Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Camila Xu and Version 2 by Raffaele Serra.
There are three main families of metalloproteinases (MPs) that are involved in human health and disease: (1) the “matrix metalloproteinase” (MMP) family, (2) the “a disintegrin and metalloprotease” (ADAM) family, and (3) the “a disintegrin and metalloproteinase with thrombospondin motifs” (ADAMTS) family. MPs are relevant to public health because of their role in several diseases and, most of all, their role as biomarkers that also impact the quality of life and the psychosocial dimension of affected patients. In this context, new pathways to precision health and precision medicine have been opened in the area of MPs.
- metalloproteinases
- MMP
- ADAM
- ADAMTS
- history
- complexity
1. Introduction
There are three main families of metalloproteinases (MPs) that are involved in human health and disease: (1) the “matrix metalloproteinase” (MMP) family, (2) the “a disintegrin and metalloprotease” (ADAM) family, and (3) the “a disintegrin and metalloproteinase with thrombospondin motifs” (ADAMTS) family. All these families belong to the superfamily of zinc endopeptidases, which are called metzincins [1,2,3,4,5,6,7][1][2][3][4][5][6][7].
In 1962, MMP families began to be studied and Woessner published the first study on this topic demonstrating that a protein derived from a rat was able to digest collagen [8]. In particular, the mammalian uterus is one of the most favorable tissues for studying protein catabolism, especially collagen, under physiological conditions, and in fact, in this study, collagen disappeared in the postpartum period during uterus involution in a rate-limiting model, suggesting the role of specific enzyme activity. Later in the same year (1962), Gross et al. [9] showed the rapid collagenolytic activity of large tissue masses in an anuran tadpole (tail, gill, gut, skin) during natural and hormone-induced metamorphosis, and this suggested the need to identify specific enzymes able to remove particular structural components during normal growth and development. Later, in 1966, Nagai et al. purified from a tadpole the first member of the MMP family, MMP-1, initially called tadpole collagenase [10]. In that period, several MMPs and tissue inhibitors of metalloproteinases (TIMPs) were characterized [11] and, ultimately, in 1988, Birkedal-Hansen published the first review on the MMP family [12].
In 1987, the first member of the ADAM family, ADAM-1, initially known as fertilization protein PH-30 or fertilin-α, was identified; subsequently, in 1992, ADAMs were actually classified as a new family of MPs. In particular, ADAM-1 was found to act as a fusion peptide during sperm–egg fusion due to cell adhesion and protease properties; hence, it was evident that it has a role in sperm–egg interactions. Furthermore, ADAMs are also similar to the snake venom metalloproteinase family (SVM), also known as the snake venom disintegrins family [13,14,15][13][14][15].
In 1997, the ADAMTS1 gene, expressed in the cachexigenic colon 26 adenocarcinoma sublines, and the relative protein ADAMTS-1, the first member of the ADAMTS family, were characterized [16]. MPs are directly related to the homeostasis of the extracellular matrix (ECM), a biochemical center that includes collagen, elastin, and other proteins that are involved in providing structural and functional support to several tissues [7].
2. The Matrix Metalloproteinase (MMP) Family
At present, there are 28 MMP family members in vertebrates, of which at least 23 are present in humans, and they may be secreted as soluble enzymes or be bound to the cell membranes (the so-called membrane-type (MT) MMPs). Furthermore, MT-MMPs may be bound to the cell membranes by a COOH-terminal transmembrane domain or by a glycosylphosphatidyl-inositol (GPI) anchor [7]. Generally, MMPs have a signal peptide that serves to lead them to the endoplasmic reticulum (ER), a prodomain that serves to maintain them as inactive zymogens, a catalytic domain with three histidine residues bound to a zinc-binding site, and a proline-rich hinge region and a C-terminal hemopexin-like (HPX) domain (not present in some MMPs, such as MMP-7, MMP-23, and MMP-26) involved in substrate binding (Figure 1).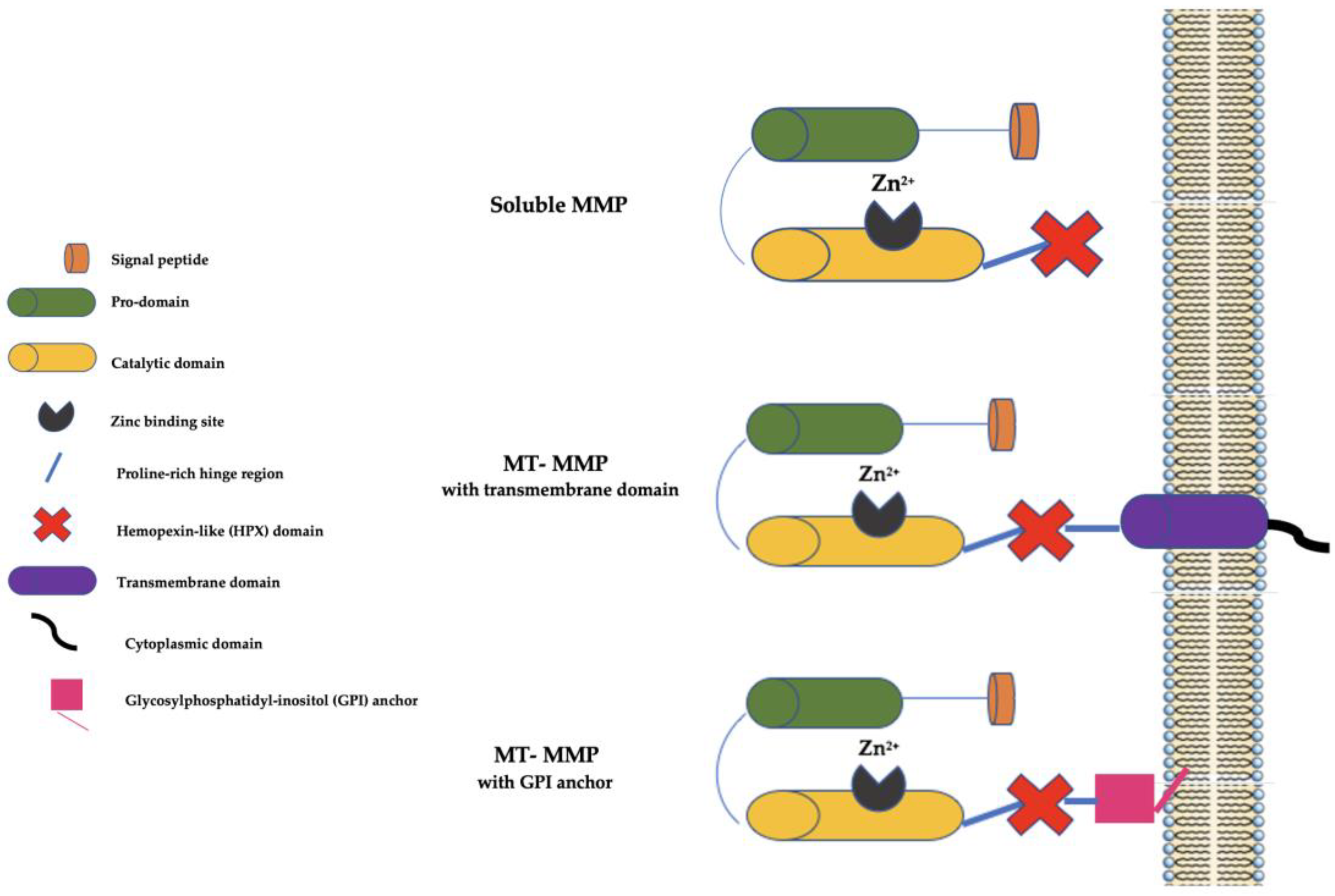
Figure 1. Schematic representation of MMPs. MMP: matrix metalloproteinase; MT-MMP: membrane type-matrix metalloproteinase; GMPI: glycosylphosphatidyl-inositol; Zn2+: Zinc ion.
Table 1.
MMP family members.
| Subgroup | Members | Enzymatic Name |
|---|---|---|
| Collagenases | MMP-1 MMP-8 MMP-13 MMP-18 |
Collagenase-1 Collagenase-2/neutrophil collagenase Collagenase-3 Collagenase-4 |
| Gelatinases | MMP-2 MMP-9 |
Gelatinase A Gelatinase B |
| Stromelysins | MMP-3 MMP-10 MMP-11 MMP-19 MMP-27 |
Stromelysin-1/transin-1 Stromelysin-2/transin-2 Stromelysin-3 Stromelysin-4/RASI-1 n/d |
| Matrilysins | MMP-7 MMP-26 |
Matrilysin-1/putative MMP (PUMP) Matrilysin-2 |
| MT-MMPs | MMP-14 MMP-15 MMP-16 MMP-4/MMP-17 MMP-5/MMP-24 MMP-6/MMP-25 |
MT1-MMP MT2-MMP MT3-MMP MT4-MMP * MT5-MMP * MT6-MMP |
| Ungrouped MMPs | MMP-12 MMP-20 MMP-21 MMP-22 MMP-23 MMP-28 |
Macrophage metalloelastase Enamelysin Xenopous MMP (XMPP) Chicken MMP (CMMP) Cysteine array MMP (CA-MMP) Epilysin |
MMP: matrix metalloproteinase; MT-MMP: membrane-type matrix metalloproteinase. * = presence of glycosylphosphatidyl-inositol (GPI) anchor.
For an enzyme to be included in the MMP family, it should meet the following criteria: (a) proteolytic activity in at least one ECM component; (b) enzymatic activity dependent on zinc at the catalytic site; (c) activation of the proenzyme by proteinases or organomercurials; (d) inhibition by TIMPs or other molecules, such as ethylenediaminetetraacetic acid or 1,10-phenantroline; and (e) related cDNA with sequence homology with MMP-1. The previous further criterion that the enzyme should be secreted in a proform is no longer valid as some MMPs are secreted in active form and others are not secreted at all [11].
