Patterning, stability, and dispersion of the semiconductor quantum dots (scQDs) are three issues strictly interconnected for successful device manufacturing. SRecently, several authors adopted direct optical patterning (DOP) as a step forward in photolithography to position the scQDs in a selected area. However, the chemistry behind the stability, dispersion, and patterning has to be carefully integrated to obtain a functional commercial device.
- semiconductor quantum dots
- ligands
1. Introduction
1.1 The Semiconductor Quantum Dots
1.2 The Semiconductor Quantum Dots
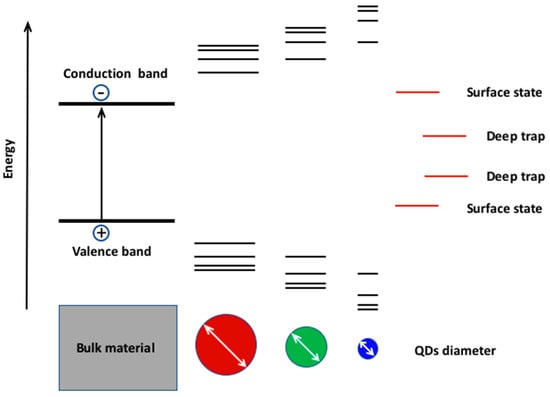
1.2. The Quantum Size Effect and Its Role in the Modulation of the Electro-Optical Properties of the scQDs
1.3 The Quantum Size Effect and Its Role in the Modulation of the Electro-Optical Properties of the scQDs
1.3. The Core@shell Systems
1.4 The Core@shell Systems
2. scQD Dispersion: Tthe Ligands and the Surrounding Environment of the scQDs
The tests of dispersion and stability which were carried out, comparing the spreading of the NPLs in three types of polymers (namely: the poly(lauryl methacrylate) (PLMA), the PIB, and the PIB block copolymer (SIBS)) showed the formation of very high transparent films[28], which indicates an optimal dispersion.
Despite a huge amount of work conducted on the study of the organic ligands, it is worth describing also the use of the inorganic ligands. Indeed, they can replace the organic ligands to improve the charge transport between the scQDs[27][28].
Talapin’s group recently showed how the native organic ligand, typically the oleic acid, can be replaced by metal-inorganic salts[27] to obtain intensely luminescent all-inorganic nanocrystals (ILANs). The metal-inorganic salts they used for surface passivation of the scQDs include the metal cations of Cd2+, Zn2+, Pb2+, and In3+ with anions like NO3−, BF4− e triftalate (OTf−).
The role of the metal cations is (i) to remove the native organic ligands, and (ii) to bind the non-metal atom, on the scQD surface. In terms of the Lewis acid-base concept, the ligand metal cation, a Lewis acid, coordinates the electron-rich chalcogenide atom, a Lewis base, at the scQD surface. On the other side, the anion acts as a charge balancer rather than a coordinating agent. The main effect of this ligand exchange is the variation of the scQDs’ solubility (dispersion) of the scQDs in solvents. Indeed, the solubility of the scQDs in non-polar solvents (hexane, toluene, etc.) switches to solubility in polar solvents (DMF, NMF, DMSO, etc.).
3. QD Stability: Tthe Effect of Oxygen and Moisture
Answering the question about the stability of the scQDs under ambient conditions in combination with light will help to adopt the necessary countermeasures to improve the life of any device equipped with this material.
Only recently, the group led by Peng clarified the role of oxygen[29] and water[30] by studying systematically their effect on a well-defined system, the CdSe/CdS core/shell scQDs, in defined experimental conditions in terms of atmosphere (only oxygen, only water, or their defined combination) and different phases at single scQD level or as an ensemble of scQDs in thin film and solution.
The study of the role of oxygen[29] shows that, at the functional level, this molecule maintains the bright state both of the single scQDs and when the scQDs are embedded together in a film (photoactivation). When the oxygen is removed, for example with argon, the scQDs enter a dim state (low emission and small PL shift). The proposed mechanism is that during the photoexcitation, there is the possibility that the scQDs form a trion (two electrons and one hole in the scQDs) bringing the scQDs in the dim charged state (off state). The “bright” state is restored with the presence of oxygen that accept one electron forming the superoxide radical (•O2−). The oxygen reduction returns the scQDs to charge neutrality restoring the scQDs’ optical properties. Another interesting conclusion of this work is that the high quality of the shell avoiding any hole and electron surface traps does not allow any effect of corrosion of the scQDs by the oxygen (the redox potential of the oxygen is quite different from the core/shell scQDs). On the other side, the redox potential of the oxygen should be able to oxidize the surface of bare CdSe scQDs (no core/shell scQDs), especially under photoexcitation producing CdO, SeO2, and CdSeOx [31][32]. The authors conclude that pure oxygen helps to maintain the photophysical properties, and it is not responsible for photo-corrosion of high-quality core/shell nanocrystals. The controversial results found in the literature on the role of oxygen may be due to the non-ideal quality of the prepared core/shell scQDs allowing the corrosion, as reported for the bare CdSe QDs.
Peng’s group studied also the role of water combined with oxygen, showing that this combination is responsible for the corrosion and the loss of the photophysical properties of the scQDs[30]. The complete “story” of the water and oxygen interaction with the scQDs starts with the “ionization by water and deionization by oxygen” step, as reported in Figure 5. In this first step, the excited nanocrystal is negatively ionized (reduced) by water that dissociates, producing a very reactive specie, the hydroxyl radical (•OH) and protons (H+). The negatively charged scQDs are now in the dim state, but the presence of the oxygen, as shown before, brings the scQDs to a neutral state, restoring its bright state and producing as a byproduct the superoxide ion (equilibrium between neutral/bright state and charged/dim state; Figure 5). The presence of the radical species, especially the hydroxyl radical (•OH) formed under the continuous presence of water and irradiation, brings to an acidic pH and a carboxylate ligand detachment from the scQDs’ surfaces. This phenomenon causes the poor solubility of the QDs in the solvent and the precipitation of the bright scQDs (precipitated/bright state, ligand-destructed/bright state; Figure 5). The loss of the surface ligand exposes the inorganic shell to the formation of surface traps bringing further chemical decomposition even of the shell of the scQDs (photo-corrosion)
with irreversible loss of their photophysical properties (etched/bleached state; Figure 5).
