization of scQDs in different conditions [40][41].
5. Quantum Dots Direct Optical Patterning (DOP)
The research on the patterning technologies of scQDs is an active area of study for their industrial application due to the high interest of companies, especially in display manufacturing [36]. Photolithography is the most widely used technique in the industrial field; however, the use of photoresists and the multiple steps of etching/washing can alter dramatically the QDs’ functionality and the production costs, respectively. Recently, different authors published some works that utilize direct optical patterning (DOP) as a step forward in photolithography.
5.1. Direct Optical Patterning of scQDs with Thiol-Ene Cross-Linkers
In Section 4.2.1 the effect of the thiolene encapsulation on scQDs stability was already reported. However, the same chemical process can be adopted for the photolithographic patterning of perovskite scQDs as shown by Zhang et al. [43].
5.2 Direct Optical Patterning of scQDs with Siloxanes
5.2. Direct Optical Patterning of scQDs with Siloxanes
The possibility using the DOP combined with the siloxane chemistry to pattern and protect the scQDs has been readily demonstrated by Bae’s group for the manufacturing of quantum dots color filters (QD-CF) for displays [44].
5.3. Direct Optical Patterning via In Situ Ligand Exchange (DOLFIN)
The patterning strategy that uses the ligands of the scQDs as active molecules for the patterning itself was proposed by Talapin’s group [45][46] (direct optical lithography of functional inorganic nanomaterials DOLFIN).
5.4 Direct Photolithography of scQDs via Photo-Active Cross- Linkers
5.4. Direct Photolithography of scQDs via Photo-Active Cross- Linkers
Another DOP strategy in which the photoresist/matrix is absent is the one using the azide cross-linkers [47][48]. The approach is similar to the one explored with the polymers and siloxanes but uses only a cross-linker molecule while the matrix is absent. The role of the cross-linker activated by light is to create a network between the scQDs through their organic ligands.
5.5 Direct Photolithography of scQDs via Their Direct Synthesis
The direct synthesis of the scQDs using a laser within a film [51] can be considered belonging to the DOP strategies. The main technical difference with respect to the previous methodologies is that the laser was used to directly synthesize the scQDs. This technique, often called direct laser patterning, combines the flexibility of the laser technique both at the industrial and technological level with the chemistry and optical properties of the QDs.
6. Conclusions
Direct optical patterning (DOP) is an emerging tool to simplify the patterning process of the scQDs for display manufacturing that, however, is strictly related to the chemical processes that lead to the stability and homogeneity (dispersion) of the QDs for the correct function of the device. The optimization of the dispersion of the scQDs within a matrix is the golden rule to homogenize the interaction of the scQDs with the matrix itself. This means that the organic ligand at the QD surface should have the same chemical nature as the matrix. On the other side, stability is ensured by the formation of a close network of covalent bonds that cages the scQDs, preventing the loss of the ligands and surface atoms and, hence, preserving their optical properties. Considering these two main boundary conditions, five different approaches of DOP that exploit different chemical processes are evaluated.
References
- Kim, B.H.; Onses, M.S.; Lim, J.B.; Nam, S.; Oh, N.; Kim, H.; Yu, K.J.; Lee, J.W.; Kim, J.-H.; Kang, S.-K.; et al. High-Resolution Patterns of Quantum Dots Formed by Electrohydrodynamic Jet Printing for Light-Emitting Diodes. Nano Lett. 2015, 15, 969–973.
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541.
- Bayer, M. Bridging Two Worlds: Colloidal versus Epitaxial Quantum Dots. Ann. Phys. 2019, 531, 1900039.
- Wu, Y.; Jia, R.; Xu, J.; Song, L.; Liu, Y.; Zhang, Y.; Ullah, S.; Dai, J. Strategies of Improving CsPbX3 Perovskite Quantum Dots Optical Performance. Front. Mater. 2022, 9, 845977.
- Song, Z.; Zhao, J.; Liu, Q. Luminescent perovskites: Recent advances in theory and experiments. Inorg. Chem. Front. 2019, 6, 2969–3011.
- Haydous, F.; Gardner, J.M.; Cappel, U.B. The impact of ligands on the synthesis and application of metal halide perovskite nanocrystals. J. Mater. Chem. A 2021, 9, 23419–23443.
- Efros, A.L.; Brus, L.E. Nanocrystal Quantum Dots: From Discovery to Modern Development. ACS Nano 2021, 15, 6192–6210.
- Weidman, M.C.; Beck, M.E.; Hoffman, R.S.; Prins, F.; Tisdale, W.A. Monodisperse, Air-Stable PbS Nanocrystals via Precursor Stoichiometry Control. ACS Nano 2014, 8, 6363–6371.
