Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Li Liao.
The decellularized extracellular matrix (dECM) is capable of promoting stem cell proliferation, migration, adhesion, and differentiation. It is a promising biomaterial for application and clinical translation in the field of periodontal tissue engineering as it most effectively preserves the complex array of ECM components as they are in native tissue, providing ideal cues for regeneration and repair of damaged periodontal tissue. dECMs of different origins have different advantages and characteristics in promoting the regeneration of periodontal tissue. dECM can be used directly or dissolved in liquid for better flowability.
- periodontal regeneration
- decellularized extracellular matrix
- decellularized cell sheet
1. Introduction
Decellularized extracellular matrix (dECM) is a promising biomaterial for repairing periodontal defects since dECM maximizes the retention of complex protein arrays, glycosaminoglycans, proteoglycans, and many other matrix components found in natural tissues [1]. Therefore, dECM provides optimal cell–ECM interactions by providing ideal biological clues to mimic native signaling events to promote the regeneration, repair, and remodeling of damaged periodontal tissue [2]. For instance, with a three-dimensional network providing a microenvironment to maintain homeostasis, support stem cell ingrowth, promote tissue formation, and initiate tissue repair, dECM has been successful in the regeneration and repair of many tissues, such as heart, nerve, and liver tissues [3]. During tissue regeneration, cell–ECM interactions coordinated with ECM component changes are vital for directing cell behaviors, functions, and fates, resulting in tissue repair and remodeling, which are regulated by specific enzymes produced by cells [3].
Periodontal defects caused by periodontitis, trauma, and tumors often result in the destruction of alveolar bone, periodontal membrane, and cementum. The current clinical treatment methods include basic treatment of periodontal disease, periodontal surgery, and guiding tissue regeneration, but their efficacy is often not ideal, and the orderly structure and the height of alveolar bone cannot be regenerated [1]. Several bio-scaffolds, with supportive effects, have been applied to avoid long junctional epithelium healing as a contact inhibition membrane, which is known as guided tissue regeneration (GTR). Recently, more and more biomimetic scaffolds with certain physiochemical characteristics and mechanical behavior have been applied in periodontal tissue engineering. However, periodontal tissue is a complicated tissue consisting of cementum, periodontium, and alveolar bone, which could hardly be regenerate by using a barrier membrane without providing regenerative niches with biological cues to activate the signaling pathway for the whole regeneration of cementum (C), periodontium (P), and alveolar bone (AB) simultaneously with certain structure. Moreover, the effect of biomaterials in clinical applications is usually hampered by the inertness of synthetic scaffolds compared with bioactive materials and could even result in a reverse result due to their foreignness and sometimes activate a severe immunologic reaction.
To the contrary, natural ECM contains useful structural and biochemical information, providing sufficient bioactive cues to trigger cell functions needed for tissue regeneration. The decellularized scaffold derived from naturally occurring tissues or cultured cells maintains a natural 3D network structure and can serve as a natural scaffold. Moreover, a dECM scaffold conserves natural ECM components such as glycosaminoglycans, proteoglycans, and growth factors and could, in return, moderate immune reactions, modulate inflammatory processes, impact periodontal-related stem cell behaviors and fates, and promote periodontal regeneration. Since the complexity of periodontal tissue and tissue-specific ECM with biological cues aimed at regeneration of certain tissue (C, P, AB), it is important to provide different regenerative niches for the recruitment and modulation of endogenous stem cells for both cementum, periodontium, and alveolar bone tissue regeneration [3,4][3][4].