It is worth mentioning that when the scQDs are confined in an area with no access to water and oxygen, like in the display applications (the QD’s film is isolated from the environment), the balance between the brightening and dimming states reaches an equilibrium and the decomposition cannot go ahead.
4. Stabilization of the QDs at Collective Level
The scQDs’ deterioration, i.e., the loss of the ligands and atoms from their structure, can be prevented by adopting two different strategies: (i) the encapsulation of each single scQD and (ii) the encapsulation of the scQDs embedding them within a matrix.
In the former, the main idea is to overcoat the single QD with a material that “freezes” its structure and hence its properties so that they can be incorporated into the final device. In the second approach, they are dispersed in a covalent network that does not protect the
single scQDs, but all of the scQDs.
4.1. The Thiol-Ene Network
4.1 The Thiol-Ene Network
A common way of collective encapsulation of the scQDs within a matrix is to use molecules forming a network through the thiol-ene chemistry [33][34]. The term thiol-ene refers to a reaction where a thiol molecule is added to a carbon–carbon double bond (“ene” bond), regardless of the reaction mechanism, that can be either radical or nucleophilic. In the following pages, the radical mechanism will be described because it is stimulated by light (Figure 11). The initiation involves the activation of the thiol with a photo-initiator (Pi) through light. The formed thiyl radical (R-S.) reacts with a carbon–carbon double bond that then activates a second molecule of thiol, propagating the reaction. A possible termination process is the radical–radical coupling process. This reaction is simple to perform, is not sensitive to water and oxygen, and the reagents are readily available. The addition of the thiol to the carbon–carbon double bond is quite general in the sense that the olefinic bond can be substitutedor not. In the same way, virtually, any type of thiol can be used for the addition. The last important property is that this reaction is quite rapid and complete in a few seconds, and there is no need to remove any byproduct. With the help of this chemical process, several papers have been published involving
the encapsulation of the scQDs. Smith et al.[35] use a triene (1,3,5,-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione) (acronym
TAIC), a three-branched allyl molecule, and a four branched thiol (pentaerythritol tetrakis(3-mercaptopropionate) (acronymPTMP) to encapsulate theQDs in amatrix ofwell-distributed scQDs. The thiol-ene reaction between the TAIC and PTMP allows the formation of a network encapsulating the scQDs (Figure 12).
Unfortunately, the mixture of the triene and the four-branched thiol includes also a urethane-based component, so it is impossible to speculate more about the chemical properties of the system. The optical properties of the scQDs are maintained because the scQDs were not subjected to further processing, like ligand exchange or other treatments, while the use of buthylamine as a ligand of the scQDs avoids any interference of the oleic acid double bond with the thiol-ene reaction [76].
4.2. The Polymers
4.2 The Polymers
The collective encapsulation with polymers was widely used [36]; however, the direct mixing of polymers and scQDs, even if it is relatively simple, suffers from the issue of scQD aggregation. When there is a chemical interaction between the scQDs and the polymer, the encapsulation strategy is more effective. Lesnyak’s group gave an interesting example of how these two obstacles, dispersion and stability, can be circumvented by using polymers [37]. The stabilization of the QDs was realized first by selecting a polymer, the polyisobuthylene (PIB), having specific properties like good flexibility, excellent oxygen and moisture barrier, thermal stability, optical transparency, and solvent resistance. Then, they modified the PIB by adding the cross-linking functions, the methacrylate (MA), which induce the formation of a covalent network around the scQDs for their effective encapsulation. In particular, the PIB-MA reticulating polymer is obtained by linking the PIB to a “tri-arm star”
aromatic center. Then, the PIB ends were functionalized with the methacrylate functions (Figure 14a,b) forming a three-arm star cross-linkable polymer (PIB-MA).
On the other side, the optimization of the scQDs’ dispersion is obtained through the exchange of the native scQDs’ ligand with a new ligand having as a spacer/tail the PIB oligomers. The ligand tail ensures the optimal interaction of the scQDs with the matrix, because they have the same chemical structure (Figure 14b,c), while the surface binding group, a thiol, ensures the strong binding of the ligand on the scQD surface during the ligand exchange procedure.
The cross-linking of the methacrylate units of the PIB completes the protective action of the polymer around the scQDs (Figure 14d,e). The optical characterization of the cross-linked film showed that the dispersion of the QDs was comparable with the one of similar polymeric films bearing the PIB without
any crosslinking. Indeed, the photoluminescence emission and the PLQY are almost equivalent between these two types of samples. This effect suggests that the scQDs do not form any aggregates that quench the photoluminescence[28] and decreases the film transparency[26].