- Mocatta, D.; Cohen, G.; Schattner, J.; Millo, O.; Rabani, E.; Banin, U. Heavily Doped Semiconductor Nanocrystal Quantum Dots. Science 2011, 332, 77–81.
- Todescato, F.; Fortunati, I.; Minotto, A.; Signorini, R.; Jasieniak, J.J.; Bozio, R. Engineering of Semiconductor Nanocrystals for Light Emitting Applications. Materials 2016, 9, 672.
- Reiss, P.; Protière, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168.
- Eagle, F.W.; Park, N.; Cash, M.; Cossairt, B.M. Surface Chemistry and Quantum Dot Luminescence: Shell Growth, Atomistic Modification, and Beyond. ACS Energy Lett. 2021, 6, 977–984.
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433.
- Cao, Z.; Shu, Y.; Qin, H.; Su, B.; Peng, X. Quantum Dots with Highly Efficient, Stable, and Multicolor Electrochemiluminescence. ACS Cent. Sci. 2020, 6, 1129–1137.
- Li, J.J.; Wang, Y.A.; Guo, W.; Keay, J.C.; Mishima, T.D.; Johnson, M.B.; Peng, X. Large-Scale Synthesis of Nearly Monodisperse CdSe/CdS Core/Shell Nanocrystals Using Air-Stable Reagents via Successive Ion Layer Adsorption and Reaction. J. Am. Chem. Soc. 2003, 125, 12567–12575.
- Bae, W.K.; Char, K.; Hur, H.; Lee, S. Single-Step Synthesis of Quantum Dots with Chemical Composition Gradients. Chem. Mater. 2008, 20, 531–539.
- Hanifi, D.A.; Bronstein, N.D.; Koscher, B.A.; Nett, Z.; Swabeck, J.K.; Takano, K.; Schwartzberg, A.M.; Maserati, L.; Vandewal, K.; van de Burgt, Y.; et al. Redefining near-unity luminescence in quantum dots with photothermal threshold quantum yield. Science 2019, 363, 1199–1202.
- Meinardi, F.; Colombo, A.; Velizhanin, K.A.; Simonutti, R.; Lorenzon, M.; Beverina, L.; Viswanatha, R.; Klimov, V.I.; Brovelli, S. Large-area luminescent solar concentrators based on ‘Stokes-shift-engineered’ nanocrystals in a mass-polymerized PMMA matrix. Nat. Photonics 2014, 8, 392–399.
- Selopal, G.S.; Abdelkarim, O.; Kumar, P.; Jin, L.; Liu, J.; Zhao, H.; Yurtsever, A.; Vidal, F.; Wang, Z.M.; Rosei, F. Role of Interfacial Engineering of “Giant” Core–Shell Quantum Dots. ACS Appl. Energy Mater. 2022, 5, 1447–1459.
- Carbone, L.; Nobile, C.; De Giorgi, M.; Sala, F.D.; Morello, G.; Pompa, P.; Hytch, M.; Snoeck, E.; Fiore, A.; Franchini, I.R.; et al. Synthesis and Micrometer-Scale Assembly of Colloidal CdSe/CdS Nanorods Prepared by a Seeded Growth Approach. Nano Lett. 2007, 7, 2942–2950.
- Srivastava, A.K.; Zhang, W.; Schneider, J.; Halpert, J.E.; Rogach, A.L. Luminescent Down-Conversion Semiconductor Quantum Dots and Aligned Quantum Rods for Liquid Crystal Displays. Adv. Sci. 2019, 6, 1901345.
- Diroll, B.T.; Guzelturk, B.; Po, H.; Dabard, C.; Fu, N.; Makke, L.; Lhuillier, E.; Ithurria, S. 2D II–VI Semiconductor Nanoplatelets: From Material Synthesis to Optoelectronic Integration. Chem. Rev. 2023, 123, 3543–3624.
- Onal, A.; Sadeghi, S.; Melikov, R.; Karatum, O.; Eren, G.O.; Nizamoglu, S. Quantum Dot to Nanorod Transition for Efficient White-Light-Emitting Diodes with Suppressed Absorption Losses. ACS Photonics 2022, 9, 3268–3278.
- Ehlert, S.; Stegelmeier, C.; Pirner, D.; Förster, S. A General Route to Optically Transparent Highly Filled Polymer Nanocomposites. Macromolecules 2015, 48, 5323-5327.