2. dECM Derived from Different Sources
As a promising biomaterial, dECM has been widely applied in periodontal tissue engineering. Natural ECM generated from decellularized ECM (dECM) can be divided into two groups as decellularized cells/cell sheets (C-dECM) or decellularized tissue-specific ECM (T-dECM) according to the origins.2.1. (Stem) Cell-Derived dECM (Decellularized Cell Sheet)
Significant efforts have been made to improve the quality and efficiency of the generation of cell-derived ECM with 3D structure. Part of these works involve harvesting cell-derived dECM membranes and then fabricating a 3D scaffold using various physical and chemical techniques [5]. As shown in Figure 1, C-dECM-laden scaffolds possess superior biological elasticity and porosity and allow more efficient nutrient exchange that favor cell proliferation, adhesion, and differentiation [6]. In recent years, scientists have applied certain decellularized cell sheets to periodontal regeneration. Decellularized cell sheet techniques refer to the effective removal of cellular and nuclear components of the cell sheet, especially DNA and RNA, while retaining the basic components, biological activity, and mechanical integrity of the extracellular matrix (dECM) of the sheet. Due to the lack of cells and major tissue structure, the foreign body reaction and immune rejection are significantly reduced or even absent in the cell sheet-derived dECM material, while the original three-dimensional structure, mechanical integrity, biological activity, and good biocompatibility are well preserved. Therefore, the use of decellularized sheets in regenerative medicine is increasing. Moreover, decellularized membrane sheets combined with cytokines or new materials to achieve better tissue regeneration effects have been used in many tissue regeneration processes, such as dental tissue regeneration [7], bone regeneration [8], vascular reconstruction [9], nerve regeneration [10], and corneal regeneration [11]. Specifically, the common C-dECM sources for periodontal regeneration are the periodontal ligament stem cell sheet (PDLSC sheet) [12], the bone marrow mesenchymal stem cell sheet (BMSC sheet) [6], the human urogenic mesenchymal stem cell sheet (hUMSC sheet), and others such as dental folicle stem cells and L929 sheet-derived dECM can also be considered.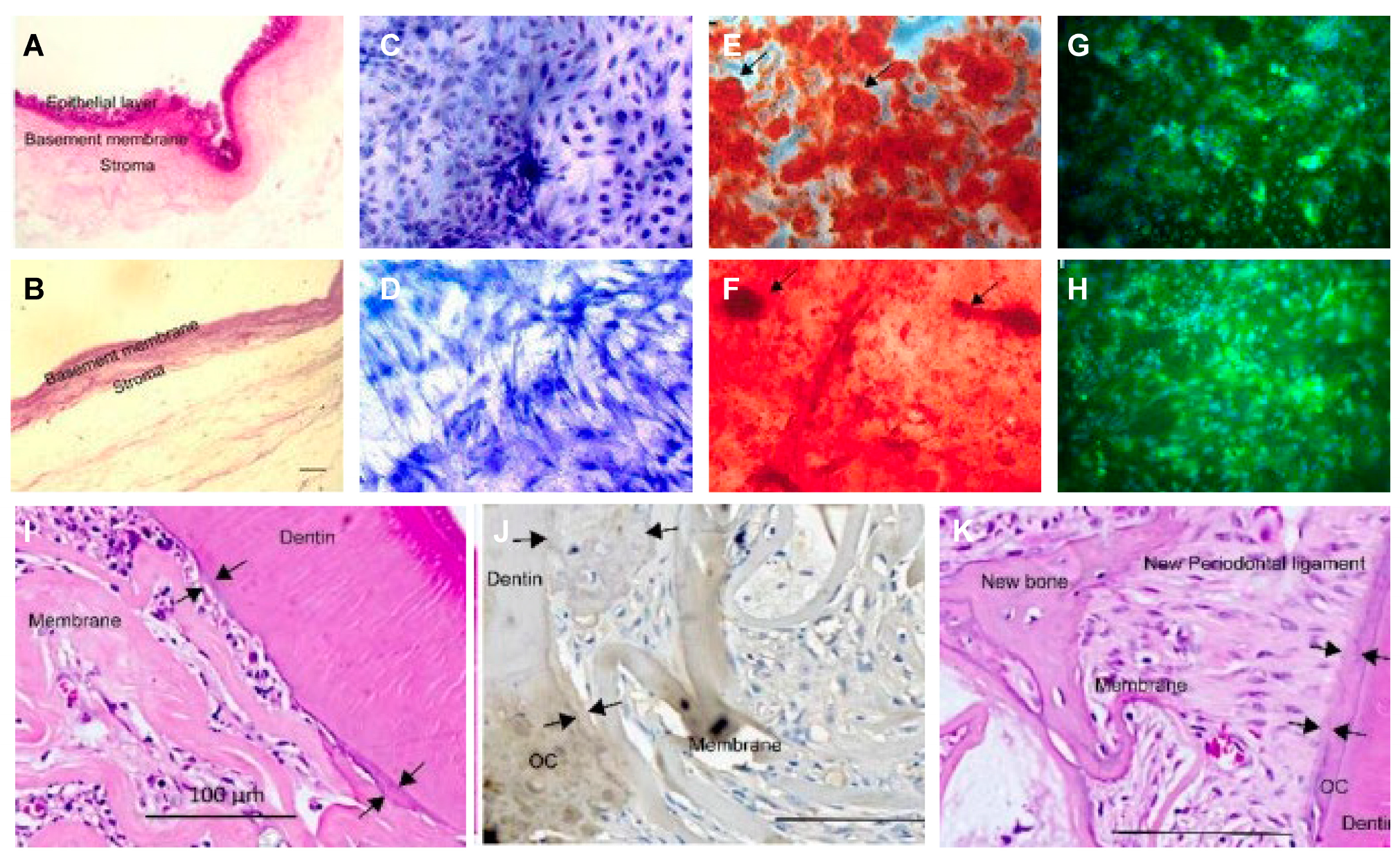