4.3. The Siloxanes
4.3 The Siloxanes
The siloxane organic–inorganic hybrid materials [38] (SHMs) are compounds that have the tetravalent silicon bonded with oxygen and one or more bonds replaced by a covalent linkage with an organic substituent [39]. With this kind of chemistry, the siloxane polymers bear ceramic-like properties joined with the ones of the organic materials. From the functional point of view, this fact means that these materials display both the ceramic “character”, i.e., high-temperature stability, hardness, chemical resistance, and optical transparency, combined with the organic “character”, i.e., low temperature and solution processing, modulation of matrix porosity, and flexibility. Here will be described the capacity of siloxanes to act as a collective encapsulating agent with high chemical thermal and mechanical stability, as recently presented by Bae’s group for the stabilization of scQDs in different conditions [40][41]. In the approach proposed by Bae’s group, the siloxane encapsulation method of scQDs consists of two steps (Figure 15): (i) the siloxane resin formation through the sol-gel method and (ii) the cross-linking of the resin with the QDs and with itself. In the first step is prepared the siloxane resin bearing the acrylate group by sol-gel condensation (Figure 15a). In the second step, the formed siloxane resin is mixed with the scQDs. The scQDs are usually passivated with oleic acid as a ligand that bears a double bond in its aliphatic chain. The acrylate group can react both with the double bond of the scQDs’ ligand (the oleic acid) or with another acrylate moiety of the resin enhancing the resin networking (Figure 15b). The binding of the acrylate group with the double bond of the scQDs’ ligand or with another acrylate moiety is mediated by UV light after the addition of a photo-initiator like 2,2-dimethoxy-2-phenylacetophenone (DMPA). It is important to point out that the siloxane resin bearing the phenyl group of the diphenylsilanediol (DPSD) helps the enhanced dispersion of the scQDs in the resin through hydrophobic interactions[42]. In these conditions, the stability of the photon siloxane encapsulated scQDs (PSE- QDs) under thermal stress at 85 ◦C for 40 days and 85% relative humidity showed that normalized PL intensity remains constant during all of the period, as well as the PL lifetime. On the contrary, the scQDs simply mixed with the resin without cross-linking lost their photophysical properties, showing a 50% decrease in PL intensity and a strong decrease in the photoluminescent lifetime, with respect to the initial values.
5. Quantum Dots Direct Optical Patterning (DOP)
The research on the patterning technologies of scQDs is an active area of study for their industrial application due to the high interest of companies, especially in display man- ufacturing [36]. Photolithography is the most widely used technique in the industrial field; however, the use of photoresists and the multiple steps of etching/washing can alter dramatically the QDs’ functionality and the production costs, respectively. Recently, different authors published some works that utilize direct optical patterning (DOP) as a step forward in photolithography. Indeed, the use of the light for direct patterning associated with smarter chemical production of the materials can ensure stability, dispersion, and a relatively simple patterning process of scQDs. Another advantage of DOP is that it can utilize the same equipment used for the photolithography so that only minor changes would be necessary in the production chain, minimizing the upgrade costs. In the following paragraphs are presented five different chemical approaches that use the same patterning methodology, the DOP, but different chemical processes to tackle the issues of stability, dispersion, and patterning itself. The purpose of this comparison is to produce a starting point for evaluating the pros and cons of the various proposed techniques.
5.1. Direct Optical Patterning of scQDs with Thiol-Ene Cross-Linkers
In Section 4.2.1 the effect of the thiolene encapsulation on scQDs stability was already reported. However, the same chemical process can be adopted for the photolithographic patterning of perovskite scQDs as shown by Zhang et al. [43].
The main novelty of this work is that the synthesis of the perovskite scQDs is realized after the thiol-ene network formation. Indeed, first, the thiol-ene matrix is formed, and then the perovskite nanocrystals are grown through the annealing of specific perovskite precursors trapped in the matrix. In particular, the process of formation can be described as four steps: (i) all the precursors for the formation of the encapsulating network (TAIC and TTMP) and the of the perovskite are dissolved together by using N,N-dimethylformamide (DMF) and dimethylsulfoxide (DMSO) solvents; (ii) this ink is then used for film deposition, and patterning with UV light that “freezes” the perovskite precursors in the patterned positions; (iii) the un-patterned areas are removed by washing them; (iv) the perovskite nanocrystals are then grown with an annealing process.
The effect of this strategy is that the final perovskite scQDs film is homogeneous because the precursors are all soluble in the prepared solution; hence, the patterned areas are also homogeneous as luminescence emission. According to the authors, the growth of the perovskite after the UV patterning avoids any perovskite damage due to UV treatment. The patterning resolution tests show the possibility reaching a limit of 5 µm, depending upon the degree of resolution of the mask.
5.2. Direct Optical Patterning of scQDs with Siloxanes
5.2 Direct Optical Patterning of scQDs with Siloxanes
The possibility using the DOP combined with the siloxane chemistry to pattern and protect the scQDs has been readily demonstrated by Bae’s group for the manufacturing of quantum dots color filters (QD-CF) for displays [44]. In this work, the authors solved three main issues encountered when building color filters, namely: (i) dispersion of high loading of QDs (>20 wt %), (ii) stability against heat and moisture; and (iii) photo-patternability. The high loading of QDs poses a problem for scQDs’ dispersion within the siloxane matrix that was bypassed by exchanging the native scQDs’ ligand, the oleic acid, with a mercaptopropyl-methyl-dimethoxy silane (MPMDMS) (Figure 19a). The tail of this ligand bears the dimethoxy groups that can react with the hydroxyl groups of diphenylsilanediol (DSPD) during the siloxane resin formation (sol-gel condensation) (Figure 19b). The pres- ence of diphenyl rings performs a dual function, i.e., (i) it ensures a good scQD dispersion, preventing further aggregation during the next steps of the patterning; and (ii) it ensures also a good optical transparency of the final film. The resin bears another important function, through its methacrylate group, introduced by the 3-methacryloxypropyltrimethoxysilane (MPTMS). This group is, indeed, necessary for the binding with the thiol cross-linker that allows the encapsulation/patterning. The siloxane solution (ink) for the encapsulation/patterning was prepared by adding to the siloxane resin a four-branch thiol (PE1) that deals as a reticulating agent coupling with the methacrylate group of the siloxane resin (Figure 19b). The photo-patterning (Figure 19c) was performed by pouring the siloxane ink (with red scQDs) into a pre-patterned array (formed by 50 µm 50 µm squares). The reaction of the thiol PE1 with the acrylate functional group is a typical process of the thiol-ene chemistry and is activated by light. The same procedure is, then, repeated by using thegreen scQDs.