MMPs’ activity is specifically regulated by several transcriptional, post-transcriptional, and post-translational modifications that can also lead to different isoforms and/or variants of MMPs. MMPs’ expression is also accurately modulated by specific control of their secretion, activation, and inhibition. MMPs are generated by several cells, such as endothelial cells (ECs), vascular smooth muscle cells (VSMCs), lymphocytes, cytotrophoblasts, fibroblasts, osteoblasts, macrophages, and neutrophils. MMPs’ expression is induced by their biochemical stimuli, such as cytokines, hormones, and growth factors, or by cellular stimuli, such as interactions between cells and matrixes and between cells [7].
Concerning MMP measurement techniques, initially, and for more than 30 years, it was thought that MMPs can be evaluated only by zymography, an electrophoretic technique that measures proteolytic activity [11,24][11][23]. In fact, modern and ultrasensitive enzyme immunoassay methods and biosensor-based measurements are able to effectively detect both pro- and active forms of MMPs [11,25][11][24]. This is an important achievement as, while in the past pro-MMPs were thought to be inactive, it was later demonstrated that even MMP proforms have certain enzymatic and biological activities [11,26][11][25]. This can be explained by considering that, in the first 30 years of research in the area of MMPs, there were no specific antibodies to recognize pro- and activated forms and zymograms were pivotal to measure MMP activity. Current technologies make it possible to measure the activity of all forms of MMPs [11].
In the past, it was also thought that MMPs are able to interact only with ECM proteins but it has been demonstrated that their ubiquitous actions also affect the extracellular and intracellular signaling of several cell types [11,27,28][11][26][27].
Moreover, in the past, MMPs were thought to have only negative effects on human health, but their roles in physiology and health have been elucidated [11]. In fact, MMPs have specific and important roles in turnover of the ECM, embryogenesis, tissue morphogenesis, bone development, angiogenesis, migration of immune cells, the menstrual cycle, involution of the endometrium after pregnancy, wound repair, learning, memory, and the cortical plasticity of the brain [29,30,31,32,33,34,35][28][29][30][31][32][33][34].
Most MMPs are located in certain cells and tissues where they regulate several physiological functions. MMP-1 is found in chondrocytes, fibroblasts, keratinocytes, endothelial cells, and macrophages; MMP-2, MMP-3, and MMP-7 are ubiquitous; MMP-8 and MMP-25 are found in polymorphonuclear leukocytes (neutrophils, macrophages, and plasma cells); MMP-9 is mainly located in macrophages, granulocytes, T-cells, dendritic cells, epithelial cells, fibroblasts, keratinocytes, and osteoblasts; MMP-10 is located in epithelial cells and hepatocytes; MMP-11 is mainly found in fibroblasts; MMP-12 is primarily found in the lung and the ear tissues; MMP-13 is found in bone, cartilage, epithelial cells, and neuronal cells; MMP-14 and MMP-15 are mainly located in the cells of the head, neck, and ear; MMP-17 is located in the brain, leukocytes, and colon, ovary, testis, and breast tissues; MMP-18 is ubiquitous in all stromal cells except in the liver and brain; MMP-19 can be found in epithelial cells and fibroblasts; MMP-20 is located in tooth enamel; MMP-21 can be found in stromal cells in the kidneys, skin, and intestines; MMP-23 is expressed in the ovary, testis, and prostate; MMP-26 is expressed in endometrial and placental tissues; MMP-27 is expressed in B-cells; and MMP-28 can be found in the testes, lungs, kidneys, pancreas, and keratinocytes [36][35].
MMPs are involved in several types of diseases and generally related to three main mechanisms: tissue destruction, such as cancer (e.g., acute myeloid leukemia and bladder, brain, breast, colorectal, endometrial, gastric, head and neck, liver, lung, ovarian, pancreas, prostate, renal, skin, and thyroid cancer), diabetes, inflammatory diseases (e.g., psoriasis, osteoarthritis, rheumatoid arthritis, inflammatory bowel disease), chronic wounds (e.g., pressure ulcers, vascular ulcers), periodontal diseases, hypertension, kidney diseases (e.g., chronic kidney disease, glomerular disease), myocardial infarction, and neurogenerative disease (e.g., multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis); fibrosis, such as liver cirrhosis, otosclerosis, gynecological disorders (e.g., polycystic ovarian syndrome, spontaneous abortion, preeclampsia), and atherosclerosis-related diseases; and weakening of the ECM, such as chronic venous disease, pulmonary embolism, aneurysms, and dilated cardiomyopathy. Often, more than one mechanism overlaps in determining the disease [2,3,7,11,19,22,37,38,39,40,41,42,43,44,45,46,47,48,49,50][2][3][7][11][18][21][36][37][38][39][40][41][42][43][44][45][46][47][48][49].
 The disintegrin is important in cell–cell and cell–matrix interactions, and the cysteine-rich domain has cell adhesive and fusogenic properties [15]. The EGF-like domain, transmembrane domain, and cytoplasmic domain are pivotal for the signaling of ADAMs [13].
ADAMs are generally in inactive form, similarly to other metalloproteinases, and can be activated by proteolytic processing of the prodomain by different molecules (e.g., cytokines, proteinases, Ca2+ ionophores, protein kinase activators) generally located intracellularly in the Golgi system. Moreover, some ADAM members may be activated in different sites (in the ECM, such as ADAM-8 and ADAM-28) or in different modalities (auto-catalytic removal of prodomains or without the necessity of activation, such as ADAM-12, which is stored as already active in the cell and internally transferred to appropriate sites to act) [13].
Between the 1990s and the first decade of the 2000s, ADAMs were widely studied and several novel characteristics that went far beyond their role as fertilization proteins were discovered. In fact, over time, some important and specific features of the ADAM family have been extensively clarified [15]. ADAM proteins have sheddase activity; that is, the ability to cleave membrane proteins in order to release the extracellular domain (ectodomain shedding). This mechanism represents a post-translational modification that is important in regulating membrane shape and controlling the levels and functions of numerous membrane-bound proteins and acts in several physiological and pathological pathways related to growth factor signaling, cell adhesion, chronic inflammation, and apoptosis [52,53,54][51][52][53]. Moreover, the released ectodomain may, after shedding, influence distant targets, and this provides evidence of the paracrine and autocrine signaling activity of ADAMs [15,55,56][15][54][55].
Another important mechanism related to sheddase activity is so-called regulated intramembrane proteolysis (RIP), which, after the year 2000, was also studied in the ADAM family. In particular, after shedding the ectodomain due to ADAM activity, other proteases may synergistically act as the intramembrane cleavers of the intracellular part of the protein, and this intracellular stub may trigger intracellular signaling activity. ADAMs may mediate the RIP activity of several proteins involved in numerous biological processes [15,57][15][56].
ADAMs are involved in several physiological conditions related to embryogenesis, the cardiovascular system, tissue repair, and the human reproductive system (in particular, the uterus, testis, and epididymis). ADAMs are also involved in several diseases, such as rheumatoid arthritis, asthma, cardiovascular diseases (e.g., myocardial infarction, aneurysms, chronic venous disease, vascular ulcers), neurologic diseases (e.g., Guillain–Barrè syndrome, multiple sclerosis, Alzheimer’s disease), gynecological disease, and cancer (e.g., breast cancer). In particular, ADAMs have important roles in vascular biology controlling vascular smooth muscle cell (VSMC) functions, proliferation, migration, and apoptosis [2,3,13,15,58][2][3][13][15][57].
The disintegrin is important in cell–cell and cell–matrix interactions, and the cysteine-rich domain has cell adhesive and fusogenic properties [15]. The EGF-like domain, transmembrane domain, and cytoplasmic domain are pivotal for the signaling of ADAMs [13].
ADAMs are generally in inactive form, similarly to other metalloproteinases, and can be activated by proteolytic processing of the prodomain by different molecules (e.g., cytokines, proteinases, Ca2+ ionophores, protein kinase activators) generally located intracellularly in the Golgi system. Moreover, some ADAM members may be activated in different sites (in the ECM, such as ADAM-8 and ADAM-28) or in different modalities (auto-catalytic removal of prodomains or without the necessity of activation, such as ADAM-12, which is stored as already active in the cell and internally transferred to appropriate sites to act) [13].
Between the 1990s and the first decade of the 2000s, ADAMs were widely studied and several novel characteristics that went far beyond their role as fertilization proteins were discovered. In fact, over time, some important and specific features of the ADAM family have been extensively clarified [15]. ADAM proteins have sheddase activity; that is, the ability to cleave membrane proteins in order to release the extracellular domain (ectodomain shedding). This mechanism represents a post-translational modification that is important in regulating membrane shape and controlling the levels and functions of numerous membrane-bound proteins and acts in several physiological and pathological pathways related to growth factor signaling, cell adhesion, chronic inflammation, and apoptosis [52,53,54][51][52][53]. Moreover, the released ectodomain may, after shedding, influence distant targets, and this provides evidence of the paracrine and autocrine signaling activity of ADAMs [15,55,56][15][54][55].
Another important mechanism related to sheddase activity is so-called regulated intramembrane proteolysis (RIP), which, after the year 2000, was also studied in the ADAM family. In particular, after shedding the ectodomain due to ADAM activity, other proteases may synergistically act as the intramembrane cleavers of the intracellular part of the protein, and this intracellular stub may trigger intracellular signaling activity. ADAMs may mediate the RIP activity of several proteins involved in numerous biological processes [15,57][15][56].