- Shiman, D.I.; Sayevich, V.; Meerbach, C.; Nikishau, P.A.; Vasilenko, I.V.; Gaponik, N.; Kostjuk, S.V.; Lesnyak, V. Robust Polymer Matrix Based on Isobutylene (Co)polymers for Efficient Encapsulation of Colloidal Semiconductor Nanocrystals. ACS Appl. Nano Mater. 2019, 2, 956-963.
- Reitinger, N.; Hohenau, A.; Köstler, S.; Krenn, J.R.; Leitner, A. Radiationless energy transfer in CdSe/ZnS quantum dot aggregates embedded in PMMA. Phys. Status Solidi A 2011, 208, 710-714.
- Xiao, P.; Zhang, Z.; Ge, J.; Deng, Y.; Chen, X.; Zhang, J.-R.; Deng, Z.; Kambe, Y.; Talapin, D.V.; Wang, Y.; et al. Surface passivation of intensely luminescent all-inorganic nanocrystals and their direct optical patterning. Nat. Commun. 2023, 14, 49.
- Kovalenko, M.V.; Scheele, M.; Talapin, D.V. Colloidal Nanocrystals with Molecular Metal Chalcogenide Surface Ligands. Science 2009, 324, 1417-1420.
- Hu, Z.; Liu, S.; Qin, H.; Zhou, J.; Peng, X. Oxygen Stabilizes Photoluminescence of CdSe/CdS Core/Shell Quantum Dots via Deionization. J. Am. Chem. Soc. 2020, 142, 4254-4264.
- Hu, Z.; Shu, Y.; Qin, H.; Hu, X.; Peng, X. Water Effects on Colloidal Semiconductor Nanocrystals: Correlation of Photophysics and Photochemistry. J. Am. Chem. Soc. 2021, 143, 18721-18732.
- Carrillo-Carrión, C.; Cárdenas, S.; Simonet, B.M.; Valcárcel, M. Quantum dots luminescence enhancement due to illumination with UV/Vis light. Chem. Commun. 2009, 35, 5214-5226.
- Hines, D.A.; Becker, M.A.; Kamat, P.V. Photoinduced Surface Oxidation and Its Effect on the Exciton Dynamics of CdSe Quantum Dots. J. Phys. Chem. C 2012, 116, 13452-13457.
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. . Polym. Chem. 2010, 1, 17-36.
- Kade, M.J.; Burke, D.J.; Hawker, C.J. The power of thiol-ene chemistry. J. Polym. Sci. Part Polym. Chem. 2010, 48, 743-750.
- Smith, M.J.; Malak, S.T.; Jung, J.; Yoon, Y.J.; Lin, C.H.; Kim, S.; Lee, K.M.; Ma, R.; White, T.J.; Bunning, T.J.; et al.et al. Robust, Uniform, and Highly Emissive Quantum Dot–Polymer Films and Patterns Using Thiol–Ene Chemistry. . ACS Appl. Mater. Interfaces 2017, 9, 17435-17448.
- Yu, M.; Saeed, M.H.; Zhang, S.; Wei, H.; Gao, Y.; Zou, C.; Zhang, L.; Yang, H. Luminescence Enhancement, Encapsulation, and Patterning of Quantum Dots Toward Display Applications. Adv. Funct. Mater. 2022, 32, 2109472.
- Design of cross-linked polyisobutylene matrix for efficient encapsulation of quantum dots. Design of cross-linked polyisobutylene matrix for efficient encapsulation of quantum dots. Nanoscale Adv. 2021, 3, 1443-1454.
- Lim, Y.-W.; Jin, J.; Bae, B.-S. Optically Transparent Multiscale Composite Films for Flexible and Wearable Electronics. . Adv. Mater. 2020, 32, 1907143.
- Ro, H.W.; Soles, C.L. Silsesquioxanes in nanoscale patterning applications.. Mater. Today 2011, 14, 20-33.
- Lee, H.E.; Lee, D.; Lee, T.-I.; Jang, J.; Jang, J.; Lim, Y.-W.; Shin, J.H.; Kang, S.-M.; Choi, G.-M.; Joe, D.J.; et al.et al. Siloxane Hybrid Material-Encapsulated Highly Robust Flexible LEDs for Biocompatible Lighting Applications. . ACS Appl. Mater. Interfaces 2022, 14, 28258-28269.
- Kuk, S.K.; Jang, J.; Han, H.J.; Lee, E.; Oh, H.; Kim, H.Y.; Jang, J.; Lee, K.T.; Lee, H.; Jung, Y.S.; et al.et al. Siloxane-Encapsulated Upconversion Nanoparticle Hybrid Composite with Highly Stable Photoluminescence against Heat and Moisture. . ACS Appl. Mater. Interfaces 2019, 11, 15952-15959.