Figure 1. Decellularized amniotic membrane promotes osteogenic differentiation and cementum regeneration both in vitro and in vivo. Histological cross-sections of fresh (A) and decellularized (B) amniotic membrane. Representative areas of adipose-derived stromal cell cultures on polystyrene (C,E,G) and decellularized amniotic membrane (DAM) (D,F,H). ASCs confluence in the presence of supplemented medium (C,D). The osteoinduction medium stimulated the deposition of the mineralized extracellular matrix, which was observed as red calcified deposits (arrows). Osteopontin expression was evidenced by green fluorescence as agglomerates on polystyrene (G) and a diffuse distribution (H) on DAM. (I,J) and exposed old cementum (J,K), as well as a thin acellular cementum layer and thick cellular cementum (J). Transplantation of decellularized amniotic membrane (DAM) (I); DAM associated with extracellular matrix and undifferentiated adipose-derived stromal cells (J); scale bars of 100µm (I–K).
- (1)
-
Periodontal ligament (stem) cell sheets
- (2)
-
BMC/BMSC sheet:
- (3)
-
Urogenic mesenchymal (stem) cell sheets:
2.2. Tissue-Derived Extracellular Matrix for Periodontal Tissue Engineering Constructs
Despite the similar ECM composition between different tissues and organs, subtle differences in function, proportion, structure, and stiffness of the ECM can influence the interaction of cells in determining cell fate (Figure 2). Tissue dECM can promote tissue-specific and non-tissue-specific stem cells/progenitor cells, primary cell proliferation, and serve as a tissue-specific scaffold for stem cells/progenitor cells. Even without a specific differentiation mediator, the stem cells or progenitor cells still have the corresponding cell lineage differentiation capacity, based on the specific interaction between the cell and the ECM [28][25].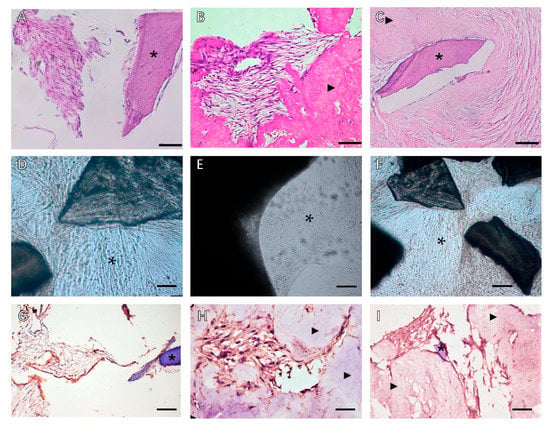
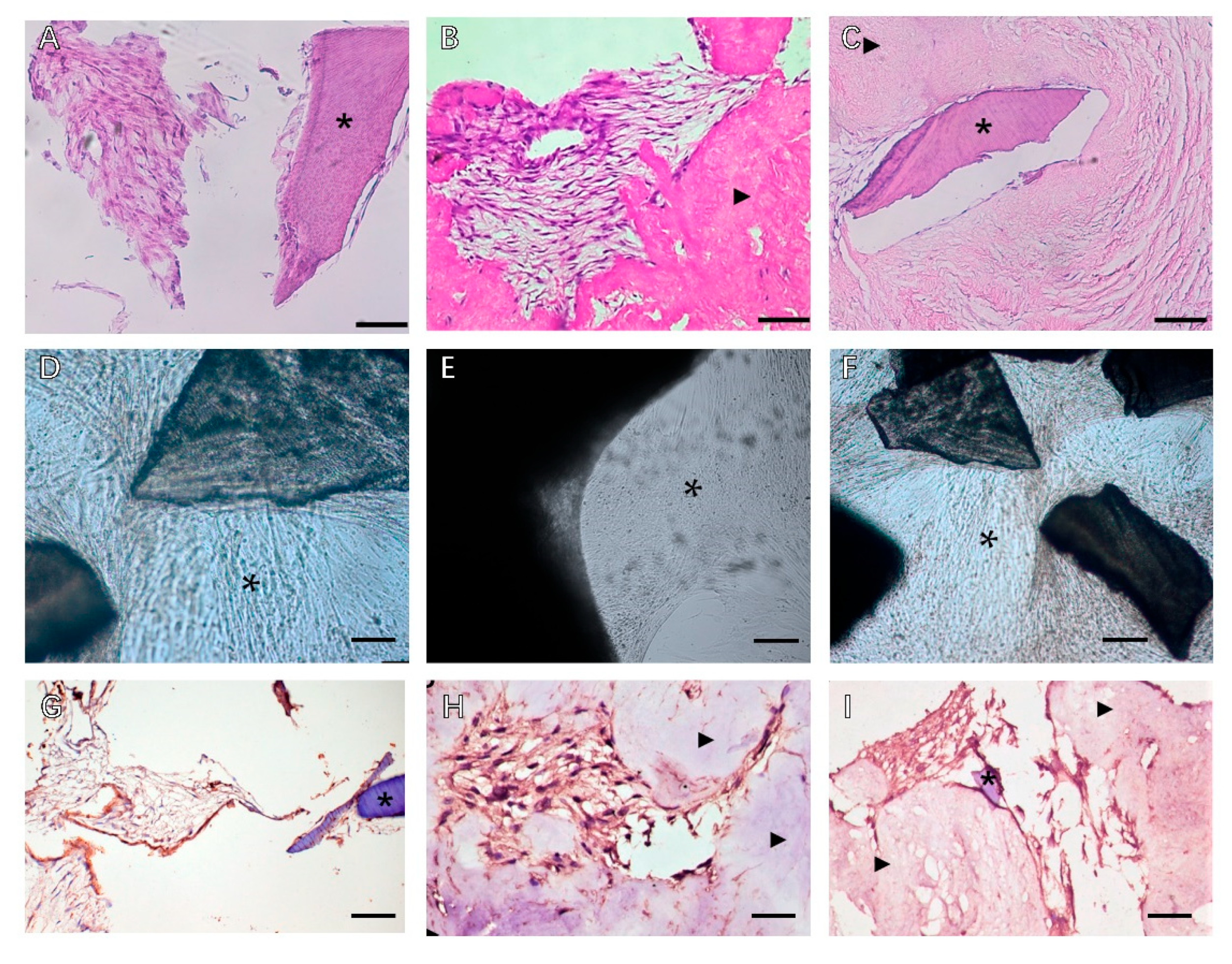 Figure 2. Phase contrast microscopy of decellularized scaffolds cultured with periodontal cells in 3D culture for 14 days. Scale bars, 200 µm in (A–C): (A) PDLSCs around the T-dECM; (B) periosteum stem cells (PSCs) around and in the dPDL; (C) PDLSCs + PSCs around the T-dECM and in the dPDL. The asterisk indicates the monolayer of the cells. Histological sections of decellularized scaffolds were cultured with