ADAMs are involved in several physiological conditions related to embryogenesis, the cardiovascular system, tissue repair, and the human reproductive system (in particular, the uterus, testis, and epididymis). ADAMs are also involved in several diseases, such as rheumatoid arthritis, asthma, cardiovascular diseases (e.g., myocardial infarction, aneurysms, chronic venous disease, vascular ulcers), neurologic diseases (e.g., Guillain–Barrè syndrome, multiple sclerosis, Alzheimer’s disease), gynecological disease, and cancer (e.g., breast cancer). In particular, ADAMs have important roles in vascular biology controlling vascular smooth muscle cell (VSMC) functions, proliferation, migration, and apoptosis [2,3,13,15,58][2][3][13][15][57].
 At present, 20 ADAMTS members are known. They are secreted metalloproteinases, and most of their targets are represented by ECM components. Similarly to ADAMs, they have several physiological roles in the same biological system (reproductive, cardiovascular, etc.) and particular functions in vascular biology where they are able to control several pathways and process. ADAMTSs, similarly to ADAMs, may act as sheddases and can activate several cell surface proteins [13,60][13][59]. ADAMTs are involved in the same diseases in which ADAMS are involved, but they have a particular tropism towards cardiovascular disease (heart valve disease, coronary artery disease, aneurysms, chronic venous disease, hypertension) [61][60]. In this family, ADAMTS-13 is one of the most studied members. ADAMTS-13 controls hemostasis and thrombosis pathways and the dysregulation of this MP may lead both to bleeding and thrombosis. Moreover, ADAMTS-13 is considered an important factor related to several diseases characterized by vascular inflammation and thrombosis, such as cancer, vascular diseases, infections, neurological diseases, and liver diseases [62][61]. Furthermore, low ADAMTS-13 levels seem to increase the risk of ischemic stroke [61,63][60][62]. Several members of this family are also pivotal in the development of the heart and vessels. In particular, ADAMTS-7 is related to coronary artery disease (CAD) and may represent a potential therapeutic target, and ADAMTS-9 and ADAMTS-19 are associated with heart valve disease [63][62]. ADAMTS-1, ADAMTS-4, and ADAMTS-7 are also involved in chronic venous disease (CVD) [3].
At present, 20 ADAMTS members are known. They are secreted metalloproteinases, and most of their targets are represented by ECM components. Similarly to ADAMs, they have several physiological roles in the same biological system (reproductive, cardiovascular, etc.) and particular functions in vascular biology where they are able to control several pathways and process. ADAMTSs, similarly to ADAMs, may act as sheddases and can activate several cell surface proteins [13,60][13][59]. ADAMTs are involved in the same diseases in which ADAMS are involved, but they have a particular tropism towards cardiovascular disease (heart valve disease, coronary artery disease, aneurysms, chronic venous disease, hypertension) [61][60]. In this family, ADAMTS-13 is one of the most studied members. ADAMTS-13 controls hemostasis and thrombosis pathways and the dysregulation of this MP may lead both to bleeding and thrombosis. Moreover, ADAMTS-13 is considered an important factor related to several diseases characterized by vascular inflammation and thrombosis, such as cancer, vascular diseases, infections, neurological diseases, and liver diseases [62][61]. Furthermore, low ADAMTS-13 levels seem to increase the risk of ischemic stroke [61,63][60][62]. Several members of this family are also pivotal in the development of the heart and vessels. In particular, ADAMTS-7 is related to coronary artery disease (CAD) and may represent a potential therapeutic target, and ADAMTS-9 and ADAMTS-19 are associated with heart valve disease [63][62]. ADAMTS-1, ADAMTS-4, and ADAMTS-7 are also involved in chronic venous disease (CVD) [3].
3. The “A Disintegrin and Metalloprotease” (ADAM) Family
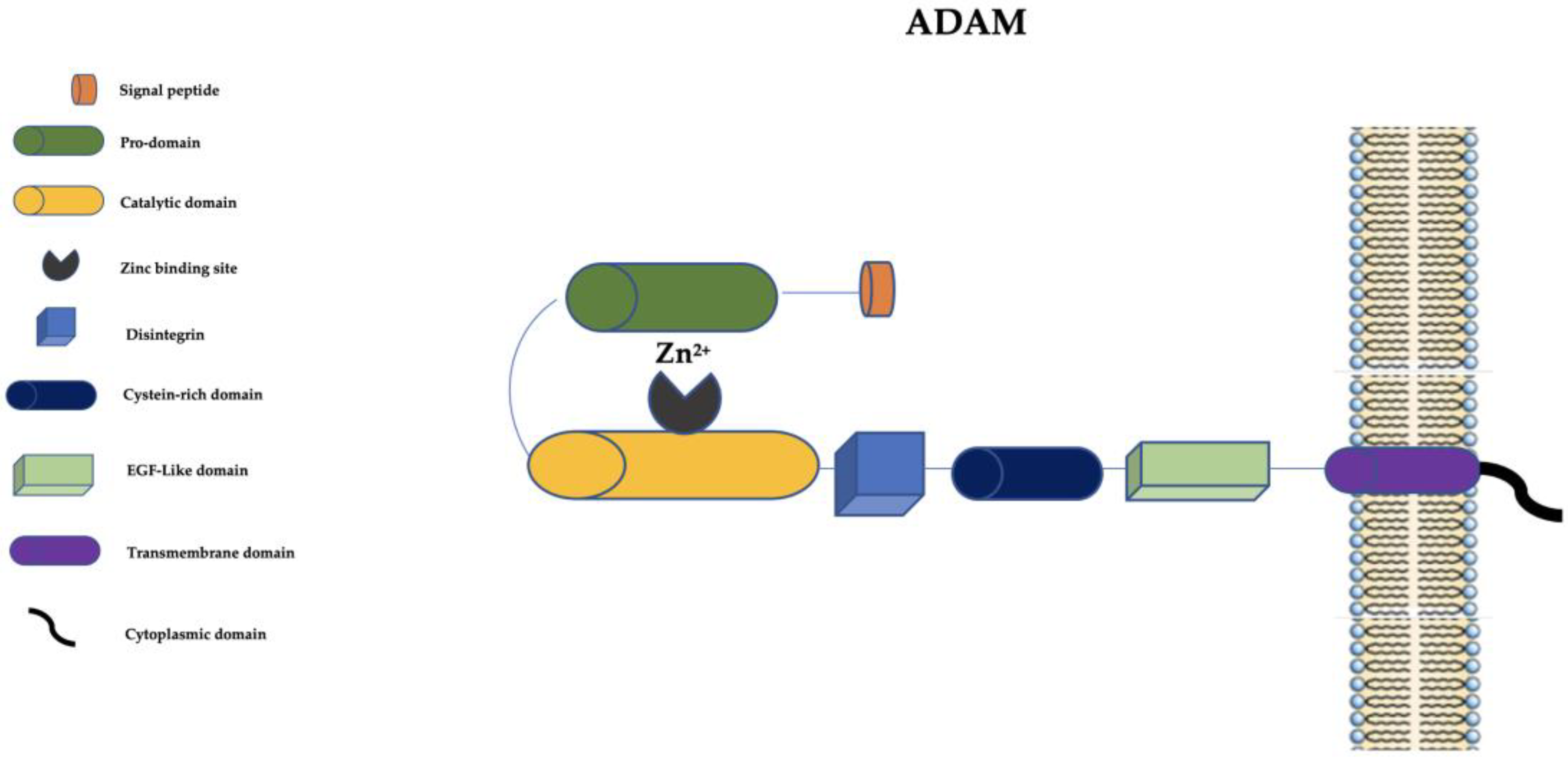
At present, in humans, there are 21 ADAM family members. Among them, 13 are catalytically active (ADAM-8, ADAM-9, ADAM-10, ADAM-12, ADAM-15, ADAM-17, ADAM-19, ADAM-20, ADAM-21, ADAM-28, ADAM-30, ADAM-33, and ADAM-DEC1) and 8 seem to be catalytically inactive [51][50]. ADAMs are structurally similar to MMPs but they have a disintegrin, a cysteine-rich domain, and an epidermal growth factor-like (EGF-like) domain (Figure 2).
Figure 2. Schematic representation of ADAMs. ADAM: a disintegrin and metalloprotease; EGF-like: epidermal growth factor-like; Zn2+: Zinc ion.