5.3. Direct Optical Patterning via In Situ Ligand Exchange (DOLFIN)
It is important to highlight the high loading of QDs used for the experiments was10 wt % for red scQDs and 20 wt % for green QDs, and with this loading the film producedis homogenous. The stability tests were carried out in conditions of high temperature and humidity, namely at 85 C and 5% and 85% of moisture, in comparison with a film realized with a standard photoresist. In these conditions, the PLQY and the time decay were stable as the initial value for the QDs encapsulated with the siloxane, while the stability of the scQDs embedded in the standard photoresist drops down after one day and it is almost 0 after 30 days. This fact further confirms that the siloxane encapsulation is effective and ensures also an optimal dispersion. The chemical stability was also verified in ethanol and strong acid and base for both encapsulants, i.e., siloxane and photoresist, for 30 days. The test was successfully surpassed only by the siloxane encapsulant in which the PLQY of the scQDs remains stable as the initial value.
5.3. Direct Optical Patterning via In Situ Ligand Exchange (DOLFIN)
The patterning strategy that uses the ligands of the scQDs as active molecules for the patterning itself was proposed by Talapin’s group [45][46] (direct optical lithography of functional inorganic nanomaterials DOLFIN). This is the first example of patterning in which the matrix is absent while the photoresist is a constant of the photolithography. This approach is particularly elegant because it forecasts the exchange of the scQDs’ ligand “in situ”. The effect of the ligand exchange is the precipitation (change in solubility) of the patterned QDs. The key molecules of this process are the so-called photoacid generators (PAGs) that have to be combined with the scQDs in special ink. Figure 20 illustrates the PAGs’ action. The 2-(4-methoxystyryl)-4,6-bis(trichloromethyl)-1,3,5-triazine (MBT), for example, after UV exposure produces chlorine radicals that in presence of solvents (toluene, acetonitrile) induce the formation of HCl that protonates and detaches the oleic acid from the scQD surface. In these conditions the scQDs become insoluble and precipitate (Figure 20a).
5.4. Direct Photolithography of scQDs via Photo-Active Cross- Linkers
Similarly, the 2-diazo-1-naphthol-4-sulfonic acid (DNS) after the UV exposure pro- duces a sulfonated derivative of the diazonaphtoquinone (ICA) that substitutes the oleic acid at the scQDs surface, which becomes insoluble (Figure 20b). The complete path of patterning can be resumed in four steps as reported in the following. The PAGs molecules and the scQDs are mixed forming the so-called photo-patternable emissive nanocrystals (PEN) ink, the ink is deposited over the substrate forming a film, the film is then irradiated, and the film is developed using a non-polar solvent that washes away the unexposed soluble scQDs. The selection of the PAG molecules is particularly important because they have to be soluble in the preparation. The patterning methodology has been tested in terms of resolution to reach up to 1.5 µm.
Talapin’s group tested also the effect of this patterning methodology over a QD-LED, showing that the electro-optical characteristics of the device are almost identical to a similar device realized with pristine scQDs. Recently, Talapin’s group also demonstrated the same approach, the change in the QDs’ solubility through the in situ modification of the ligands, by using intensely luminescent all-inorganic nanocrystals (ILANs) [12] for the direct optical patterning. In this work, the ILANs were combined with a molecule that, after irradiation, produces a type L ligand that complexes the surface of the ILANs themselves, changing their solubility. In particular, the ILANs were combined with a PAmG-BTA molecule and then deposited. The PAmG-BTA has the property that, decomposes after UV exposure, giving the n-butylamine (BTA) (Figure 21a). The butylamine is an L-type ligand (two-electron donor) that form complexes with the exposed metal sites at the scQDs surface. This change in ligand switches the solubility of the scQDs in polar solvent so it is possible to obtain patterned areas (Figure 21b). The resolution of the patterning arrives up to 2 µm, and the thickness of the layer can be modulated by controlling the spin coating parameters and the density of the solutions. The limitation of this work is the decrease in the PLQY always associated with the inorganic ligand exchange; indeed, the PLQY of the red scads decreases from 80% (un-patterned) to 75% (patterned), from 76% (un-patterned) to 68% (patterned) for green scQDs, and from 78% (un-patterned) to 58% (patterned) for the blue-emitting scQDs.
5.4 Direct Photolithography of scQDs via Photo-Active Cross- Linkers
Another DOP strategy in which the photoresist/matrix is absent is the one using the azide cross-linkers [47][48]. The approach is similar to the one explored with the polymers and siloxanes but uses only a cross-linker molecule while the matrix is absent. The role of the cross-linker activated by light is to create a network between the scQDs through their organic ligands. With this methodology, all the steps that require the scQDs manipulation, like the ligand exchange or solvent change, or other chemical manipulation necessary for the deposition and patterning of the scQDs, are avoided or limited. In particular, the patterning strategy proposed by the Kang group uses a bis-Per Fluoro Phenyl Azide cross-linker (bis-PFPA) that can bind two different carbon atoms of the perovskite QDs ligands after light activation[49][50].
When the bifunctional cross-linker is mixed with the scQDs, it reacts with two different carbon groups of two aliphatic ligand chains of the neighboring scQDs (Figure 23). A careful evaluation of the cross-linker amount is necessary because it can damage the QDs’ optical properties. However, this drawback has been solved by the same research group by modifying the structure of the PFPA by introducing a multiple-arm PFPA[48]. Indeed, the bulky structure of the multiple-arm PFPA prevents the photoactivated nitrene radicals to join the scQD surface, compromising the PLQY.
