Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Raffaele Serra and Version 3 by Camila Xu.
There are three main families of metalloproteinases (MPs) that are involved in human health and disease: (1) the “matrix metalloproteinase” (MMP) family, (2) the “a disintegrin and metalloprotease” (ADAM) family, and (3) the “a disintegrin and metalloproteinase with thrombospondin motifs” (ADAMTS) family. MPs are relevant to public health because of their role in several diseases and, most of all, their role as biomarkers that also impact the quality of life and the psychosocial dimension of affected patients. In this context, new pathways to precision health and precision medicine have been opened in the area of MPs.
- metalloproteinases
- MMP
- ADAM
- ADAMTS
- history
- complexity
1. Introduction
There are three main families of metalloproteinases (MPs) that are involved in human health and disease: (1) the “matrix metalloproteinase” (MMP) family, (2) the “a disintegrin and metalloprotease” (ADAM) family, and (3) the “a disintegrin and metalloproteinase with thrombospondin motifs” (ADAMTS) family. All these families belong to the superfamily of zinc endopeptidases, which are called metzincins [1][2][3][4][5][6][7][1,2,3,4,5,6,7].
In 1962, MMP families began to be studied and Woessner published the first study on this topic demonstrating that a protein derived from a rat was able to digest collagen [8]. In particular, the mammalian uterus is one of the most favorable tissues for studying protein catabolism, especially collagen, under physiological conditions, and in fact, in this study, collagen disappeared in the postpartum period during uterus involution in a rate-limiting model, suggesting the role of specific enzyme activity. Later in the same year (1962), Gross et al. [9] showed the rapid collagenolytic activity of large tissue masses in an anuran tadpole (tail, gill, gut, skin) during natural and hormone-induced metamorphosis, and this suggested the need to identify specific enzymes able to remove particular structural components during normal growth and development. Later, in 1966, Nagai et al. purified from a tadpole the first member of the MMP family, MMP-1, initially called tadpole collagenase [10]. In that period, several MMPs and tissue inhibitors of metalloproteinases (TIMPs) were characterized [11] and, ultimately, in 1988, Birkedal-Hansen published the first review on the MMP family [12].
In 1987, the first member of the ADAM family, ADAM-1, initially known as fertilization protein PH-30 or fertilin-α, was identified; subsequently, in 1992, ADAMs were actually classified as a new family of MPs. In particular, ADAM-1 was found to act as a fusion peptide during sperm–egg fusion due to cell adhesion and protease properties; hence, it was evident that it has a role in sperm–egg interactions. Furthermore, ADAMs are also similar to the snake venom metalloproteinase family (SVM), also known as the snake venom disintegrins family [13][14][15][13,14,15].
In 1997, the ADAMTS1 gene, expressed in the cachexigenic colon 26 adenocarcinoma sublines, and the relative protein ADAMTS-1, the first member of the ADAMTS family, were characterized [16]. MPs are directly related to the homeostasis of the extracellular matrix (ECM), a biochemical center that includes collagen, elastin, and other proteins that are involved in providing structural and functional support to several tissues [7].
2. The Matrix Metalloproteinase (MMP) Family
At present, there are 28 MMP family members in vertebrates, of which at least 23 are present in humans, and they may be secreted as soluble enzymes or be bound to the cell membranes (the so-called membrane-type (MT) MMPs). Furthermore, MT-MMPs may be bound to the cell membranes by a COOH-terminal transmembrane domain or by a glycosylphosphatidyl-inositol (GPI) anchor [7]. Generally, MMPs have a signal peptide that serves to lead them to the endoplasmic reticulum (ER), a prodomain that serves to maintain them as inactive zymogens, a catalytic domain with three histidine residues bound to a zinc-binding site, and a proline-rich hinge region and a C-terminal hemopexin-like (HPX) domain (not present in some MMPs, such as MMP-7, MMP-23, and MMP-26) involved in substrate binding (Figure 1).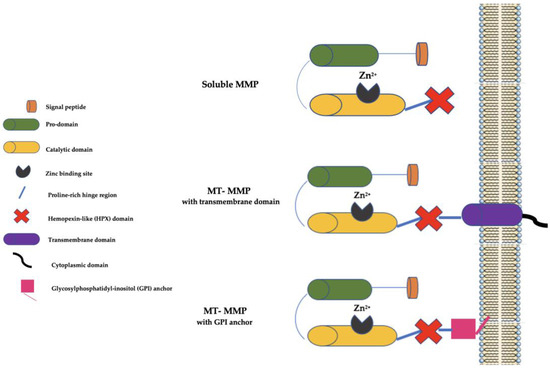
Figure 1. Schematic representation of MMPs. MMP: matrix metalloproteinase; MT-MMP: membrane type-matrix metalloproteinase; GMPI: glycosylphosphatidyl-inositol; Zn2+: Zinc ion.