periodontal cells in 3D culture for 14 days. Hematoxylin-eosin staining. Scale bars, 100 µm in (D), 50 µm in (E,F): *: PDLSCs next to the dTM indicated by an asterisk; (E) PSCs in the dPDL (F) PDLSCs + PSCs around the T-dECM and in the dPDL (D) original magnification ×200; (E,F) original magnification ×100. Evaluation of the expression of osteogenic differentiation markers in periodontal cells cultured on decellularized scaffolds under 3D culture for 14 days: (G–I) Immunohistochemical staining. The positive cells have a brown color. The nuclei were counterstained with hematoxylin. Scale bars, 100 µm in (G–I): (G) OPN staining of PDLSCs next to the T-dECM indicated by an asterisk; (H) OC staining of PSCs in the dPDL indicated by arrowheads; (I) OPN staining of PDLSCs + PSCs around the T-dECM and in the dPDL indicated by an asterisk and arrowheads, respectively. *: MSCs around T-dECM(dTM);
Figure 2. Phase contrast microscopy of decellularized scaffolds cultured with periodontal cells in 3D culture for 14 days. Scale bars, 200 µm in (A–C): (A) PDLSCs around the T-dECM; (B) periosteum stem cells (PSCs) around and in the dPDL; (C) PDLSCs + PSCs around the T-dECM and in the dPDL. The asterisk indicates the monolayer of the cells. Histological sections of decellularized scaffolds were cultured with periodontal cells in 3D culture for 14 days. Hematoxylin-eosin staining. Scale bars, 100 µm in (D), 50 µm in (E,F): *: PDLSCs next to the dTM indicated by an asterisk; (E) PSCs in the dPDL (F) PDLSCs + PSCs around the T-dECM and in the dPDL (D) original magnification ×200; (E,F) original magnification ×100. Evaluation of the expression of osteogenic differentiation markers in periodontal cells cultured on decellularized scaffolds under 3D culture for 14 days: (G–I) Immunohistochemical staining. The positive cells have a brown color. The nuclei were counterstained with hematoxylin. Scale bars, 100 µm in (G–I): (G) OPN staining of PDLSCs next to the T-dECM indicated by an asterisk; (H) OC staining of PSCs in the dPDL indicated by arrowheads; (I) OPN staining of PDLSCs + PSCs around the T-dECM and in the dPDL indicated by an asterisk and arrowheads, respectively. *: MSCs around T-dECM(dTM); : MSCs around T-dECM(dPDL).
: MSCs around T-dECM(dPDL).2.2.1. Human (Allogenic) Tissue Derived dECMs
- (1)
-
Dental (craniofacial)-related human tissues derived dECM