With the use of a six-arm PFPA, the final pixel diameter obtained with the photo- patterning is 6 µm, 4 µm, and 2.5 µm. This methodology was utilized to manufacture an electron-driven QD-LED as a test for the effect of the cross-linker and the device stability in comparison with a QD-LED without any cross-linker[48]. In both devices, the current- voltage characteristics are almost identical; however, the time at which the luminance became 90% of the initial one (L = 1000 cd m−2) is 156 h for the cross-linked device with respect to 15 h of the QD-LED without cross-linking. The authors suggest that the increased robustness of the device is a consequence of the scQDs cross-linking that decreases the interfacial defects between the scQDs layer and the ZnMgO electron transporting layer.
Progress with the cross-linkers was made by introducing a benzophenone-derived ligand for direct optical patterning[47]. This strategy introduces the benzophenone cross- linker as an end group of the scQDs’ ligand instead of adding an external molecule like the bis-azides. Of course, this new ligand has to be exchanged with the native ligand of the synthesized scQDs. The benzophenone acts as the azide cross-linker by binding a four-valence carbon group of a neighboring ligand of another scQD (Figure 24a). The patterning strategy starts with the QDs’ ligand exchange to replace some of the native ligands with the benzophenone-modified ligand (Figure 24b–d). Then, the light activates the process of cross-linking, and patterning (Figure 24d). The authors tested both the optical properties of the patterned scQDs and the pattern- ing resolution. The PLQY of the ligand-exchanged scQDs remains unchanged after the ligand displacement due to the mild exchanging procedure and even after the cross-linking reaction. The patterning resolution was high indeed; the authors were able to reach patterns with widths from 3.8 µm up to 0.8 µm. Another interesting feature of the chemistry of the patterning is that it can be repeated layer-by-layer without any buffering coating.The authors also tested the patterned scQDs in an electron-driven QD-LED and all of the electrical and optical characteristics are almost identical to the ones using the pristine scQDs.
5.5 Direct Photolithography of scQDs via Their Direct Synthesis
The direct synthesis of the scQDs using a laser within a film [51] can be considered belonging to the DOP strategies. The main technical difference with respect to the previous methodologies is that the laser was used to directly synthesize the scQDs. This technique, often called direct laser patterning, combines the flexibility of the laser technique both at the industrial and technological level with the chemistry and optical properties of the QDs. Indeed, recently it was demonstrated that by changing the laser parameters, like the pulse power and frequency, it is possible to pattern (to grow) the scQDs, modulating their optical properties[52]. This kind of result has been possible by designing the chemical precursors within the film to be patterned in a proper way. This means including in the chemical formulation the suitable scQDs precursors, polymeric matrix, and other chemicals, allowing the laser-stimulated scQDs’ growth (Figure 25a).
Among these components, it is worth mentioning the BZT, (2-(2H-Benzotriazol-2-yl)- 4,6-ditertpentylphenol), which is a molecule that absorbs the laser light converting it into thermal energy, that is the driving force of the QDs’ synthesis.
On the other side, equally importantly, are the laser parameters that condition the achievement of the correct optical properties of the scQDs. Indeed, given a film chemical formulation, only specific combinations of pulse frequency (laser repetition rate), laser power, and beam speed allow to reach the proper conditions to grow the green- or red- emitting CdTe scQDs regions (Figure 25b).
The heat generated by BZT after the absorption of the UV laser radiation induces the formation of the CdTe scQDs. Indeed, the CdTe precursors were designed to form the CdTe scQDs by a thermal process when incorporated in a similar polymeric matrix[53]. By observing the parameters necessary to obtain red or green crystals, it was evident that the laser dose and the laser repetition rate are the key factors determining the optical properties of the CdTe scQDs. In particular, the pulse frequency is the key factor to obtain the red and green squares, because the pulse frequency is correlated with the temperature of the film. Indeed, it was shown that an annealing process carried out at relatively low temperatures favors the green scQDs, while at higher temperatures the growth of the red scQDs is stimulated. The limitation of this work is about the scQDs’ stability. Indeed, the PMMA matrix does not protect the CdTe scQDs from environmental conditions (oxygen and moisture), so the PL intensity and the time stability are poor. However, this work demonstrates that direct optical patterning can be a suitable strategy to modulate the QDs’ optical properties and that the use of a laser can replace the masks’ manufacturing maintaining a high resolution and automation typical of lasers.
6. Conclusions
Direct optical patterning (DOP) is an emerging tool to simplify the patterning process of the scQDs for display manufacturing that, however, is strictly related to the chemical processes that lead to the stability and homogeneity (dispersion) of the QDs for the correct function of the device. The optimization of the dispersion of the scQDs within a matrix is the golden rule to homogenize the interaction of the scQDs with the matrix itself. This means that the organic ligand at the QD surface should have the same chemical nature as the matrix. On the other side, stability is ensured by the formation of a close network of covalent bonds that cages the scQDs, preventing the loss of the ligands and surface atoms and, hence, preserving their optical properties. Considering these two main boundary conditions, five different approaches of DOP that exploit different chemical processes are evaluated. Even if often a comparison is complex, due to the different tests carried out in different types of samples, some general evaluation can be carried out.
The thiol-ene approach to DOP uses two simple multi-branched molecules, one thiol and one with a double bond, to encapsulate and pattern the scQDs[43]. The limitation of these works lies in the fact that the encapsulating network is still not designed to improve the interaction between the network itself and the QDs. In this sense, the work presented by Lesnyak’s group with the PIB polymer is more suitable[37]. Indeed, the PIB cross-linkable polymer and the scQDs functionalized with PIB-ligand improve the dispersion and stability of the QDs in the matrix. Even if this approach was not been tested for patterning, the fact that, with the help of light, the polymeric network is formed means that the DOP can reasonably be applied.