4. The “A Disintegrin and Metalloprotease with Thrombospondin Motifs” (ADAMTS) Family
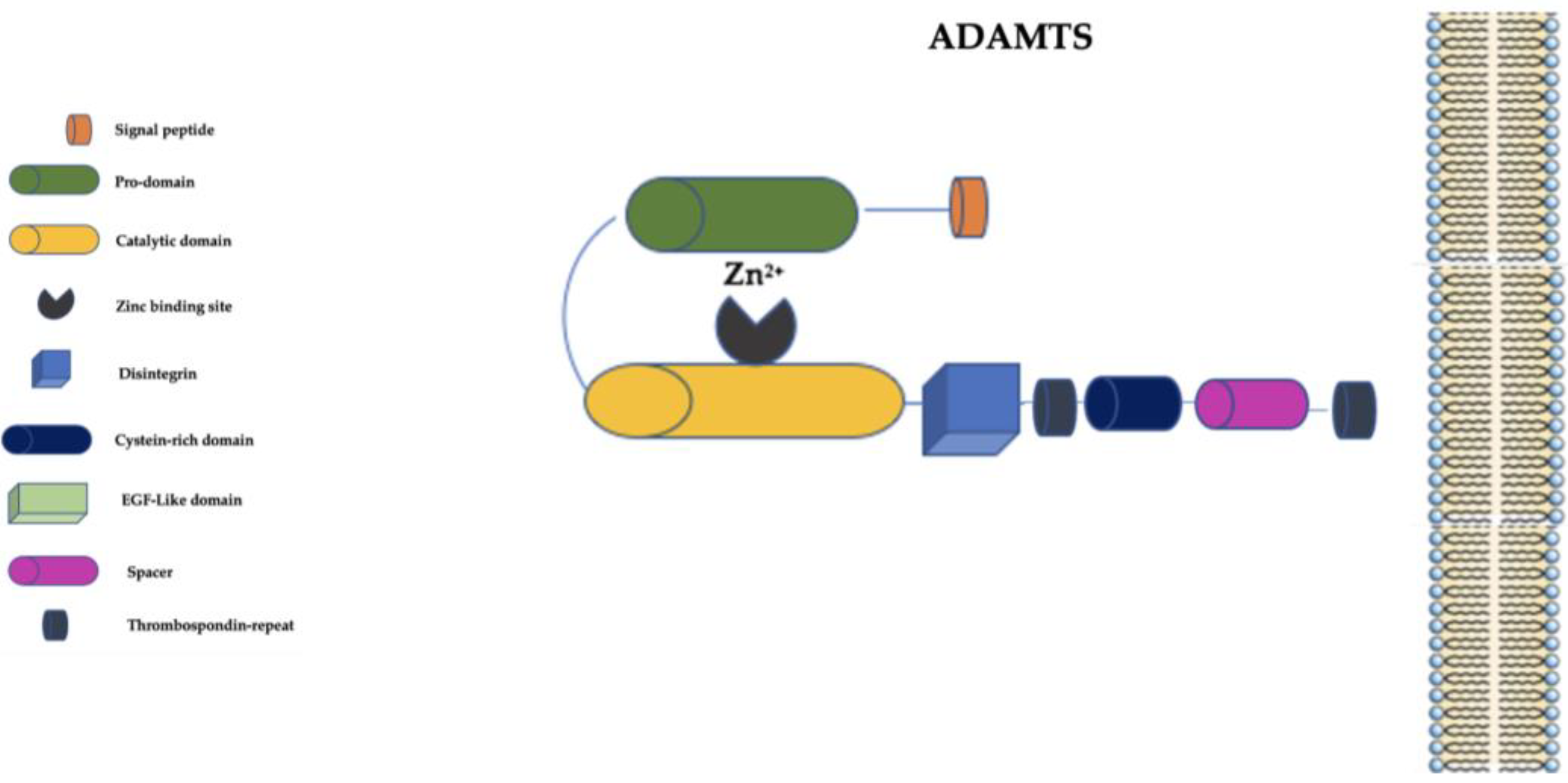
The ADAMTS family is distinguished from the ADAM family primarily by the presence of a thrombospondin-repeat domain and the lack of EGF-like transmembrane and cytoplasmic domains (Figure 3) [59][58].
Figure 3. Schematic representation of ADAMTS family. ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs; EGF-Like: epidermal growth factor-like; Zn2+: Zinc ion.
5. Inhibition of Metalloproteinases
The inhibitory activity of TIMPs was discovered in the early 1970s when an enzyme was found in the form of a collagenase inhibitor isolated from cultured human skin fibroblasts and human serum and in bovine cartilage and aorta tissues [64][63]. This was the first member of TIMP family (TIMP-1), which was definitely characterized in 1975 [65][64]. Later, three further members of this family (TIMP-2, TIMP-3, and TIMP-4) were also discovered [66][65]. TIMPs have multiple roles not limited to MP inhibition, including as growth factors with mitogenic effects and as regulators of apoptosis and several immunological processes [66][65]. In a physiological environment, MPs are controlled by tissue inhibitors of metalloproteinases (TIMPs), which may inhibit both the proforms and activated forms of MPs [22][21]. In 1994, the crystal structure of a collagenase–inhibitor complex was identified in human fibroblasts, and soon after, in 1997, the first MMP-null mouse was created. From this moment on, several MMP-null mouse models have been available for research, thus highlighting the importance of inhibiting MPs’ activity in various circumstances [11]. In the context of MPs, TIMPs have wide-range inhibitory effects on most MMP family members but specific inhibitory effects on ADAMs and ADAMTSs. In particular, the ratios of MP families and TIMPs are important for the maintenance of the normal structure of the ECM, and when this ratio is altered, ECM imbalance can lead to weaknesses in several tissues’ environments. Furthermore, there are also several broad cellular effects from TIMP activity [13,58,67,68,69][13][57][66][67][68]. Several studies have tried to find artificial MP inhibitors to contrast with the effects of MMPs in tissues, especially in the inhibition of metastasis and cancer progression and several vascular diseases, such as chronic ulceration of the lower limbs. In particular, some drugs used primarily for a variety of diseases, such as tetracyclines (doxycycline and minocycline), sulodexide, cilostazol, and statins, seem to have also inhibitory effects on MMPs in vascular disease contexts, but their specific use for MP inhibition has not been definitely validated at this time [22,70,71,72,73,74][21][69][70][71][72][73]. With regard to specific MP inhibition, several molecules have been studied. The first generation of MP inhibitors (MPIs), studied in the middle and late 1990s, are represented by hydroxamate-based inhibitors (containing high-affinity zinc-binding groups able to act through peptidomimetics’ activity). Batimastat and marimastat are the most well-known in this category, and they have demonstrated broad inhibitory effects towards the MMP, ADAM, and ADAMTS families and more specific anticancer effects in animal models. Batimastat reached only phase-one clinical trials, while marimastat reached phase-two and -three clinical trials. Both showed several important side effects related to musculoskeletal syndrome or other inflammatory effects. Moreover, these molecules were pharmacologically unstable with overly short half-lives. All these issues prevented the clinical utilization of the first generation of MPIs [22,75][21][74]. In the first decade of the 21st century, a new MPI group with non-hydroxamate-based inhibitors was tested. Considering that the side effects seem to be related to the strong anchoring to the zinc-binding site, which causes the alteration of the orientation of the molecules, this group has less affinity for Zn2+ compared to hydroxamate-based inhibitors and has been proved to reduce side effects but with less specificity and a weaker therapeutic effect. This group includes tanomastat, metastat, and doxycycline. Although these compounds show better tolerability, their efficacy is controversial. Catalytic-domain non-zinc-binding inhibitors represent the third generation of MPIs, which specifically target the catalytic domain of MPs without affinity for the zinc binding group but with high affinity for some pockets near the zinc binding site (respectively, on the right and left sides). The aim of these new compounds was to increase the affinity and thus reduce side effects. Some natural compounds were identified in this group, such as caffeates and flavonoids, but the collected data show no superiority compared to the previous groups of inhibitors. The fourth generation of MPIs is represented by allosteric and exosite inhibitors, which target other MP domains, such as hemopexin or the propeptide domain. At the moment, the efficacy of inhibition at these sites is quite low. At this time, no specific MPs inhibitor has been found that has both safety and specificity characteristics and can be effectively used in clinical practice [22,75][21][74].6. Metalloproteinases as Biomarkers
As MPs and TIMPs can be secreted by several cells and found in all body fluids (e.g., plasma, serum, saliva, urine, etc.) and tissues, they can be easily sampled and measured for use as biomarkers of disease in the areas of precision health and precision medicine, which are novel and specific areas of medicine that seek to identify personalized solutions to diseases, integrating biological variables, interindividual variability, and a wide range of determinants of health. In this context, a biomarker is any analyte related to biological functions or to clinical, imaging, or other related factors, such as social, psychological, economic, and environmental determinants, that can give useful information on disease susceptibility, characteristics, staging, evolution, and response to treatment [1]. Several diseases are based on ECM imbalance and disruption. Staging and progression to complications often parallel MP levels. In other circumstances, MPs’ level patterns are arranged typically in certain patients, and this may account for different treatment options. Specific MP patterns can even predict that some patient populations may have a greater risk for certain complications compared to others, suggesting tailored follow-up programs. There is evidence of these applications with several diseases, such as cardiovascular disease (e.g., atrial fibrillation, aneurysms, chronic venous disease, venous thromboembolism, vascular ulcers), cancer (i.e., lung carcinoma, ovarian cancer), inflammatory diseases (i.e., rheumatoid arthritis), oral diseases (i.e., periodontal disease), proctologic diseases (i.e., hemorrhoids), infective diseases (i.e., COVID-19), and neurodegenerative diseases (i.e., Alzheimer’s disease) [76,77,78,79,80,81,82,83,84,85,86][75][76][77][78][79][80][81][82][83][84][85].7. Metalloproteinases and the Paradigm of Complexity
The history of MPs is complex, just as the world of molecular biology is complex. The concept of complexity and its relationship with a broad and multidisciplinary approach found its first formulation between the 1960s and 1970s [87][86]. Thus, the paradigm of complexity can be helpful in understanding and studying health and disease as a set of environmental, biological, social, political, economic, and natural factors [88][87]. MPs are evidently “complex” in the usual sense of the word, as they are endowed with the emergent properties of spontaneity and self-organization [89][88]. Thus, the complex approach has become a challenge for every form of knowledge, all of which are called to integrate, mix, and deal with apparently distant disciplines, such as the human sciences [87][86]. In this context, Edgar Morin was one of the first intellectuals who proposed mixing knowledge through the paradigm of complexity [90][89]. In the context of MPs, Morin’s theories find a significant application given their great influence on the concepts of health and disease [91][90]. All this stimulates a move away from the reductionist vision of biology, and biomedical sciences in general, to arrive at a complex vision of phenomena, including the biomolecular phenomena, that is no longer only bound to the cellular dimension but in constant interchange with the material and social environment, both physical and metaphysical. The effects mediated by MPs, as Morin explains, only exist when they are interpreted by living beings, and they are inseparable from life [92][91]. That is why today the paradigm of complexity still finds wide applications in biology [87][86]. The idea that proteins and other molecules always exist in one environment from which they receive information is largely outdated, as is the idea of emergent properties that cannot be predicted from knowledge of linear, mechanistic interactions between the constituent components of a system [93][92]. In this regard, MPs are related to a series of diseases that have strong complex connotations; i.e., they also have implications for and impacts on the psychosocial sphere [94][93]. In the context of diabetes and the complications associated with it, the psychosocial impact is particularly relevant [95][94]; in fact, alongside the biomedical dimension of this pathology, forms of diabetes distress, psychiatric comorbidities, inadequate support from society and family, and diabetes hearsay emerge [96][95]. Even in the field of wounds, in which MPs play a central role in the etiopathogenesis, a complex vision is required, since wounds have an important impact on the social and psychological lives of patients [97][96]. In particular, there is evidence of a certain link between psychological stress and the wound healing process and, furthermore, there are important positive results from the transdisciplinary approach in wound management [98][97]. In the context of diabetic foot ulcers (DFUs), MPs are considered specific targets for wound healing [99][98]. In particular MMP-9 is considered a specific molecular target for wound healing in relation to DFUs [100[99][100],101], and cilostazol, a drug used for peripheral artery disease (PAD), seems to have an effect that lowers MMP-9 levels, suggesting a beneficial effect that could be used to prevent or retard the onset of DFUs in diabetic patients [73,102][72][101]. MMP-9 is an important contributor to the pathophysiology of depression [103][102], and depression is widely prevalent among DFU patients. This strict interplay between more biological and clinical variables explains the complexity of MPs’ effects. With regard to chronic venous disease (CVD), observation of MPs’ families and levels may be used to describe the entire course of this chronic disease, from onset and progression to response to clinical management. In particular, serum elevation of MMP-2, ADAMTS-1, and ADAMTS-7 correlates well with the initial stages of CVD, whereas the serum elevation of MMP-1, MMP-8, MMP-9, ADAM-10, ADAM-17, and ADAMTS-4 is particularly involved in skin change complications, including chronic venous leg ulcers (CVLUs) [3]. In the context of kidney diseases, the relationship between the biological and psychosocial dimensions becomes very interesting when the psychosocial sphere impacts the perception of a nephrological disease: participation in the monitoring process in the initial stages of a nephrological disease; the ability to collaborate in hospital treatment and/or at home; the possible acceptance of a transplant; etc. [104][103]. For example, in this type of disease, the relationship between the biological sphere of chronic kidney disease and depression is very strong, as the disease has a negative impact on quality of life, self-care behavior, and mortality [105][104]. Furthermore, the complexity paradigm applied to MPs highlights an important correlation with neurodegenerative diseases [106][105]. Moreover, in this case, there are even psychosocial repercussions that underline the need for an overall vision capable of integrating the biological dimension with the psychological, social, environmental, etc., since neurodegenerative diseases are associated with emotional lability, reduced social interactions, declining to engage in mealtime tasks, and increased distractibility [107][106]. MPs, as has already been reported, also have a crucial role in cardiovascular diseases, which, once again, have a “complex” aspect. In fact, several biological, social, psychological, and environmental triggers that cause cardiovascular diseases have been identified in recent years [108,109,110][107][108][109]. For example, among patients with cardiovascular disease, psychosocial problems, such as depression and/or anxiety, are very frequent and have significant effects, such as rehospitalization and effects related to all-cause and cardiovascular mortality; unhealthy lifestyles; reduced social interactions; poor quality of life, etc. [110,111,112][109][110][111]. Since these are problems that affect sexuality, the psychological, social, and environmental impacts become central not only in the interaction with the partner but first of all in the bodies of women with gynecological problems, with which depression, stigma, and subsequent reductions in social interactions are related [113,114,115][112][113][114]. Another complex aspect of MPs is their peculiar and constant relationship with the same cell population across different diseases. For example, there is a strict link between MPs and the phenotype of macrophages M1–M2 in different diseases, such as atherosclerosis, and related clinical manifestations, such as in vascular diseases, cancer, wound healing, and osteoarthritis [116,117,118,119][115][116][117][118]. Most pivotal activation pathways for MPs in the macrophage population probably share the same pathological mechanisms, independently of the specific disease and the specific biologic environment. With regard to cardiac surgery procedures, including cardiopulmonary bypasses (CPBs), MPs have been found to be involved in the inflammatory response to heart surgery and CPBs. In particular, MMP-2 and MMP-9 are released at high levels during surgery, whereas MMP-9 levels increase after the start of CPBs and during their entire duration. MPs are overexpressed both in the myocardial tissue and in circulation in the bloodstream. Surgical complications seem to be strictly related to MP elevations during surgery, and future research on specific MP inhibitions may improve clinical outcomes for patients undergoing cardiac surgery [42,120][41][119]. Moreover, elevated MMP-2 levels are able to affect cardiac ECM’s collagen turnover, leading to hypertrophic cardiomyopathy (HCM). MMP-2 levels may help in monitoring myocardial remodeling process following surgical or percutaneous treatments for HCM [121][120]. Finally, in the case of inflammatory diseases also, such as arthritic diseases and chronic obstructive pulmonary disease (COPD), the interconnection between the biological sphere and the psychological and social spheres is very relevant. In the case of osteoarthritis, the social context, age, and any work activity have important relevance during the disease and are linked to the degree of perception and acceptance of the disability that this disease entails [122,123][121][122]. Moreover, in the case of COPD, the difficulty entailed by the disease significantly reduces the quality of life in social, psychological, and environmental terms such that positive or negative variations significantly affect the disease [124][123]. In the case of cancer, the paradigm of complexity finds its maximum application since many researchers have noticed how evident the interaction between biological, psychological, and social factors is [125,126,127][124][125][126]. Specifically, the experience of this disease involves the interaction of a multitude of variables, such as aspects relating to illness, treatment, coping, social support, social determinants of health (SDHs) distress, and psychological distress [128,129][127][128]. Interestingly, a recent study showed how important social disparities in the context of cardiovascular disease are significantly associated with probable biological pathways linked to protein imbalance, including MPs. In particular, among the 48 studied biomarkers, MMP-12 was strongly and negatively associated with the length of education of the studied subjects. All biomarkers in the studied subjects also related to inflammation pathways. Nevertheless, at the moment, the underlying pathophysiologic mechanisms cannot yet be explained [130][129]. From all this, it can be seen that the complexity paradigm is particularly promising in the field of health and disease [131][130]. Thus, this complex view of MPs could be adopted by researchers to realize multidisciplinary practices and theories capable of responding to biological, individual, social, and systemic needs [132][131]. In this context, the complexity paradigm—and considering actual growing computing power—could stimulate the possibility of developing, implementing, and studying mathematical predictive models using clinical data, biological data, SDHs, and knowledge of MPs to describe disease-related characteristics. For example, fuzzy logic is a flexible mathematical system that has been seen considered a powerful tool for decision making and pattern classification in clinical settings. In fact, this mathematical system can allow very fast computations of large sets of heterogeneous data from patients; therefore, it uses a large amount of information [133,134][132][133]. Considering heart disease, Garvin et al., in an eight-year follow-up study of a community-based, middle-aged population, showed that plasma MMP-9 levels are able to predict first-time coronary heart disease (CHD) [135][134]. Psychological stress is able to release MMP-9 in patients with coronary artery disease, triggering inflammation [136][135]. SDHs, which include economic, social, environmental, and psychosocial factors, have been shown to play a significant role in CHD [137][136]. Thus, it is important to find a mathematically based predictive system that would allow, in the near future, evaluation of the complexity of the effects that MPs can have on several characteristics of human health. This predictive approach based on the complexity paradigm may help health professionals better tailor diagnostic pathways and treatments with optimal sensitivity, specificity, and accuracy using a range of readily available data from patients. Most healthcare organizations consider predictive modeling a fundamental tool to support patient segmentation and service matching to achieve the most efficient and effective delivery of healthcare services [138][137].References
- Ielapi, N.; Andreucci, M.; Licastro, N.; Faga, T.; Grande, R.; Buffone, G.; Mellace, S.; Sapienza, P.; Serra, R. Precision Medicine and Precision Nursing: The Era of Biomarkers and Precision Health. Int. J. Gen. Med. 2020, 13, 1705–1711.
- Costa, D.; Andreucci, M.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Bracale, U.M.; Serra, R. Molecular Determinants of Chronic Venous Disease: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 1928.
- Serra, R.; Gallelli, L.; Butrico, L.; Buffone, G.; Caliò, F.G.; De Caridi, G.; Massara, M.; Barbetta, A.; Amato, B.; Labonia, M.; et al. From varices to venous ulceration: The story of chronic venous disease described by metalloproteinases. Int. Wound J. 2017, 14, 233–240.
- Costa, D.; Andreucci, M.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Bracale, U.M.; Serra, R. Vascular Biology of arterial aneurysms. Ann. Vasc. Surg. 2023; in press.
- Stöcker, W.; Bode, W. Structural features of a superfamily of zinc-endopeptidases: The metzincins. Curr. Opin. Struct. Biol. 1995, 5, 383–390.
- Wächter, J.; Shannon, M.J.; Beristain, A.G. Transcriptomic mapping of the metzincin landscape in human trophoblasts. Gene Expr. Patterns GEP 2022, 46, 119283.
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076.
- Woessner, J.F., Jr. Catabolism of collagen and non-collagen protein in the rat uterus during post-partum involution. Biochem. J. 1962, 83, 304–314.
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022.
- Nagai, Y.; Lapiere, C.M.; Gross, J. Tadpole collagenase. Preparation and purification. Biochemistry 1966, 5, 3123–3130.
- Iyer, R.P.; Patterson, N.L.; Fields, G.B.; Lindsey, M.L. The history of matrix metalloproteinases: Milestones, myths, and misperceptions. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H919–H930.
- Birkedal-Hansen, H. From tadpole collagenase to a family of matrix metalloproteinases. J. Oral Pathol. 1988, 17, 445–451.
- Zhong, S.; Khalil, R.A. A Disintegrin and Metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) family in vascular biology and disease. Biochem. Pharmacol. 2019, 164, 188–204.