- Kim, H.Y.; Yoon, D.-E.; Jang, J.; Lee, D.; Choi, G.-M.; Chang, J.H.; Lee, J.Y.; Lee, D.C.; Bae, B.-S. Quantum Dot/Siloxane Composite Film Exceptionally Stable against Oxidation under Heat and Moisture. . J. Am. Chem. Soc. 2016, 138, 16478-16485.
- Zhang, P.; Yang, G.; Li, F.; Shi, J.; Zhong, H. Direct in situ photolithography of perovskite quantum dots based on photocatalysis of lead bromide complexes.. Nat. Commun. 2022, 13, 6713.
- Kim, Y.H.; Koh, S.; Lee, H.; Kang, S.-M.; Lee, D.C.; Bae, B.-S. Photo-Patternable Quantum Dots/Siloxane Composite with Long-Term Stability for Quantum Dot Color Filters. . ACS Appl. Mater. Interfaces 2020, 12, 3961-3968.
- Wang, Y.; Fedin, I.; Zhang, H.; Talapin, D.V. Direct optical lithography of functional inorganic nanomaterials. Science 2017, 357, 385-388.
- Cho, H.; Pan, J.-A.; Wu, H.; Lan, X.; Coropceanu, I.; Wang, Y.; Cho, W.; Hill, E.A.; Anderson, J.S.; Talapin, D.V.; et al. Direct Optical Patterning of Quantum Dot Light-Emitting Diodes via In Situ Ligand Exchange. Adv. Mater. 2020, 32, 2003805.
- Hahm, D.; Lim, J.; Kim, H.; Shin, J.-W.; Hwang, S.; Rhee, S.; Chang, J.H.; Yang, J.; Lim, C.H.; Jo, H.; et al.et al. Direct patterning of colloidal quantum dots with adaptable dual-ligand surface. . Nat. Nanotechnol. 2022, 17, 952-958.
- Yang, J.; Lee, M.; Park, S.Y.; Park, M.; Kim, J.; Sitapure, N.; Hahm, D.; Rhee, S.; Lee, D.; Jo, H.; et al.et al. Nondestructive Photopatterning of Heavy-Metal-Free Quantum Dots.. Adv. Mater. 2022, 34, 2205504.
- Yang, J.; Hahm, D.; Kim, K.; Rhee, S.; Lee, M.; Kim, S.; Chang, J.H.; Park, H.W.; Lim, J.; Lee, M.; et al.et al. High-resolution patterning of colloidal quantum dots via non-destructive, light-driven ligand crosslinking. . Nat. Commun. 2020, 11, 2874.
- Liu, D.; Weng, K.; Lu, S.; Li, F.; Abudukeremu, H.; Zhang, L.; Yang, Y.; Hou, J.; Qiu, H.; Fu, Z.; et al.et al. Direct optical patterning of perovskite nanocrystals with ligand cross-linkers.. Sci. Adv. 2022, 8, eabm8433.
- Antolini, F.; Orazi, L. Quantum Dots Synthesis through Direct Laser Patterning: A Review. F. Front. Chem. 2019, 7, 752.
- Antolini, F.; Limosani, F.; Carcione, R. Direct Laser Patterning of CdTe QDs and Their Optical Properties Control through Laser Parameters. . Nanomaterials 2022, 12, 1551.
- Carcione, R.; Limosani, F.; Antolini, F. Cadmium Telluride Nanocomposite Films Formation from Thermal Decomposition of Cadmium Carboxylate Precursor and Their Photoluminescence Shift from Green to Red. . Crystals 2021, 11, 253.
- Lu, S.; Fu, Z.; Li, F.; Weng, K.; Zhou, L.; Zhang, L.; Yang, Y.; Qiu, H.; Liu, D.; Qing, W.; et al.et al. Beyond a Linker: The Role of Photochemistry of Crosslinkers in the Direct Optical Patterning of Colloidal Nanocrystals. . Angew. Chem. Int. Ed. 2022, 61, e202202633.
- Jang, J.; Yoon, D.-E.; Kang, S.-M.; Kim, Y.H.; Lee, I.; Lee, H.; Kim, Y.H.; Lee, D.C.; Bae, B.-S. Exceptionally stable quantum dot/siloxane hybrid encapsulation material for white light-emitting diodes with a wide color gamut. . Nanoscale 2019, 11, 14887-14895.
- Qiao, W.; Huang, W.; Liu, Y.; Li, X.; Chen, L.-S.; Tang, J.-X. Toward Scalable Flexible Nanomanufacturing for Photonic Structures and Devices. Adv. Mater. 2016, 28, 10353-10380.