The DOP associated with the chemistry of the cross-linkers, like azides[54] or benzophenone[47], is one of the matrix-free methods. This method requests a moderate impact in chemistry for the synthesis of specific azides or ligands bearing the benzophenone end group. Once the impact of the chemical manipulation has been overcome and this should be taken into consideration for an industrial application, the patterning is relatively simple. The patterning approach of Talapin’s group [95,96] is an elegant way to control the position of the QDs, but the ligand exchange presents a decrease, even if modest, in the PLQY that influences the efficiency of the whole system. In these matrix-free methods, the absence of an encapsulating agent does not ensure the stability of the material over time and in harsh conditions, even if it is possible to conceive that the presence of a matrix should not compromise the patterning strategy, as also reported by Hahm et al.[47].
The direct synthesis of the scQDs by laser[52] is interesting from the scientific point of view. However, without the setup of an encapsulating agent it is not suitable for market exploitation.
In general, all of the techniques showed the possibility to obtain patterns even with high resolution for pixel manufacturing (below 10 µm or even less). Only in three cases the devices were successfully realized. The main difference in these works is the evaluation of the device’s stability. As stated above, the functional tests of the realized devices are quite different and only Bae’s group carried out a test in harsh conditions (high humidity for a long time and in the presence of acids/bases).
The patterning strategy using the siloxanes[44] seems to be the most mature approach because it was developed over time to surpass dispersion, moisture, temperature, mechan- ical and biocompatibility tests. Bae’s group, indeed, “played” with siloxane chemistry introducing time by time different chemical groups to improve the dispersion of the scQDs, the optical transparency of the matrix[42], the stability at higher temperatures[55], or the mechanical performances of the siloxanes[40]. In addition, the chemistry of the siloxanes is well-developed and almost all the reagents described are commercially available.
In conclusion, the combination of the use of the laser for DOP with the mentioned chemical strategies starting from the chemistry of the siloxanes could be an interesting benchmark to find the best chemical path for the patterning of functional scQDs. Indeed, what is appealing in the use of the laser as patterning equipment is that (i) it is not necessary to have a mask for patterning, (ii) the laser is easily driven by a PC (beam position and energy), (iii) it maintains a high resolution[56], and finally, (iv) from an industrial point of view, it is a mature technology with a broad industrial penetration.
References
- Kim, B.H.; Onses, M.S.; Lim, J.B.; Nam, S.; Oh, N.; Kim, H.; Yu, K.J.; Lee, J.W.; Kim, J.-H.; Kang, S.-K.; et al. High-Resolution Patterns of Quantum Dots Formed by Electrohydrodynamic Jet Printing for Light-Emitting Diodes. Nano Lett. 2015, 15, 969–973.
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541.
- Bayer, M. Bridging Two Worlds: Colloidal versus Epitaxial Quantum Dots. Ann. Phys. 2019, 531, 1900039.
- Wu, Y.; Jia, R.; Xu, J.; Song, L.; Liu, Y.; Zhang, Y.; Ullah, S.; Dai, J. Strategies of Improving CsPbX3 Perovskite Quantum Dots Optical Performance. Front. Mater. 2022, 9, 845977.
- Song, Z.; Zhao, J.; Liu, Q. Luminescent perovskites: Recent advances in theory and experiments. Inorg. Chem. Front. 2019, 6, 2969–3011.
- Haydous, F.; Gardner, J.M.; Cappel, U.B. The impact of ligands on the synthesis and application of metal halide perovskite nanocrystals. J. Mater. Chem. A 2021, 9, 23419–23443.
- Efros, A.L.; Brus, L.E. Nanocrystal Quantum Dots: From Discovery to Modern Development. ACS Nano 2021, 15, 6192–6210.
- Weidman, M.C.; Beck, M.E.; Hoffman, R.S.; Prins, F.; Tisdale, W.A. Monodisperse, Air-Stable PbS Nanocrystals via Precursor Stoichiometry Control. ACS Nano 2014, 8, 6363–6371.
- Mocatta, D.; Cohen, G.; Schattner, J.; Millo, O.; Rabani, E.; Banin, U. Heavily Doped Semiconductor Nanocrystal Quantum Dots. Science 2011, 332, 77–81.
- Todescato, F.; Fortunati, I.; Minotto, A.; Signorini, R.; Jasieniak, J.J.; Bozio, R. Engineering of Semiconductor Nanocrystals for Light Emitting Applications. Materials 2016, 9, 672.
- Reiss, P.; Protière, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168.
- Eagle, F.W.; Park, N.; Cash, M.; Cossairt, B.M. Surface Chemistry and Quantum Dot Luminescence: Shell Growth, Atomistic Modification, and Beyond. ACS Energy Lett. 2021, 6, 977–984.
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433.
- Cao, Z.; Shu, Y.; Qin, H.; Su, B.; Peng, X. Quantum Dots with Highly Efficient, Stable, and Multicolor Electrochemiluminescence. ACS Cent. Sci. 2020, 6, 1129–1137.
- Li, J.J.; Wang, Y.A.; Guo, W.; Keay, J.C.; Mishima, T.D.; Johnson, M.B.; Peng, X. Large-Scale Synthesis of Nearly Monodisperse CdSe/CdS Core/Shell Nanocrystals Using Air-Stable Reagents via Successive Ion Layer Adsorption and Reaction. J. Am. Chem. Soc. 2003, 125, 12567–12575.
- Bae, W.K.; Char, K.; Hur, H.; Lee, S. Single-Step Synthesis of Quantum Dots with Chemical Composition Gradients. Chem. Mater. 2008, 20, 531–539.
- Hanifi, D.A.; Bronstein, N.D.; Koscher, B.A.; Nett, Z.; Swabeck, J.K.; Takano, K.; Schwartzberg, A.M.; Maserati, L.; Vandewal, K.; van de Burgt, Y.; et al. Redefining near-unity luminescence in quantum dots with photothermal threshold quantum yield. Science 2019, 363, 1199–1202.