- Wong, G.E.; Zhu, X.; Prater, C.E.; Oh, E.; Evans, J.P. Analysis of fertilin alpha (ADAM1)-mediated sperm-egg cell adhesion during fertilization and identification of an adhesion-mediating sequence in the disintegrin-like domain. J. Biol. Chem. 2001, 276, 24937–24945.
- Giebeler, N.; Zigrino, P. A Disintegrin and Metalloprotease (ADAM): Historical Overview of Their Functions. Toxins 2016, 8, 122.
- Kuno, K.; Kanada, N.; Nakashima, E.; Fujiki, F.; Ichimura, F.; Matsushima, K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J. Biol. Chem. 1997, 272, 556–562.
- Noël, A.; Gutiérrez-Fernández, A.; Sounni, N.E.; Behrendt, N.; Maquoi, E.; Lund, I.K.; Cal, S.; Hoyer-Hansen, G.; López-Otín, C. New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Front. Pharmacol. 2012, 3, 140.
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739.
- Dufour, A.; Sampson, N.S.; Zucker, S.; Cao, J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J. Cell. Physiol. 2008, 217, 643–651.
- Alford, V.M.; Kamath, A.; Ren, X.; Kumar, K.; Gan, Q.; Awwa, M.; Tong, M.; Seeliger, M.A.; Cao, J.; Ojima, I.; et al. Targeting the Hemopexin-like Domain of Latent Matrix Metalloproteinase-9 (proMMP-9) with a Small Molecule Inhibitor Prevents the Formation of Focal Adhesion Junctions. ACS Chem. Biol. 2017, 12, 2788–2803.
- Shi, Y.; Ma, X.; Fang, G.; Tian, X.; Ge, C. Matrix metalloproteinase inhibitors (MMPIs) as attractive therapeutic targets: Recent progress and current challenges. NanoImpact 2021, 21, 100293.
- Jones, L.; Ghaneh, P.; Humphreys, M.; Neoptolemos, J.P. The matrix metalloproteinases and their inhibitors in the treatment of pancreatic cancer. Ann. N. Y. Acad. Sci. 1999, 880, 288–307.
- Leber, T.M.; Balkwill, F.R. Zymography: A single-step staining method for quantitation of proteolytic activity on substrate gels. Anal. Biochem. 1997, 249, 24–28.
- Lei, Z.; Jian, M.; Li, X.; Wei, J.; Meng, X.; Wang, Z. Biosensors and bioassays for determination of matrix metalloproteinases: State of the art and recent advances. J. Mater. Chem. B 2020, 8, 3261–3291.
- Hadler-Olsen, E.; Fadnes, B.; Sylte, I.; Uhlin-Hansen, L.; Winberg, J.O. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011, 278, 28–45.
- Zhu, P.; Chen, C.; Wu, D.; Chen, G.; Tan, R.; Ran, J. AGEs-induced MMP-9 activation mediated by Notch1 signaling is involved in impaired wound healing in diabetic rats. Diabetes Res. Clin. Pract. 2022, 186, 109831.
- Cook, L.; Sengelmann, M.; Winkler, B.; Nagl, C.; Koch, S.; Schlomann, U.; Slater, E.P.; Miller, M.A.; von Strandmann, E.P.; Dörsam, B.; et al. ADAM8-Dependent Extracellular Signaling in the Tumor Microenvironment Involves Regulated Release of Lipocalin 2 and MMP-9. Int. J. Mol. Sci. 2022, 23, 1976.
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290.
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768.
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J. Neurochem. 2016, 139 (Suppl. S2), 91–114.
- Braundmeier, A.G.; Fazleabas, A.T.; Nowak, R.A. Extracellular matrix metalloproteinase inducer expression in the baboon endometrium: Menstrual cycle and endometriosis. Reproduction 2010, 140, 911–920.
- Morris, S.A.; Korach, K.S.; Burns, K.A. Unique Sensitivity of Uterine Tissue and the Immune System for Endometriotic Lesion Formation. Front. Physiol. 2021, 12, 805784.
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1940–1951.
- Kaczmarek, L.; Lapinska-Dzwonek, J.; Szymczak, S. Matrix metalloproteinases in the adult brain physiology: A link between c-Fos, AP-1 and remodeling of neuronal connections? EMBO J. 2002, 21, 6643–6648.
- Mittal, R.; Patel, A.P.; Debs, L.H.; Nguyen, D.; Patel, K.; Grati, M.; Mittal, J.; Yan, D.; Chapagain, P.; Liu, X.Z. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J. Cell. Physiol. 2016, 231, 2599–2621.
- Serra, R.; Buffone, G.; Falcone, D.; Molinari, V.; Scaramuzzino, M.; Gallelli, L.; de Franciscis, S. Chronic venous leg ulcers are associated with high levels of metalloproteinases-9 and neutrophil gelatinase-associated lipocalin. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2013, 21, 395–401.
- Horecka, A.; Hordyjewska, A.; Biernacka, J.; Dąbrowski, W.; Zubilewicz, T.; Malec, A.; Musik, I.; Kurzepa, J. Intense remodeling of extracellular matrix within the varicose vein: The role of gelatinases and vascular endothelial growth factor. Ir. J. Med. Sci. 2021, 190, 255–259.
- Chen, Y.; Peng, W.; Raffetto, J.D.; Khalil, R.A. Matrix Metalloproteinases in Remodeling of Lower Extremity Veins and Chronic Venous Disease. Prog. Mol. Biol. Transl. Sci. 2017, 147, 267–299.
- Busceti, M.T.; Grande, R.; Amato, B.; Gasbarro, V.; Buffone, G.; Amato, M.; Gallelli, L.; Serra, R.; de Franciscis, S. Pulmonary embolism, metalloproteinsases and neutrophil gelatinase associated lipocalin. Acta Phlebol. 2013, 14, 115–121.
- Raffetto, J.D.; Ross, R.L.; Khalil, R.A. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: Relevance to varicose vein formation. J. Vasc. Surg. 2007, 45, 373–380.
- Serraino, G.F.; Jiritano, F.; Costa, D.; Ielapi, N.; Battaglia, D.; Bracale, U.M.; Mastroroberto, P.; Andreucci, M.; Serra, R. Metalloproteinases in Cardiac Surgery: A Systematic Review. Biomolecules 2023, 13, 113.
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806.
- Andreucci, M.; Provenzano, M.; Faga, T.; Michael, A.; Patella, G.; Mastroroberto, P.; Serraino, G.F.; Bracale, U.M.; Ielapi, N.; Serra, R. Aortic Aneurysms, Chronic Kidney Disease and Metalloproteinases. Biomolecules 2021, 11, 194.
- Provenzano, M.; Andreucci, M.; Garofalo, C.; Faga, T.; Michael, A.; Ielapi, N.; Grande, R.; Sapienza, P.; Franciscis, S.; Mastroroberto, P.; et al. The Association of Matrix Metalloproteinases with Chronic Kidney Disease and Peripheral Vascular Disease: A Light at the End of the Tunnel? Biomolecules 2020, 10, 154.
- Serra, R.; Grande, R.; Montemurro, R.; Butrico, L.; Caliò, F.G.; Mastrangelo, D.; Scarcello, E.; Gallelli, L.; Buffone, G.; de Franciscis, S. The role of matrix metalloproteinases and neutrophil gelatinase-associated lipocalin in central and peripheral arterial aneurysms. Surgery 2015, 157, 155–162.
- de Franciscis, S.; Mastroroberto, P.; Gallelli, L.; Buffone, G.; Montemurro, R.; Serra, R. Increased plasma levels of metalloproteinase-9 and neutrophil gelatinase-associated lipocalin in a rare case of multiple artery aneurysm. Ann. Vasc. Surg. 2013, 27, 1185.e5–1185.e7.
- Mei, K.; Chen, Z.; Wang, Q.; Luo, Y.; Huang, Y.; Wang, B.; Gu, R. The role of intestinal immune cells and matrix metalloproteinases in inflammatory bowel disease. Front. Immunol. 2023, 13, 1067950.
- Chowkwale, M.; Lindsey, M.L.; Saucerman, J.J. Intercellular model predicts mechanisms of inflammation-fibrosis coupling after myocardial infarction. J. Physiol. 2022; online ahead of print.
- Wagner, J.; Kumar, Y.; Lautenbach, A.; von Kroge, P.; Wolter, S.; Mann, O.; Izbicki, J.; Gagliani, N.; Duprée, A. Fatty acid-binding protein-4 (FABP4) and matrix metalloproteinase-9 (MMP9) as predictive values for nonalcoholic steatohepatitis (NASH). Lipids Health Dis. 2023, 22, 1.
- Seegar, T.C.; Blacklow, S.C. Domain integration of ADAM family proteins: Emerging themes from structural studies. Exp. Biol. Med. 2019, 244, 1510–1519.
- Meyer-Schwesinger, C.; Seipold, L.; Saftig, P. Ectodomain shedding by ADAM proteases as a central regulator in kidney physiology and disease. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119165.
- Lichtenthaler, S.F.; Lemberg, M.K.; Fluhrer, R. Proteolytic ectodomain shedding of membrane proteins in mammals-hardware, concepts, and recent developments. EMBO J. 2018, 37, e99456.
- Clark, P. Protease-mediated ectodomain shedding. Thorax 2014, 69, 682–684.
- Tatsumi, M.; Kishi, T.; Ishida, S.; Kawana, H.; Uwamizu, A.; Ono, Y.; Kawakami, K.; Aoki, J.; Inoue, A. Ectodomain shedding of EGFR ligands serves as an activation readout for TRP channels. PLoS ONE 2023, 18, e0280448.
- Ma, X.; Takahashi, Y.; Wu, W.; Liang, W.; Chen, J.; Chakraborty, D.; Li, Y.; Du, Y.; Benyajati, S.; Ma, J.X. ADAM17 mediates ectodomain shedding of the soluble VLDL receptor fragment in the retinal epithelium. J. Biol. Chem. 2021, 297, 101185.
