Antibiotics are among the most important discoveries of the 20th century, having saved millions of lives from infectious diseases. Microbes have developed acquired antimicrobial resistance (AMR) to many drugs due to high selection pressure from increasing use and misuse of antibiotics over the years. The transmission and acquisition of AMR occur primarily via a human–human interface both within and outside of healthcare facilities. A huge number of interdependent factors related to healthcare and agriculture govern the development of AMR through various drug-resistance mechanisms. The emergence and spread of AMR from the unrestricted use of antimicrobials in livestock feed has been a major contributing factor. The prevalence of antimicrobial-resistant bacteria has attained an incongruous level worldwide and threatens global public health as a silent pandemic, necessitating urgent intervention.
- antibiotics
- antimicrobial resistance
- mechanisms of resistance
- drivers of resistance
- measures to combat resistance
1. Introduction
2. Superbugs
Superbugs refer to germs that have shown resistance to antimicrobial agents used to treat them and include multidrug- or pan-drug-resistant bacteria and fungi. In reality, there is scarce or no treatment at all available for infections caused by superbugs. The term “ESKAPE” is the acronym for six highly drug-resistant bacteria (Enterobacterales, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter) and at present, carbapenem-resistant enterobacterales (CRE), carbapenem-resistant Klebsiella pneumoniae (CRKP), methicillin-resistant Staphylococcus aureus (MRSA), ESBL-producing enterobacterales, vancomycin-resistant Enterococcus (VRE), multidrug-resistant Pseudomonas aeruginosa, and multidrug-resistant Acinetobacter are among the topmost encountered superbugs worldwide. Multidrug-resistant bacteria have emerged only after long-continued and widespread use of antibiotics to treat infections caused by them. For example, M. tuberculosis has turned out as MDR-TB after decades of treatment with antitubercular drugs, now found as a major superbug prevalent in both underdeveloped and developing countries. Hospital-acquired or healthcare-associated infections (HAIs) caused by both gram-positive (e.g., Staphylococcus epidermidis, Clostridioides difficile, and Streptococcus pneumoniae) and gram-negative (e.g., Burkholderia cepacia, Stenotrophomonas maltophilia, Campylobacter jejuni, Citrobacter freundii, Enterobacter spp., Haemophilus influenzae, Proteus mirabilis, Salmonella spp., and Serratia spp.) bacteria are considered as superbugs because most of the available antibiotics have been proven ineffective to treat them [10][22]. Infections with superbugs enhance the rate of morbidity and mortality, as therapeutic options for these bacteria are seriously jeopardized and also there are high treatment costs and extended periods of hospital stay associated with these infections [11][23].3. Basis of Antibiotic Resistance
Antibiotic resistance is an evolutionary response of bacteria that develops against the challenge of therapeutic antibiotics. From a clinical perspective, all targeted pathogens remain susceptible to an antibiotic when it is first launched, but with sustained use, bacteria develop resistance to it. From an evolutionary perspective, bacteria adapt the action of antibiotics by either (1) chromosomal gene mutations, or (2) acquisition of foreign DNA through horizontal gene transfer (HGT) that codes for resistance determinants. Mutations principally involve three different types of genes, viz., genes encoding the targets of the antibiotic, transporters of the antibiotic, and regulators that repress the expression of transporters (e.g., antibiotic-modifying enzymes and multidrug efflux pumps) to give rise to antibiotic resistance. There is intriguing evidence to support the notion that commensal or environmental bacteria are the source of the antibiotic-resistance gene(s) that are transmitted to human pathogenic bacteria through HGT [12][24]. Bacteria exhibiting antibiotic resistance can have gene(s) from intrinsic, acquired, or adaptive sources [13][26]. Intrinsic resistance refers to bacteria’s inherent natural capacity to show resistance to certain classes of antibiotics due to the presence of their own chromosomal genes without mutation or gain of further genes. The implication of intrinsic resistance is that these bacteria will show inevitable resistance against certain antibiotics if used to treat infections by them. As far as the drug-resistance mechanisms are concerned, both efflux pumps and reduced permeability are involved in intrinsic resistance. It can also affect the multidrug efflux pumps frequently [14][15][27,28]. Acquired resistance is defined as an evolutionary process of exhibiting the resistance by a previously sensitive bacterium due to acquisition of chromosomal gene mutation or gaining an exogenous new genetic material via HGT. There are three main mechanisms for HGT, viz., transformation, transposition, and conjugation. The acquired resistance is most often transmitted through a plasmid acquired via conjugation, and it may be temporary or permanent [16][17][29,30]. Adaptive resistance is a phenotype that is conditional to environmental changes, and depending on the ability and duration of selection pressure, it may be interim or permanent. When bacterial growth is influenced by subinhibitory concentrations of antibiotics along with specific environmental signals such as growth factors, nutrition, stress, pH, concentrations of ions, etc., bacteria can develop adaptive resistance in both humans and livestock. As opposed to intrinsic and acquired resistance phenotypes, adaptive resistance is usually developed transiently and generally reverts back to the original state upon removal of the inducing signals. Although the exact biological processes involved in the evolution of adaptive resistance are not well understood, several factors including high mutation rates, gene amplification, efflux pumps, biofilm formation, epigenetic inheritance, population structure, and heterogeneity have been mentioned as possible explanations for its development [18][19][31,32].4. Sources and Routes of Transmission of AMR