- Meinardi, F.; Colombo, A.; Velizhanin, K.A.; Simonutti, R.; Lorenzon, M.; Beverina, L.; Viswanatha, R.; Klimov, V.I.; Brovelli, S. Large-area luminescent solar concentrators based on ‘Stokes-shift-engineered’ nanocrystals in a mass-polymerized PMMA matrix. Nat. Photonics 2014, 8, 392–399.
- Selopal, G.S.; Abdelkarim, O.; Kumar, P.; Jin, L.; Liu, J.; Zhao, H.; Yurtsever, A.; Vidal, F.; Wang, Z.M.; Rosei, F. Role of Interfacial Engineering of “Giant” Core–Shell Quantum Dots. ACS Appl. Energy Mater. 2022, 5, 1447–1459.
- Carbone, L.; Nobile, C.; De Giorgi, M.; Sala, F.D.; Morello, G.; Pompa, P.; Hytch, M.; Snoeck, E.; Fiore, A.; Franchini, I.R.; et al. Synthesis and Micrometer-Scale Assembly of Colloidal CdSe/CdS Nanorods Prepared by a Seeded Growth Approach. Nano Lett. 2007, 7, 2942–2950.
- Srivastava, A.K.; Zhang, W.; Schneider, J.; Halpert, J.E.; Rogach, A.L. Luminescent Down-Conversion Semiconductor Quantum Dots and Aligned Quantum Rods for Liquid Crystal Displays. Adv. Sci. 2019, 6, 1901345.
- Diroll, B.T.; Guzelturk, B.; Po, H.; Dabard, C.; Fu, N.; Makke, L.; Lhuillier, E.; Ithurria, S. 2D II–VI Semiconductor Nanoplatelets: From Material Synthesis to Optoelectronic Integration. Chem. Rev. 2023, 123, 3543–3624.
- Onal, A.; Sadeghi, S.; Melikov, R.; Karatum, O.; Eren, G.O.; Nizamoglu, S. Quantum Dot to Nanorod Transition for Efficient White-Light-Emitting Diodes with Suppressed Absorption Losses. ACS Photonics 2022, 9, 3268–3278.
- Ehlert, S.; Stegelmeier, C.; Pirner, D.; Förster, S. A General Route to Optically Transparent Highly Filled Polymer Nanocomposites. Macromolecules 2015, 48, 5323-5327.
- Shiman, D.I.; Sayevich, V.; Meerbach, C.; Nikishau, P.A.; Vasilenko, I.V.; Gaponik, N.; Kostjuk, S.V.; Lesnyak, V. Robust Polymer Matrix Based on Isobutylene (Co)polymers for Efficient Encapsulation of Colloidal Semiconductor Nanocrystals. ACS Appl. Nano Mater. 2019, 2, 956-963.
- Reitinger, N.; Hohenau, A.; Köstler, S.; Krenn, J.R.; Leitner, A. Radiationless energy transfer in CdSe/ZnS quantum dot aggregates embedded in PMMA. Phys. Status Solidi A 2011, 208, 710-714.
- Xiao, P.; Zhang, Z.; Ge, J.; Deng, Y.; Chen, X.; Zhang, J.-R.; Deng, Z.; Kambe, Y.; Talapin, D.V.; Wang, Y.; et al. Surface passivation of intensely luminescent all-inorganic nanocrystals and their direct optical patterning. Nat. Commun. 2023, 14, 49.
- Kovalenko, M.V.; Scheele, M.; Talapin, D.V. Colloidal Nanocrystals with Molecular Metal Chalcogenide Surface Ligands. Science 2009, 324, 1417-1420.
- Hu, Z.; Liu, S.; Qin, H.; Zhou, J.; Peng, X. Oxygen Stabilizes Photoluminescence of CdSe/CdS Core/Shell Quantum Dots via Deionization. J. Am. Chem. Soc. 2020, 142, 4254-4264.
- Hu, Z.; Shu, Y.; Qin, H.; Hu, X.; Peng, X. Water Effects on Colloidal Semiconductor Nanocrystals: Correlation of Photophysics and Photochemistry. J. Am. Chem. Soc. 2021, 143, 18721-18732.
- Carrillo-Carrión, C.; Cárdenas, S.; Simonet, B.M.; Valcárcel, M. Quantum dots luminescence enhancement due to illumination with UV/Vis light. Chem. Commun. 2009, 35, 5214-5226.
- Hines, D.A.; Becker, M.A.; Kamat, P.V. Photoinduced Surface Oxidation and Its Effect on the Exciton Dynamics of CdSe Quantum Dots. J. Phys. Chem. C 2012, 116, 13452-13457.
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. . Polym. Chem. 2010, 1, 17-36.
- Kade, M.J.; Burke, D.J.; Hawker, C.J. The power of thiol-ene chemistry. J. Polym. Sci. Part Polym. Chem. 2010, 48, 743-750.
- Smith, M.J.; Malak, S.T.; Jung, J.; Yoon, Y.J.; Lin, C.H.; Kim, S.; Lee, K.M.; Ma, R.; White, T.J.; Bunning, T.J.; et al.et al. Robust, Uniform, and Highly Emissive Quantum Dot–Polymer Films and Patterns Using Thiol–Ene Chemistry. . ACS Appl. Mater. Interfaces 2017, 9, 17435-17448.
- Yu, M.; Saeed, M.H.; Zhang, S.; Wei, H.; Gao, Y.; Zou, C.; Zhang, L.; Yang, H. Luminescence Enhancement, Encapsulation, and Patterning of Quantum Dots Toward Display Applications. Adv. Funct. Mater. 2022, 32, 2109472.
- Design of cross-linked polyisobutylene matrix for efficient encapsulation of quantum dots. Design of cross-linked polyisobutylene matrix for efficient encapsulation of quantum dots. Nanoscale Adv. 2021, 3, 1443-1454.