- (2)
-
Non-dental-related tissue-derived dECM
- a.
-
Human amnion dECM
- b.
-
Human umbilical vein dECM
2.2.2. Heterogenous Tissue-Derived dECMs
- (1)
-
Heterogenous dental (craniofacial)-related tissues derived from dECM
- a.
-
Decellularized porcine dental matrix
- b.
-
Decellularized matrix of dog periodontal ligament
- c.
-
Decellularized rat mandible matrix
- (2)
-
Heterogenous, non-dental-related tissue-derived dECM
- a.
-
SIS
- b.
-
Decellularized Amnion
- c.
-
Decellularized pericardium
- d.
-
To sum up, decellularization of natural tissues to produce extracellular matrix is a promising method for 3D scaffolding and for investigating cell-ECM interaction during regeneration of target tissue [3,51][3][48]. The fate and behavior of mesenchymal stem cells are influenced by the stem cell niches ideal biochemical and physical cues. Ana Rita Pereira et al. compared the biological behaviors of BMSCs when exposed to C-dECM and T-dECM, and better outcomes were observed in 3D decellularized bone tissue for greater architecture complexity and physicochemical properties [52][49]. To sum up, tissue-derived dECM has great potential in the context of endogenous periodontal regeneration, with a better effect on preserving the tissue niche intended for different tissues in periodontal defects.
References
- Cho, Y.D.; Kim, K.H.; Lee, Y.M.; Ku, Y.; Seol, Y.J. Periodontal Wound Healing and Tissue Regeneration: A Narrative Review. Pharmaceuticals 2021, 14, 456.
- Feng, Z.; Su, X.; Wang, T.; Guo, S. Identification of Biomarkers That Modulate Osteogenic Differentiation in Mesenchymal Stem Cells Related to Inflammation and Immunity: A Bioinformatics-Based Comprehensive Study. Pharmaceuticals 2022, 15, 1094.
- Liu, C.; Pei, M.; Li, Q.; Zhang, Y. Decellularized extracellular matrix mediates tissue construction and regeneration. Front. Med. 2022, 16, 56–82.
- Wen, Y.; Yang, H.; Wu, J.; Wang, A.; Chen, X.; Hu, S.; Zhang, Y.; Bai, D.; Jin, Z. COL4A2 in the tissue-specific extracellular matrix plays important role on osteogenic differentiation of pessssriodontal ligament stem cells. Theranostics 2019, 9, 4265–4286.
- Kim, B.; Ventura, R.; Lee, B.T. Functionalization of porous BCP scaffold by generating cell-derived extracellular matrix from rat bone marrow stem cells culture for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e1256–e1267.