- Brown, M.S.; Ye, J.; Rawson, R.B.; Goldstein, J.L. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria to humans. Cell 2000, 100, 391–398.
- Butrico, L.; Barbetta, A.; Ciranni, S.; Mastroroberto, P.; Andreucci, M.; De Franciscis, S.; Serra, R. Role of metalloproteinases and their inhibitors in the development of abdominal aortic aneurysm: Current insights and systematic review of the literature. Chirurgia 2017, 30, 151–159.
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015, 16, 113.
- Porter, S.; Clark, I.M.; Kevorkian, L.; Edwards, D.R. The ADAMTS metalloproteinases. Biochem. J. 2005, 386, 15–27.
- Santamaria, S.; de Groot, R. ADAMTS proteases in cardiovascular physiology and disease. Open Biol. 2020, 10, 200333.
- DeYoung, V.; Singh, K.; Kretz, C.A. Mechanisms of ADAMTS13 regulation. J. Thromb. Haemost. 2022, 20, 2722–2732.
- Grosse, G.M.; Leotescu, A.; Sieweke, J.T.; Schneppenheim, S.; Budde, U.; Ziegler, N.L.; Biber, S.; Gabriel, M.M.; Ernst, J.; Schuppner, R.; et al. ADAMTS-13 activity in stroke of known and unknown cause: Relation to vascular risk factor burden. Front. Neurol. 2023, 13, 1045478.
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71.
- Bauer, E.A.; Stricklin, G.P.; Jeffrey, J.J.; Eisen, A.Z. Collagenase production by human skin fibroblasts. Biochem. Biophys. Res. Commun. 1975, 64, 232–240.
- Verstappen, J.; Von den Hoff, J.W. Tissue inhibitors of metalloproteinases (TIMPs): Their biological functions and involvement in oral disease. J. Dent. Res. 2006, 85, 1074–1084.
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 233.
- Rose, K.W.J.; Taye, N.; Karoulias, S.Z.; Hubmacher, D. Regulation of ADAMTS Proteases. Front. Mol. Biosci. 2021, 8, 701959.
- Khanafer, K.; Ghosh, A.; Vafai, K. Correlation between MMP and TIMP levels and elastic moduli of ascending thoracic aortic aneurysms. Cardiovasc. Revascularization Med. Incl. Mol. Interv. 2019, 20, 324–327.
- Serra, R.; Gallelli, L.; Buffone, G.; Molinari, V.; Stillitano, D.M.; Palmieri, C.; de Franciscis, S. Doxycycline speeds up healing of chronic venous ulcers. Int. Wound J. 2015, 12, 179–184.
- Serra, R.; Gallelli, L.; Conti, A.; De Caridi, G.; Massara, M.; Spinelli, F.; Buffone, G.; Caliò, F.G.; Amato, B.; Ceglia, S.; et al. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and venous ulcers of lower limbs. Drug Des. Dev. Ther. 2014, 8, 519–527.
- Serra, R.; Grande, R.; Buffone, G.; Gallelli, L.; De Franciscis, S. The effects of minocycline on extracellular matrix in patients with chronic venous leg ulcers. Acta Phlebol. 2013, 14, 99–107.
- de Franciscis, S.; Gallelli, L.; Battaglia, L.; Molinari, V.; Montemurro, R.; Stillitano, D.M.; Buffone, G.; Serra, R. Cilostazol prevents foot ulcers in diabetic patients with peripheral vascular disease. Int. Wound J. 2015, 12, 250–253.
- Cione, E.; Piegari, E.; Gallelli, G.; Caroleo, M.C.; Lamirata, E.; Curcio, F.; Colosimo, F.; Cannataro, R.; Ielapi, N.; Colosimo, M.; et al. Expression of MMP-2, MMP-9, and NGAL in Tissue and Serum of Patients with Vascular Aneurysms and Their Modulation by Statin Treatment: A Pilot Study. Biomolecules 2020, 10, 359.
- Li, K.; Tay, F.R.; Yiu, C.K.Y. The past, present and future perspectives of matrix metalloproteinase inhibitors. Pharmacol. Ther. 2020, 207, 107465.
- Serra, R.; Ielapi, N.; Barbetta, A.; Andreucci, M.; de Franciscis, S. Novel biomarkers for cardiovascular risk. Biomark. Med. 2018, 12, 1015–1024.
- Galliera, E.; Tacchini, L.; Corsi Romanelli, M.M. Matrix metalloproteinases as biomarkers of disease: Updates and new insights. Clin. Chem. Lab. Med. 2015, 53, 349–355.
- Fonseca, F.L.; da Costa Aguiar Alves, B.; Azzalis, L.A.; Belardo, T.M. Matrix Metalloproteases as Biomarkers of Disease. Methods Mol. Biol. 2017, 1579, 299–311.
- Liu, C.H.; Di, Y.P. Matrix Metallopeptidase-Gene Signature Predicts Stage I Lung Adenocarcinoma Survival Outcomes. Int. J. Mol. Sci. 2023, 24, 2382.
- Sun, W.P.; Du, X.; Chen, J.J. Biomarkers for Predicting the Occurrence and Progression of Atrial Fibrillation: Soluble Suppression of Tumorigenicity 2 Protein and Tissue Inhibitor of Matrix Metalloproteinase-1. Int. J. Clin. Pract. 2022, 2022, 6926510.
- Stojanovic, S.K.; Stamenkovic, B.N.; Cvetkovic, J.M.; Zivkovic, V.G.; Apostolovic, M.R.A. Matrix Metalloproteinase-9 Level in Synovial Fluid-Association with Joint Destruction in Early Rheumatoid Arthritis. Medicina 2023, 59, 167.
- Brusa, S.; Terracciano, D.; Bruzzese, D.; Fiorenza, M.; Stanziola, L.; Pinchera, B.; Valente, V.; Gentile, I.; Cittadini, A.; Mormile, I.; et al. Circulating tissue inhibitor of metalloproteinases 1 (TIMP-1) at COVID-19 onset predicts severity status. Front. Med. 2022, 9, 1034288.
- Kicman, A.; Niczyporuk, M.; Kulesza, M.; Motyka, J.; Ławicki, S. Utility of Matrix Metalloproteinases in the Diagnosis, Monitoring and Prognosis of Ovarian Cancer Patients. Cancer Manag. Res. 2022, 14, 3359–3382.
- Noh, J.W.; Jang, J.H.; Yoon, H.S.; Kim, K.B.; Heo, M.H.; Jang, H.E.; Kim, Y.J.; Lee, Y. Evaluation of Salivary Biomarkers of Periodontal Disease Based on Smoking Status: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 14619.
- Serra, R.; Gallelli, L.; Grande, R.; Amato, B.; De Caridi, G.; Sammarco, G.; Ferrari, F.; Butrico, L.; Gallo, G.; Rizzuto, A.; et al. Hemorrhoids and matrix metalloproteinases: A multicenter study on the predictive role of biomarkers. Surgery 2016, 159, 487–494.
- Tsiknia, A.A.; Sundermann, E.E.; Reas, E.T.; Edland, S.D.; Brewer, J.B.; Galasko, D.; Banks, S.J.; Alzheimer’s Disease Neuroimaging Initiative. Sex differences in Alzheimer’s disease: Plasma MMP-9 and markers of disease severity. Alzheimer’s Res. Ther. 2022, 14, 160.
- Marks, J. Dossier: Le groupe des Dix, des précurseurs de l’interdisciplinarité–Biology and complexity: Edgar Morin and Henri Atlan. Nat. Sci. Sociétés 2019, 27, 159–168.
- Waldrop, M.M. Complexity: The Emerging Science at the Edge of Order and Chaos; Simon and Schuster: New York, NY, USA, 1993.
- Coveney, P.V. Self-Organization and Complexity: A New Age for Theory, Computation and Experiment. Philos. Trans. Math. Phys. Eng. Sci. 2003, 361, 1057–1079.
- Morin, E. Introduction à la Pensée Complexe; Editors du Seuil: Paris, France, 2005.
- Serra, R. Matrix Metalloproteinases in Health and Disease. Biomolecules 2020, 10, 1138.
- Morin, E. Restricted complexity, general complexity. In Worldviews, Science and Us: Philosophy and Complexity; Gershenson, C., Aerts, D., Edmonds, B., Eds.; World Scientific: Singapore, 2007; pp. 5–29.
- Heath-Carpentier, A. The Challenge of Complexity: Essays by Edgar Morin; Liverpool University Press: Liverpool, UK, 2022.
- Costa, D. Diversity and Health: Two Sides of the Same Coin. Ital. Sociol. Rev. 2023, 13, 69–90.
- Costa, D.; Ielapi, N.; Caprino, F.; Giannotta, N.; Sisinni, A.; Abramo, A.; Ssempijja, L.; Andreucci, M.; Bracale, U.M.; Serra, R. Social Aspects of Diabetic Foot: A Scoping Review. Soc. Sci. 2022, 11, 149.
- Kalra, S.; Baruah, M.P.; Sahay, R. Salutogenesis in Type 2 Diabetes Care: A Biopsychosocial Perspective. Indian J. Endocrinol. Metab. 2018, 22, 169–172.
- Soon, K.; Acton, C. Pain-induced stress: A barrier to wound healing. Wounds UK 2006, 2, 92–101.
- Alexander, S.J. Time to get serious about assessing- and managing-psychosocial issues associated with chronic wounds. Curr. Opin. Support. Palliat. Care 2013, 7, 95–100.
- Fu, K.; Zheng, X.; Chen, Y.; Wu, L.; Yang, Z.; Chen, X.; Song, W. Role of matrix metalloproteinases in diabetic foot ulcers: Potential therapeutic targets. Front. Pharmacol. 2022, 13, 1050630.