The transmission and acquisition of AMR occur primarily via the human–human interface both within and outside of healthcare facilities. Humans, animals, water, and the environment are found to be reservoirs, and antimicrobial-resistance genes can be transmitted between and within these reservoirs. As far as the transmission routes are concerned, there is significant difference between bacterial species and resistance elements [20][33]. Transmission of antimicrobial-resistant bacteria is much facilitated by certain hotspot sources such as wastewater and sludge from urban wastewater treatment plants, and natural fertilizers such as pig slurry, cow manure, and fertilizer from poultry farming [21][34]. Animal feeds treated with antibiotics and their subsequent transfer to humans through consumption of these animals constitute the direct route of acquisition of antimicrobial resistance from animals [22][35]. Further, ingestion of fecal-contaminated food or water and direct contact between animals and humans constitute other common routes of transmission [23][36].5. Mechanisms of Drug Resistance
Antimicrobials and bacteria coexist in the same ecological niche, and bacteria develop defenses against the harmful effects of antibiotic molecules. There are four essential targets in a bacterial cell for antibiotics (e.g., cell wall, cell membrane, protein synthesis, and nucleic acid synthesis). Primary mechanisms for antimicrobial resistance include limiting drug uptake, altering a drug target, inactivating a drug, and increasing active drug efflux (Figure 12).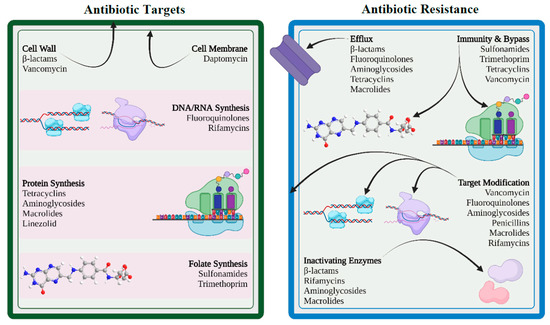
5.1. Limiting Drug Uptake
5.2. Modification of Targets for Drug
Bacteria can modify the targets required for drug binding so that the drug cannot bind or binds poorly to the modified target. This modification results from spontaneous mutations of the gene or genes that encode the protein that acts as the drug target. For instance, when mutations impact the quinolone-resistance-determining region (QRDR) in the DNA gyrase (topoisomerase II and topoisomerase IV), fluoroquinolone resistance develops in both gram-positive and gram-negative bacteria [26][43]. Another way of target modification is methylation, which is considered to be a very efficient method in developing resistance. Examples of methylation include erm methylases against macrolides, lincosamides, and streptogramin B antibiotics in both gram-positive and gram-negative bacteria.5.3. Inactivation of Drug
Drug resistance may result from the inactivation of antibiotics by certain bacterial species that follows in one of two ways: either the antibiotic is really degraded, or a chemical group is transferred to the antibiotic. The hydrolyzing enzymes known as β-lactamases, produced by members of the enterobacterales family, are particularly effective at inactivating β-lactam antibiotics. The β-lactamases originally known as penicillinases and cephalosporinases inactivate the β-lactam ring structure by opening at a specific point rendering it ineffective to bind with the target, called penicillin-binding proteins. Several members of the enterobacterales family as well as many species of gram-positive bacteria such as Staphylococcus aureus, Enterococcus faecalis, and Enterococcus faecium, are known to harbor β-lactamase genes that are transmitted by HGT.5.4. Efflux of Drug
By using an energy-dependent efflux pump located on the cytoplasmic membrane, bacteria are able to control the accumulation of antibacterial chemicals, including antibiotics, inside bacterial cells. By expelling harmful compounds such as antibiotics, metabolites, and quorum-sensing signal molecules from the cell, efflux pumps enable bacteria to control their internal environment. In 1980, researchers described the first plasmid-encoded efflux pump in Escherichia coli, which pushed tetracycline out of the bacterial cell. Since then, numerous gram-positive and gram-negative resistant bacteria with diverse efflux mechanisms have been found. It is interesting to note that the majority of efflux systems engage in multidrug efflux mechanisms that are always chromosomally encoded to ensure bacterial intrinsic drug resistance [27][48].6. Drivers to AMR
6.1. Misuse and Overuse of Antibiotics