- Xu, Y.; Xu, G.Y.; Tang, C.; Wei, B.; Pei, X.; Gui, J.C.; Min, B.H.; Jin, C.Z.; Wang, L.M. Preparation and characterization of bone marrow mesenchymal stem cell-derived extracellular matrix scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 670–678.
- Jiang, Y.; Liu, J.M.; Huang, J.P.; Lu, K.X.; Sun, W.L.; Tan, J.Y.; Li, B.X.; Chen, L.L.; Wu, Y. Regeneration potential of decellularized periodontal ligament cell sheets combined with 15-Deoxy-Δ12,14-prostaglandin J2 nanoparticles in a rat periodontal defect. Biomed. Mater. 2021, 16, 045008.
- Hoang Thi, T.T.; Tran Nguyen, D.H.; Nguyen, D.T.D.; Nguyen, D.H.; Truong, M.D. Decellularized Porcine Epiphyseal Plate-Derived Extracellular Matrix Powder: Synthesis and Characterization. Cells Tissues Organs 2020, 209, 101–109.
- Liu, Y.; Zhang, Y.; Mei, T.; Cao, H.; Hu, Y.; Jia, W.; Wang, J.; Zhang, Z.; Wang, Z.; Le, W.; et al. hESCs-Derived Early Vascular Cell Spheroids for Cardiac Tissue Vascular Engineering and Myocardial Infarction Treatment. Adv. Sci. (Weinh) 2022, 9, e2104299.
- Xu, Y.; Zhou, J.; Liu, C.; Zhang, S.; Gao, F.; Guo, W.; Sun, X.; Zhang, C.; Li, H.; Rao, Z.; et al. Understanding the role of tissue-specific decellularized spinal cord matrix hydrogel for neural stem/progenitor cell microenvironment reconstruction and spinal cord injury. Biomaterials 2021, 268, 120596.
- Kim, H.; Jang, J.H.; Han, W.; Hwang, H.J.; Jang, J.; Kim, J.Y.; Cho, D.W. Extracellular matrix-based sticky sealants for scar-free corneal tissue reconstruction. Biomaterials 2023, 292, 121941.
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Bartold, P.M.; Hutmacher, D.W.; Ivanovski, S. The effect of decellularized tissue engineered constructs on periodontal regeneration. J. Clin. Periodontol. 2018, 45, 586–596.
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Volpato, F.Z.; Hutmacher, D.W.; Ivanovski, S. Assessment of static and perfusion methods for decellularization of PCL membrane-supported periodontal ligament cell sheet constructs. Arch. Oral Biol. 2018, 88, 67–76.
- Xu, F.; Zheng, Z.; Yao, M.; Zhu, F.; Shen, T.; Li, J.; Zhu, C.; Yang, T.; Shao, M.; Wan, Z.; et al. A regulatory mechanism of a stepwise osteogenesis-mimicking decellularized extracellular matrix on the osteogenic differentiation of bone marrow-derived mesenchymal stem cells. J. Mater. Chem. B 2022, 10, 6171–6180.
- Yang, X.; Xiong, X.; Zhou, W.; Feng, G.; Zhang, Y.; Dai, H.; Zhou, J. Effects of human urine-derived stem cells on the cementogenic differentiation of indirectly-cocultured periodontal ligament stem cells. Am. J. Transl. Res. 2020, 12, 361–378.
- Xiong, X.; Yang, X.; Dai, H.; Feng, G.; Zhang, Y.; Zhou, J.; Zhou, W. Extracellular matrix derived from human urine-derived stem cells enhances the expansion, adhesion, spreading, and differentiation of human periodontal ligament stem cells. Stem. Cell Res. Ther. 2019, 10, 396.
- Hoshiba, T.; Lu, H.; Yamada, T.; Kawazoe, N.; Tateishi, T.; Chen, G. Effects of extracellular matrices derived from different cell sources on chondrocyte functions. Biotechnol. Prog. 2011, 27, 788–795.
- Huang, J.P.; Wu, Y.M.; Liu, J.M.; Zhang, L.; Li, B.X.; Chen, L.L.; Ding, P.H.; Tan, J.Y. Decellularized matrix could affect the proliferation and differentiation of periodontal ligament stem cells in vitro. J. Periodontal. Res. 2021, 56, 929–939.