- Lim, Y.-W.; Jin, J.; Bae, B.-S. Optically Transparent Multiscale Composite Films for Flexible and Wearable Electronics. . Adv. Mater. 2020, 32, 1907143.
- Ro, H.W.; Soles, C.L. Silsesquioxanes in nanoscale patterning applications.. Mater. Today 2011, 14, 20-33.
- Lee, H.E.; Lee, D.; Lee, T.-I.; Jang, J.; Jang, J.; Lim, Y.-W.; Shin, J.H.; Kang, S.-M.; Choi, G.-M.; Joe, D.J.; et al.et al. Siloxane Hybrid Material-Encapsulated Highly Robust Flexible LEDs for Biocompatible Lighting Applications. . ACS Appl. Mater. Interfaces 2022, 14, 28258-28269.
- Kuk, S.K.; Jang, J.; Han, H.J.; Lee, E.; Oh, H.; Kim, H.Y.; Jang, J.; Lee, K.T.; Lee, H.; Jung, Y.S.; et al.et al. Siloxane-Encapsulated Upconversion Nanoparticle Hybrid Composite with Highly Stable Photoluminescence against Heat and Moisture. . ACS Appl. Mater. Interfaces 2019, 11, 15952-15959.
- Kim, H.Y.; Yoon, D.-E.; Jang, J.; Lee, D.; Choi, G.-M.; Chang, J.H.; Lee, J.Y.; Lee, D.C.; Bae, B.-S. Quantum Dot/Siloxane Composite Film Exceptionally Stable against Oxidation under Heat and Moisture. . J. Am. Chem. Soc. 2016, 138, 16478-16485.
- Zhang, P.; Yang, G.; Li, F.; Shi, J.; Zhong, H. Direct in situ photolithography of perovskite quantum dots based on photocatalysis of lead bromide complexes.. Nat. Commun. 2022, 13, 6713.
- Kim, Y.H.; Koh, S.; Lee, H.; Kang, S.-M.; Lee, D.C.; Bae, B.-S. Photo-Patternable Quantum Dots/Siloxane Composite with Long-Term Stability for Quantum Dot Color Filters. . ACS Appl. Mater. Interfaces 2020, 12, 3961-3968.
- Wang, Y.; Fedin, I.; Zhang, H.; Talapin, D.V. Direct optical lithography of functional inorganic nanomaterials. Science 2017, 357, 385-388.
- Cho, H.; Pan, J.-A.; Wu, H.; Lan, X.; Coropceanu, I.; Wang, Y.; Cho, W.; Hill, E.A.; Anderson, J.S.; Talapin, D.V.; et al. Direct Optical Patterning of Quantum Dot Light-Emitting Diodes via In Situ Ligand Exchange. Adv. Mater. 2020, 32, 2003805.
- Hahm, D.; Lim, J.; Kim, H.; Shin, J.-W.; Hwang, S.; Rhee, S.; Chang, J.H.; Yang, J.; Lim, C.H.; Jo, H.; et al.et al. Direct patterning of colloidal quantum dots with adaptable dual-ligand surface. . Nat. Nanotechnol. 2022, 17, 952-958.
- Yang, J.; Lee, M.; Park, S.Y.; Park, M.; Kim, J.; Sitapure, N.; Hahm, D.; Rhee, S.; Lee, D.; Jo, H.; et al.et al. Nondestructive Photopatterning of Heavy-Metal-Free Quantum Dots.. Adv. Mater. 2022, 34, 2205504.
- Yang, J.; Hahm, D.; Kim, K.; Rhee, S.; Lee, M.; Kim, S.; Chang, J.H.; Park, H.W.; Lim, J.; Lee, M.; et al.et al. High-resolution patterning of colloidal quantum dots via non-destructive, light-driven ligand crosslinking. . Nat. Commun. 2020, 11, 2874.
- Liu, D.; Weng, K.; Lu, S.; Li, F.; Abudukeremu, H.; Zhang, L.; Yang, Y.; Hou, J.; Qiu, H.; Fu, Z.; et al.et al. Direct optical patterning of perovskite nanocrystals with ligand cross-linkers.. Sci. Adv. 2022, 8, eabm8433.
- Antolini, F.; Orazi, L. Quantum Dots Synthesis through Direct Laser Patterning: A Review. F. Front. Chem. 2019, 7, 752.
- Antolini, F.; Limosani, F.; Carcione, R. Direct Laser Patterning of CdTe QDs and Their Optical Properties Control through Laser Parameters. . Nanomaterials 2022, 12, 1551.
- Carcione, R.; Limosani, F.; Antolini, F. Cadmium Telluride Nanocomposite Films Formation from Thermal Decomposition of Cadmium Carboxylate Precursor and Their Photoluminescence Shift from Green to Red. . Crystals 2021, 11, 253.
- Lu, S.; Fu, Z.; Li, F.; Weng, K.; Zhou, L.; Zhang, L.; Yang, Y.; Qiu, H.; Liu, D.; Qing, W.; et al.et al. Beyond a Linker: The Role of Photochemistry of Crosslinkers in the Direct Optical Patterning of Colloidal Nanocrystals. . Angew. Chem. Int. Ed. 2022, 61, e202202633.
- Jang, J.; Yoon, D.-E.; Kang, S.-M.; Kim, Y.H.; Lee, I.; Lee, H.; Kim, Y.H.; Lee, D.C.; Bae, B.-S. Exceptionally stable quantum dot/siloxane hybrid encapsulation material for white light-emitting diodes with a wide color gamut. . Nanoscale 2019, 11, 14887-14895.
- Qiao, W.; Huang, W.; Liu, Y.; Li, X.; Chen, L.-S.; Tang, J.-X. Toward Scalable Flexible Nanomanufacturing for Photonic Structures and Devices. Adv. Mater. 2016, 28, 10353-10380.