Although the process of development of antibiotic resistance occurs as a natural phenomenon, it has been accelerated by the misuse of antibiotics in both humans and animals over the years. There is a causal relationship between overuse and development of microbial resistance to antibiotics as revealed by epidemiological studies [28][52]. Despite being warned repeatedly by health organizations, unfortunately, misuse and overuse of antibiotics continue at a disproportionate level worldwide, and the current scenario seems to be at the point of no return. Surveys have revealed that people across the globe, especially noneducated sections, do have misconceptions and beliefs about antibiotics, for example, that they help to recover from most common viral diseases, such as the common cold or flu. Moreover, it has been observed that antibiotics are a frequently prescribed medicine for patient management, particularly observed in many developing countries where there is lack of adequate diagnostic facilities [29][53]. Administering antibiotics without a clear indication is a good example of common misuse. The emergence and spread of drug-resistant pathogens are facilitated further when antibiotics can be bought for human as well as animal use as over-the-counter (OTC) drugs. Antibiotic abuse is also contributed to by lack of antibiotic policy and standard treatment guidelines, frequently seen in developing countries.6.2. Increase in Gross Domestic Product (GDP)
The significant rise in antibiotic use globally is predominantly accredited to the rise in the GDP especially in many developing countries. With the rise in GDP, there has been substantial improvement in the quality of life of people from low- and middle-income countries (LMICs) that positively correlates with increased antibiotic consumption. It is estimated that between 2000 and 2015, global antibiotic use has been elevated by 65%, according to Klein et al. [30][55].6.3. Inappropriate Prescribing Patterns
Inappropriately prescribed antibiotics contribute significantly to promoting AMR [31][57]. Inappropriate antibiotic prescribing refers to prescription of antibiotics where it is not necessary or selection of inappropriate antibiotics or the wrong dose and duration of an antibiotic [32][58]. It has been shown in a study that at least one antibiotic was received by 50% of patients without compelling reasons during their stay at the hospital. The introduction of antibiotics should ideally be guided by prior isolation and antimicrobial susceptibility testing of bacteria, but according to a CDC (Centers for Disease Control and Prevention) report from 2017, antibiotic prescriptions were made for about one-third of hospital patients without adequate testing and continued for longer durations [33][59].6.4. Paucity in Futuristic Antibiotics
The looming problem of antibiotic resistance demands urgent response by the pharma companies with new novel antibiotics [34][61]. Unfortunately, there is a dearth of development in new antibiotics, despite having repeated calls from the WHO. Surprisingly, only 8 out of the 51 newly developed antibiotics can be catalogued as innovative drugs to treat infections caused by antibiotic-resistant bacteria; the overwhelming majority are just reformations of previous drugs. As a consequence, it is speculated that these new drugs are likely to show resistance shortly. The current scenario states that management of drug-resistant TB, urinary tract infections, pneumonia, and other gram-negative infections has been seriously jeopardized because of lack of available treatment options. The paucity of new drugs has made patients of extreme of age much more vulnerable to life-threatening infections [35][62].6.5. Agricultural Use of Antibiotics
Use of antibiotics in livestock farming has been markedly increased in most developing countries for various purposes, including increased demand of animal protein in recent years. In turn, it is contributing to AMR due to the presence of antibiotic residues in animal-derived products (e.g., muscles, kidney, liver, fat, milk, and egg). Antibiotics are being used randomly for various purposes including treating animal diseases, preparation of animal feed for growth promotion, improved feed conversion efficiency, as well as for disease prevention [36][63].6.6. Easy Travel Routes
There is growing evidence that the emergence and global spread of antibiotic-resistant bacteria are much facilitated by human movement. Dissemination of AMR across the globe is contributed to significantly by easy and modern travelling routes available not only for humans but also for animals and goods [37][66]. Human travellers are highly likely to return to their own countries, unknowingly with colonization or infection by antimicrobial-resistant organisms, from the countries they have visited. It has been shown that antimicrobial-resistant bacteria may persist for up to 12 months carried in the body after a person has travelled to highly endemic AMR regions, amplifying the risk of transmission among susceptible populations [38][67].6.7. Knowledge Gap
There is substantial evidence that both healthcare workers (HCWs) and members of the public have knowledge gaps about appropriate use of antibiotics and mechanisms of antibiotic resistance [39][68]. Surveillance is a prerequisite to estimate the magnitude of the AMR burden and to establish any intervention strategies, such as antimicrobial stewardship. Unfortunately, actual statistical data regarding the use of antibiotics and the status of AMR in both healthcare and agriculture sectors have yet to be gathered worldwide [40][69].7. Clinical Implications of AMR
There are many clinical implications of AMR, and following are some of the major concerns [41][70]:-
Successful treatment of microbial infections including bacterial, fungal, and viral infections is hindered by antimicrobial resistance.
