There are three main families of metalloproteinases (MPs) that are involved in human health and disease: (1) the “matrix metalloproteinase” (MMP) family, (2) the “a disintegrin and metalloprotease” (ADAM) family, and (3) the “a disintegrin and metalloproteinase with thrombospondin motifs” (ADAMTS) family. MPs are relevant to public health because of their role in several diseases and, most of all, their role as biomarkers that also impact the quality of life and the psychosocial dimension of affected patients. In this context, new pathways to precision health and precision medicine have been opened in the area of MPs. This review describes, from the initial studies, the complex dimensions of MPs and related issues centered on health and disease dimensions.
- metalloproteinases
- MMP
- ADAM
- ADAMTS
- history
- complexity
1. Introduction
2. The Matrix Metalloproteinase (MMP) Family
At present, there are 28 MMP family members in vertebrates, of which at least 23 are present in humans, and they may be secreted as soluble enzymes or be bound to the cell membranes (the so-called membrane-type (MT) MMPs). Furthermore, MT-MMPs may be bound to the cell membranes by a COOH-terminal transmembrane domain or by a glycosylphosphatidyl-inositol (GPI) anchor [7]. Generally, MMPs have a signal peptide that serves to lead them to the endoplasmic reticulum (ER), a prodomain that serves to maintain them as inactive zymogens, a catalytic domain with three histidine residues bound to a zinc-binding site, and a proline-rich hinge region and a C-terminal hemopexin-like (HPX) domain (not present in some MMPs, such as MMP-7, MMP-23, and MMP-26) involved in substrate binding (Figure 1).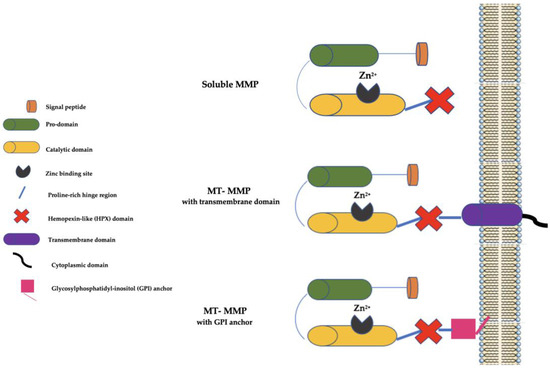
| Subgroup | Members | Enzymatic Name |
|---|---|---|
| Collagenases | MMP-1 MMP-8 MMP-13 MMP-18 |
Collagenase-1 Collagenase-2/neutrophil collagenase Collagenase-3 Collagenase-4 |
| Gelatinases | MMP-2 MMP-9 |
Gelatinase A Gelatinase B |
| Stromelysins | MMP-3 MMP-10 MMP-11 MMP-19 MMP-27 |
Stromelysin-1/transin-1 Stromelysin-2/transin-2 Stromelysin-3 Stromelysin-4/RASI-1 n/d |
| Matrilysins | MMP-7 MMP-26 |
Matrilysin-1/putative MMP (PUMP) Matrilysin-2 |
| MT-MMPs | MMP-14 MMP-15 MMP-16 MMP-4/MMP-17 MMP-5/MMP-24 MMP-6/MMP-25 |
MT1-MMP MT2-MMP MT3-MMP MT4-MMP * MT5-MMP * MT6-MMP |
| Ungrouped MMPs | MMP-12 MMP-20 MMP-21 MMP-22 MMP-23 MMP-28 |
Macrophage metalloelastase Enamelysin Xenopous MMP (XMPP) Chicken MMP (CMMP) Cysteine array MMP (CA-MMP) Epilysin |
3. The “A Disintegrin and Metalloprotease” (ADAM) Family
At present, in humans, there are 21 ADAM family members. Among them, 13 are catalytically active (ADAM-8, ADAM-9, ADAM-10, ADAM-12, ADAM-15, ADAM-17, ADAM-19, ADAM-20, ADAM-21, ADAM-28, ADAM-30, ADAM-33, and ADAM-DEC1) and 8 seem to be catalytically inactive [51]. ADAMs are structurally similar to MMPs but they have a disintegrin, a cysteine-rich domain, and an epidermal growth factor-like (EGF-like) domain (Figure 2).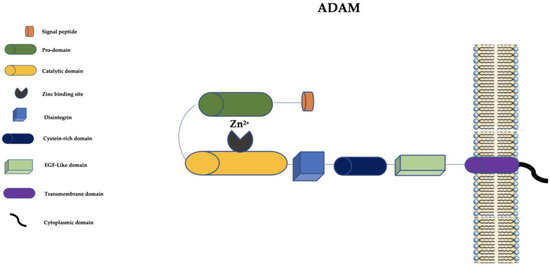
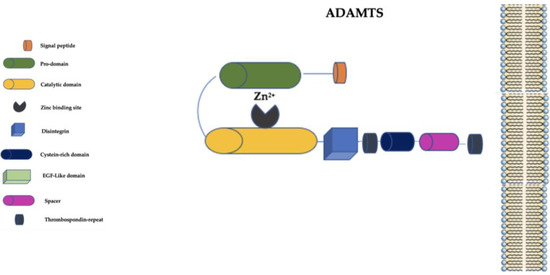
4. The “A Disintegrin and Metalloprotease with Thrombospondin Motifs” (ADAMTS) Family
The ADAMTS family is distinguished from the ADAM family primarily by the presence of a thrombospondin-repeat domain and the lack of EGF-like transmembrane and cytoplasmic domains (Figure 3) [59].