- Hariono, M.; Yuliani, S.H.; Istyastono, E.P.; Riswanto, F.D.O.; Adhipandito, C.F. Matrix metalloproteinase 9 (MMP9) in wound healing of diabetic foot ulcer: Molecular target and structure-based drug design. Vasc. Med. 2018, 22, 1–13.
- Jones, J.I.; Nguyen, T.T.; Peng, Z.; Chang, M. Targeting MMP-9 in Diabetic Foot Ulcers. Pharmaceuticals 2019, 12, 79.
- Chuang, S.Y.; Yang, S.H.; Chen, T.Y.; Pang, J.H. Cilostazol inhibits matrix invasion and modulates the gene expressions of MMP-9 and TIMP-1 in PMA-differentiated THP-1 cells. Eur. J. Pharmacol. 2011, 670, 419–426.
- Li, H.; Sheng, Z.; Khan, S.; Zhang, R.; Liu, Y.; Zhang, Y.; Yong, V.W.; Xue, M. Matrix Metalloproteinase-9 as an Important Contributor to the Pathophysiology of Depression. Front. Neurol. 2022, 13, 861843.
- Cukor, D. Introduction: Psychosocial Issues in Kidney Disease. Semin. Nephrol. 2021, 41, 485–486.
- Cukor, D.; Cohen, S.D.; Kimmel, P.L. Psychosocial Aspects of Chronic Kidney Disease: Exploring the Impact of CKD, Dialysis, and Transplantation on Patients; Academic Press: Cambridge, MA, USA, 2021.
- Bayne, D.F.; Shune, S.E. A Biopsychosocial Model of Mealtime Management in Persons with Dementia, an Asset-Based Approach to Patient-Centered Care. Geriatrics 2022, 7, 112.
- Ismail, Z.; Smith, E.E.; Geda, Y.; Sultzer, D.; Brodaty, H.; Smith, G.; Agüera-Ortiz, L.; Sweet, R.; Miller, D.; Lyketsos, C.G.; et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimer’s Dement. 2015, 12, 195–202.
- Edmondson, D.; Newman, J.; Whang, W.; Davidson, K. Emotional triggers in myocardial infarction: Do they matter? Eur. Heart J. 2013, 34, 300–306.
- Mittleman, M.; Mostofsky, E. Physical, psychological and chemical triggers of acute cardiovascular events. Circulation 2011, 124, 346–354.
- Cilli, E.; Ranieri, J.; Guerra, F.; Ferri, C.; Di Giacomo, D. Cardiovascular disease, self-care and emotional regulation processes in adult patients: Balancing unmet needs and quality of life. BioPsychoSocial Med. 2022, 16, 20.
- Monami, M.; Marchionni, N. Psychological disorders and cardiovascular diseases. G. Ital. Cardiol. 2007, 8, 335–348.
- WHO. Adherence to Long-Term Therapies: Evidence for Action; WHO: Geneva, Switzerland, 2003.
- Thomas, H.N.; Thurston, R.C. A biopsychosocial approach to women’s sexual function and dysfunction at midlife: A narrative review. Maturitas 2016, 87, 49–60.
- Ventegodt, S. Sex and the quality of life in Denmark. Arch. Sex Behav. 1998, 27, 295–307.
- Mercer, C.H.; Fenton, K.A.; Johnson, A.M.; Wellings, K.; Macdowall, W.; McManus, S.; Nanchahal, K.; Erens, B. Sexual function problems and help seeking behaviour in Britain: National probability sample survey. BMJ Clin. Res. Ed. 2003, 327, 426–427.
- Newby, A.C. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2108–2114.
- Zhang, N.; Liu, C.; Jin, L.; Zhang, R.; Wang, T.; Wang, Q.; Chen, J.; Yang, F.; Siebert, H.C.; Zheng, X. Ketogenic Diet Elicits Antitumor Properties through Inducing Oxidative Stress, Inhibiting MMP-9 Expression, and Rebalancing M1/M2 Tumor-Associated Macrophage Phenotype in a Mouse Model of Colon Cancer. J. Agric. Food Chem. 2020, 68, 11182–11196.
- Seraphim, P.M.; Leal, E.C.; Moura, J.; Gonçalves, P.; Gonçalves, J.P.; Carvalho, E. Lack of lymphocytes impairs macrophage polarization and angiogenesis in diabetic wound healing. Life Sci. 2020, 254, 117813.
- Chen, B.; Hong, H.; Sun, Y.; Chen, C.; Wu, C.; Xu, G.; Bao, G.; Cui, Z. Role of macrophage polarization in osteoarthritis (Review). Exp. Ther. Med. 2022, 24, 757.
- Serra, R.; Jiritano, F.; Bracale, U.M.; Ielapi, N.; Licastro, N.; Provenzano, M.; Andreucci, M.; Rizzuto, A.; Mastroroberto, P.; Serraino, G.F. Novel biomarkers in cardiovascular surgery. Biomark. Med. 2021, 15, 307–318.
- Serraino, G.F.; Jiritano, F.; Costa, D.; Ielapi, N.; Napolitano, D.; Mastroroberto, P.; Bracale, U.M.; Andreucci, M.; Serra, R. Metalloproteinases and Hypertrophic Cardiomyopathy: A Systematic Review. Biomolecules 2023, 13, 665.
- Hunt, M.A.; Birmingham, T.B.; Skarakis-Doyle, E.; Vandervoort, A.A. Towards a biopsychosocial framework of osteoarthritis of the knee. Disabil. Rehabil. 2008, 30, 54–61.
- Ali, S.A.; Lee, K.; MacDermid, J.C. Applying the International Classification of Functioning, Disability and Health to understand osteoarthritis management in urban and rural community-dwelling seniors. Osteoarthr. Cartil. Open 2021, 3, 100132.
- Chen, Y.W.; Camp, P.G.; Coxson, H.O.; Road, J.D.; Guenette, J.A.; Hunt, M.A.; Reid, W.D. A Comparison of Pain, Fatigue, Dyspnea and their Impact on Quality of Life in Pulmonary Rehabilitation Participants with Chronic Obstructive Pulmonary Disease. COPD 2018, 15, 65–72.
- Novy, D.M.; Aigner, C.J. The biopsychosocial model in cancer pain. Curr. Opin. Support. Palliat. Care 2014, 8, 117–123.
- Syrjala, K.L.; Chapko, M.E. Evidence for a biopsychosocial model of cancer treatment-related pain. Pain 1995, 61, 69–79.
- Badura, A.S.; Grohmann, J.M. Psychological issues in pain perception and treatment in the elderly. Ann. Long-Term Care 2002, 10, 29–34.
- Masselin-Dubois, A.; Attal, N.; Fletcher, D.; Jayr, C.; Albi, A.; Fermanian, J.; Bouhassira, D.; Baudic, S. Are psychological predictors of chronic postsurgical pain dependent on the surgical model? A comparison of total knee arthroplasty and breast surgery for cancer. J. Pain 2013, 14, 854–864.
- Costa, D.; Ielapi, N.; Bevacqua, E.; Ciranni, S.; Cristodoro, L.; Torcia, G.; Serra, R. Social Determinants of Health and Vascular Diseases: A Systematic Review and Call for Action. Soc. Sci. 2023, 12, 214.
- Shafi, B.H.; Bøttcher, M.; Ejupi, A.; Jensen, G.; Osler, M.; Lange, T.; Prescott, E. Socioeconomic disparity in cardiovascular disease: Possible biological pathways based on a proteomic approach. Atherosclerosis 2022, 352, 62–68.
- Carey, G.; Malbon, E.; Carey, N.; Joyce, A.; Crammond, B.; Carey, A. Systems science and systems thinking for public health: A systematic review of the field. BMJ Open 2015, 5, e009002.
- Cabral, M.D.F.C.T.; Viana, A.L.; Gontijo, D.T. Use of the complexity paradigm in the field of health: Scope review. Esc. Anna Nery 2020, 24, e20190235.
- de Franciscis, S.; Fregola, S.; Gallo, A.; Argirò, G.; Barbetta, A.; Buffone, G.; Caliò, F.G.; De Caridi, G.; Amato, B.; Serra, R. PredyCLU: A prediction system for chronic leg ulcers based on fuzzy logic; part I—Exploring the venous side. Int. Wound J. 2016, 13, 1349–1353.
- Serra, R.; Bracale, U.M.; Barbetta, A.; Ielapi, N.; Licastro, N.; Gallo, A.; Fregola, S.; Turchino, D.; Gasbarro, V.; Mastroroberto, P.; et al. PredyCLU: A prediction system for chronic leg ulcers based on fuzzy logic; part II-Exploring the arterial side. Int. Wound J. 2020, 17, 987–991.
- Garvin, P.; Jonasson, L.; Nilsson, L.; Falk, M.; Kristenson, M. Plasma Matrix Metalloproteinase-9 Levels Predict First-Time Coronary Heart Disease: An 8-Year Follow-Up of a Community-Based Middle Aged Population. PLoS ONE 2015, 10, e0138290.
- Lundberg, A.K.; Jönsson, S.; Stenmark, J.; Kristenson, M.; Jonasson, L. Stress-induced release of matrix metalloproteinase-9 in patients with coronary artery disease: The possible influence of cortisol. Psychoneuroendocrinology 2016, 73, 117–124.
- Powell-Wiley, T.M.; Baumer, Y.; Baah, F.O.; Baez, A.S.; Farmer, N.; Mahlobo, C.T.; Pita, M.A.; Potharaju, K.A.; Tamura, K.; Wallen, G.R. Social determinants of cardiovascular disease. Circ. Res. 2022, 130, 782–799.
- Kasthurirathne, S.N.; Vest, J.R.; Menachemi, N.; Halverson, P.K.; Grannis, S.J. Assessing the capacity of social determinants of health data to augment predictive models identifying patients in need of wraparound social services. J. Am. Med. Inform. Assoc. 2018, 25, 47–53.
More