- Junka, R.; Zhou, X.; Wang, W.; Yu, X. Albumin-Coated Polycaprolactone (PCL)-Decellularized Extracellular Matrix (dECM) Scaffold for Bone Regeneration. ACS Appl. Bio. Mater. 2022, 5, 5634–5644.
- Ventura, R.D.; Padalhin, A.R.; Kim, B.; Park, M.; Lee, B.T. Evaluation of bone regeneration potential of injectable extracellular matrix (ECM) from porcine dermis loaded with biphasic calcium phosphate (BCP) powder. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110663.
- Gao, C.Y.; Huang, Z.H.; Jing, W.; Wei, P.F.; Jin, L.; Zhang, X.H.; Cai, Q.; Deng, X.L.; Yang, X.P. Directing osteogenic differentiation of BMSCs by cell-secreted decellularized extracellular matrixes from different cell types. J. Mater. Chem. B 2018, 6, 7471–7485.
- Safari, F.; Fani, N.; Eglin, D.; Alini, M.; Stoddart, M.J.; Baghaban Eslaminejad, M. Human umbilical cord-derived scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. A 2019, 107, 1793–1802.
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683.
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243.
- Zhang, Y.; He, Y.; Bharadwaj, S.; Hammam, N.; Carnagey, K.; Myers, R.; Atala, A.; Van Dyke, M. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 2009, 30, 4021–4028.
- Son, H.; Jeon, M.; Choi, H.J.; Lee, H.S.; Kim, I.H.; Kang, C.M.; Song, J.S. Decellularized human periodontal ligament for periodontium regeneration. PLoS ONE 2019, 14, e0221236.
- Kim, I.H.; Jeon, M.; Cheon, K.; Kim, S.H.; Jung, H.S.; Shin, Y.; Kang, C.M.; Kim, S.O.; Choi, H.J.; Lee, H.S.; et al. In Vivo Evaluation of Decellularized Human Tooth Scaffold for Dental Tissue Regeneration. Appl. Sci. 2021, 11, 8472.
- Iwasaki, K.; Peng, Y.; Kanda, R.; Umeda, M.; Ishikawa, I. Stem Cell Transplantation and Cell-Free Treatment for Periodontal Regeneration. Int. J. Mol. Sci. 2022, 23, 1011.
- Liang, J.; Yi, P.; Wang, X.; Huang, F.; Luan, X.; Zhao, Z.; Liu, C. Acellular matrix hydrogel for repair of the temporomandibular joint disc. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2995–3007.
- Lee, D.J.; Miguez, P.; Kwon, J.; Daniel, R.; Padilla, R.; Min, S.; Zalal, R.; Ko, C.C.; Shin, H.W. Decellularized pulp matrix as scaffold for mesenchymal stem cell mediated bone regeneration. J. Tissue Eng. 2020, 11, 2041731420981672.
- Dziedzic, D.S.M.; Mogharbel, B.F.; Irioda, A.C.; Stricker, P.E.F.; Perussolo, M.C.; Franco, C.R.C.; Chang, H.W.; Abdelwahid, E.; de Carvalho, K.A.T. Adipose-Derived Stromal Cells and Mineralized Extracellular Matrix Delivery by a Human Decellularized Amniotic Membrane in Periodontal Tissue Engineering. Membranes 2021, 11, 606.
- Iwasaki, K.; Akazawa, K.; Nagata, M.; Komaki, M.; Honda, I.; Morioka, C.; Yokoyama, N.; Ayame, H.; Yamaki, K.; Tanaka, Y.; et al. The Fate of Transplanted Periodontal Ligament Stem Cells in Surgically Created Periodontal Defects in Rats. Int. J. Mol. Sci. 2019, 20, 192.
- Venkatesan, N.; Lavu, V.; Balaji, S.K. Clinical efficacy of amniotic membrane with biphasic calcium phosphate in guided tissue regeneration of intrabony defects- a randomized controlled clinical trial. Biomater. Res. 2021, 25, 15.
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147.
- Imamura, K.; Hamada, Y.; Yoshida, W.; Murakami, T.; Nakane-Koyachi, S.; Yoshikawa, K.; Saito, A. Investigating the Effects of Dehydrated Human Amnion-Chorion Membrane on Periodontal Healing. Biomolecules 2022, 12, 857.