Table 1.
MMP family members.
| Subgroup | Members | Enzymatic Name |
|---|---|---|
| Collagenases | MMP-1 MMP-8 MMP-13 MMP-18 |
Collagenase-1 Collagenase-2/neutrophil collagenase Collagenase-3 Collagenase-4 |
| Gelatinases | MMP-2 MMP-9 |
Gelatinase A Gelatinase B |
| Stromelysins | MMP-3 MMP-10 MMP-11 MMP-19 MMP-27 |
Stromelysin-1/transin-1 Stromelysin-2/transin-2 Stromelysin-3 Stromelysin-4/RASI-1 n/d |
| Matrilysins | MMP-7 MMP-26 |
Matrilysin-1/putative MMP (PUMP) Matrilysin-2 |
| MT-MMPs | MMP-14 MMP-15 MMP-16 MMP-4/MMP-17 MMP-5/MMP-24 MMP-6/MMP-25 |
MT1-MMP MT2-MMP MT3-MMP MT4-MMP * MT5-MMP * MT6-MMP |
| Ungrouped MMPs | MMP-12 MMP-20 MMP-21 MMP-22 MMP-23 MMP-28 |
Macrophage metalloelastase Enamelysin Xenopous MMP (XMPP) Chicken MMP (CMMP) Cysteine array MMP (CA-MMP) Epilysin |
MMP: matrix metalloproteinase; MT-MMP: membrane-type matrix metalloproteinase. * = presence of glycosylphosphatidyl-inositol (GPI) anchor.
For an enzyme to be included in the MMP family, it should meet the following criteria: (a) proteolytic activity in at least one ECM component; (b) enzymatic activity dependent on zinc at the catalytic site; (c) activation of the proenzyme by proteinases or organomercurials; (d) inhibition by TIMPs or other molecules, such as ethylenediaminetetraacetic acid or 1,10-phenantroline; and (e) related cDNA with sequence homology with MMP-1. The previous further criterion that the enzyme should be secreted in a proform is no longer valid as some MMPs are secreted in active form and others are not secreted at all [11].
MMPs’ activity is specifically regulated by several transcriptional, post-transcriptional, and post-translational modifications that can also lead to different isoforms and/or variants of MMPs. MMPs’ expression is also accurately modulated by specific control of their secretion, activation, and inhibition. MMPs are generated by several cells, such as endothelial cells (ECs), vascular smooth muscle cells (VSMCs), lymphocytes, cytotrophoblasts, fibroblasts, osteoblasts, macrophages, and neutrophils. MMPs’ expression is induced by their biochemical stimuli, such as cytokines, hormones, and growth factors, or by cellular stimuli, such as interactions between cells and matrixes and between cells [7].
Concerning MMP measurement techniques, initially, and for more than 30 years, it was thought that MMPs can be evaluated only by zymography, an electrophoretic technique that measures proteolytic activity [11][23][11,24]. In fact, modern and ultrasensitive enzyme immunoassay methods and biosensor-based measurements are able to effectively detect both pro- and active forms of MMPs [11][24][11,25]. This is an important achievement as, while in the past pro-MMPs were thought to be inactive, it was later demonstrated that even MMP proforms have certain enzymatic and biological activities [11][25][11,26]. This can be explained by considering that, in the first 30 years of research in the area of MMPs, there were no specific antibodies to recognize pro- and activated forms and zymograms were pivotal to measure MMP activity. Current technologies make it possible to measure the activity of all forms of MMPs [11].