-
Emergence and dissemination of new resistant mechanisms threaten the scope of treatment for many common illnesses such as urinary tract infections, upper respiratory tract infections, typhoid, and flu, resulting in treatment failure, permanent disability, or even death.
-
The success of cancer chemotherapy, transplantation surgery, and even minor dental procedures will be seriously jeopardized by virtue of AMR unless novel drugs are available.
-
AMR infections impose mandatory prolonged treatment with higher healthcare costs and may require expensive alternate drugs.
8. How to Combat AMR
8.1. International Measures
9.1. International Measures
The following are international measures that can be taken:-
Establishing and strengthening collaboration among international agencies, governments, nongovernmental organizations, and professional groups.
-
Establishing surveillance networks for antimicrobial use and AMR globally.
-
Building laboratory capacity for the detection and reporting of pathogens with AMR that have global health impacts.
-
Establishing and strengthening international tracking systems for quick identification and mitigation of emerging pathogens.
-
International monitoring to control counterfeit antimicrobials across the globe.
-
Investing in research, new drug discovery, and vaccines.
8.2. National Strategies
9.2. National Strategies
The following are national measures that can be taken:-
Implementing an “Antibiotic policy” for judicious use in healthcare and agricultural settings.
-
Strengthening of national surveillance, monitoring, and evaluation efforts by integration of public health and veterinary sectors.
-
Developing innovative point-of-care diagnostic tests for pathogen identification and resistance monitoring.
-
Investing in basic and applied research on new antibiotics and vaccines.
-
Building capacity and strengthening international collaboration to combat AMR.
-
Adopting antimicrobial stewardship in healthcare settings with essential drug list.
8.3. Rational Use of Antibiotics
9.3. Rational Use of Antibiotics
“Rational use of medicine” has been defined by the WHO as using correct medications including antibiotics appropriate for clinical needs of patients, in exact doses of individual needs, for an adequate period of time, and at the lowest cost [41][70]. The optimal results of treating infections can only be achieved when the selection of pathogens, drug toxicity, and development of resistance are minimized through the rational use of antibiotics. Antibiotic stewardship programs (ASPs) in healthcare settings are primarily aimed at maintaining the rational use of antibiotics.8.4. Ban on Over-the-Counter (OTC) Antibiotics
9.4. Ban on Over-the-Counter (OTC) Antibiotics
Stringent regulatory control should be imposed on OTC selling and dispensing of oral and injectable antibiotics, which is unfortunately still a common practice in many underdeveloped and developing countries. Antibiotics should only be dispensed to serve the prescription from a qualified physician. Continuous awareness programs on the use of antibiotics and AMR among patients and pharmacy drug dispensers is strongly recommended, along with reappraisal of existing antibiotic policies based on local and regional AMR surveillance data [42][74].8.5. Infection Prevention and Control (IPC)
9.5. Infection Prevention and Control (IPC)
Recommended measures related to IPC in a healthcare facility include:-
Formation of “infection prevention and control committee”.
-
Practices of good hand hygiene.
-
Proper diagnosis and successful treatment of infection.
-
Responsible use of antimicrobial agents.
-
Continuous surveillance and monitoring of antibiotic use and antibiotic resistance.
-
Establishing quality antimicrobials supply chain.
-
Good microbiological laboratory practices.