- Adachi, K.; Amemiya, T.; Nakamura, T.; Honjyo, K.; Kumamoto, S.; Yamamoto, T.; Bentley, A.J.; Fullwood, N.J.; Kinoshita, S.; Kanamura, N. Human periodontal ligament cell sheets cultured on amniotic membrane substrate. Oral Dis. 2014, 20, 582–590.
- Goktas, S.; Pierre, N.; Abe, K.; Dmytryk, J.; McFetridge, P.S. Cellular interactions and biomechanical properties of a unique vascular-derived scaffold for periodontal tissue regeneration. Tissue Eng. Part A 2010, 16, 769–780.
- Goktas, S.; Matuska, A.M.; Pierre, N.; Gibson, T.M.; Dmytryk, J.J.; McFetridge, P.S. Decellularization method influences early remodeling of an allogenic tissue scaffold. J. Biomed. Mater. Res. A 2014, 102, 8–16.
- Han, X.; Liao, L.; Zhu, T.; Xu, Y.; Bi, F.; Xie, L.; Li, H.; Huo, F.; Tian, W.; Guo, W. Xenogeneic native decellularized matrix carrying PPARγ activator RSG regulating macrophage polarization to promote ligament-to-bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111224.
- Lee, S.J.; Nah, H.; Heo, D.N.; Kim, K.-H.; Seok, J.M.; Heo, M.; Moon, H.-J.; Lee, D.; Lee, J.S.; An, S.Y.; et al. Induction of osteogenic differentiation in a rat calvarial bone defect model using an In situ forming graphene oxide incorporated glycol chitosan/oxidized hyaluronic acid injectable hydrogel. Carbon 2020, 168, 264–277.
- Nakamura, N.; Ito, A.; Kimura, T.; Kishida, A. Extracellular Matrix Induces Periodontal Ligament Reconstruction In Vivo. Int. J. Mol. Sci. 2019, 20, 3277.
- Gou, M.; Huang, Y.Z.; Hu, J.G.; Jiang, Y.L.; Zhang, X.Z.; Su, N.C.; Lei, Y.; Zhang, H.; Wang, H.; Xie, H.Q. Epigallocatechin-3-gallate Cross-Linked Small Intestinal Submucosa for Guided Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 5024–5035.
- Wilshaw, S.P.; Kearney, J.; Fisher, J.; Ingham, E. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng. Part A 2008, 14, 463–472.
- Semyari, H.; Rajipour, M.; Sabetkish, S.; Sabetkish, N.; Abbas, F.M.; Kajbafzadeh, A.M. Evaluating the bone regeneration in calvarial defect using osteoblasts differentiated from adipose-derived mesenchymal stem cells on three different scaffolds: An animal study. Cell Tissue Bank. 2016, 17, 69–83.
- Bianchi, S.; Bernardi, S.; Simeone, D.; Torge, D.; Macchiarelli, G.; Marchetti, E. Proliferation and Morphological Assessment of Human Periodontal Ligament Fibroblast towards Bovine Pericardium Membranes: An In Vitro Study. Materials 2022, 15, 8284.
- Suzuki, M.; Kimura, T.; Yoshida, Y.; Kobayashi, M.; Hashimoto, Y.; Takahashi, H.; Shimizu, T.; Anzai, S.; Nakamura, N.; Kishida, A. In Vitro Tissue Reconstruction Using Decellularized Pericardium Cultured with Cells for Ligament Regeneration. Polymers 2022, 14, 2351.
- Suzuki, M.; Kimura, T.; Nakano, Y.; Kobayashi, M.; Okada, M.; Matsumoto, T.; Nakamura, N.; Hashimoto, Y.; Kishida, A. Preparation of mineralized pericardium by alternative soaking for soft-hard interregional tissue application. J. Biomed. Mater. Res. A 2023, 111, 198–208.
- Moffat, D.; Ye, K.; Jin, S. Decellularization for the retention of tissue niches. J. Tissue Eng. 2022, 13, 20417314221101151.
- Pereira, A.R.; Trivanović, D.; Stahlhut, P.; Rudert, M.; Groll, J.; Herrmann, M. Preservation of the naïve features of mesenchymal stromal cells in vitro: Comparison of cell- and bone-derived decellularized extracellular matrix. J. Tissue Eng. 2022, 13.
More