In the past, it was also thought that MMPs are able to interact only with ECM proteins but it has been demonstrated that their ubiquitous actions also affect the extracellular and intracellular signaling of several cell types [11][26][27][11,27,28].
Moreover, in the past, MMPs were thought to have only negative effects on human health, but their roles in physiology and health have been elucidated [11]. In fact, MMPs have specific and important roles in turnover of the ECM, embryogenesis, tissue morphogenesis, bone development, angiogenesis, migration of immune cells, the menstrual cycle, involution of the endometrium after pregnancy, wound repair, learning, memory, and the cortical plasticity of the brain [28][29][30][31][32][33][34][29,30,31,32,33,34,35].
Most MMPs are located in certain cells and tissues where they regulate several physiological functions. MMP-1 is found in chondrocytes, fibroblasts, keratinocytes, endothelial cells, and macrophages; MMP-2, MMP-3, and MMP-7 are ubiquitous; MMP-8 and MMP-25 are found in polymorphonuclear leukocytes (neutrophils, macrophages, and plasma cells); MMP-9 is mainly located in macrophages, granulocytes, T-cells, dendritic cells, epithelial cells, fibroblasts, keratinocytes, and osteoblasts; MMP-10 is located in epithelial cells and hepatocytes; MMP-11 is mainly found in fibroblasts; MMP-12 is primarily found in the lung and the ear tissues; MMP-13 is found in bone, cartilage, epithelial cells, and neuronal cells; MMP-14 and MMP-15 are mainly located in the cells of the head, neck, and ear; MMP-17 is located in the brain, leukocytes, and colon, ovary, testis, and breast tissues; MMP-18 is ubiquitous in all stromal cells except in the liver and brain; MMP-19 can be found in epithelial cells and fibroblasts; MMP-20 is located in tooth enamel; MMP-21 can be found in stromal cells in the kidneys, skin, and intestines; MMP-23 is expressed in the ovary, testis, and prostate; MMP-26 is expressed in endometrial and placental tissues; MMP-27 is expressed in B-cells; and MMP-28 can be found in the testes, lungs, kidneys, pancreas, and keratinocytes [35][36].
MMPs are involved in several types of diseases and generally related to three main mechanisms: tissue destruction, such as cancer (e.g., acute myeloid leukemia and bladder, brain, breast, colorectal, endometrial, gastric, head and neck, liver, lung, ovarian, pancreas, prostate, renal, skin, and thyroid cancer), diabetes, inflammatory diseases (e.g., psoriasis, osteoarthritis, rheumatoid arthritis, inflammatory bowel disease), chronic wounds (e.g., pressure ulcers, vascular ulcers), periodontal diseases, hypertension, kidney diseases (e.g., chronic kidney disease, glomerular disease), myocardial infarction, and neurogenerative disease (e.g., multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis); fibrosis, such as liver cirrhosis, otosclerosis, gynecological disorders (e.g., polycystic ovarian syndrome, spontaneous abortion, preeclampsia), and atherosclerosis-related diseases; and weakening of the ECM, such as chronic venous disease, pulmonary embolism, aneurysms, and dilated cardiomyopathy. Often, more than one mechanism overlaps in determining the disease [2][3][7][11][18][21][36][37][38][39][40][41][42][43][44][45][46][47][48][49][2,3,7,11,19,22,37,38,39,40,41,42,43,44,45,46,47,48,49,50].
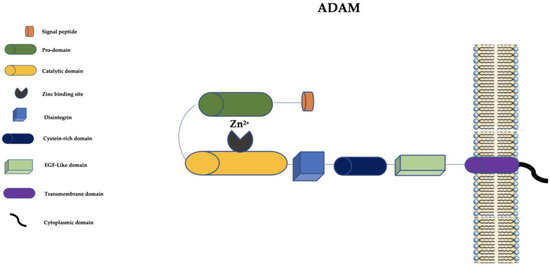 The disintegrin is important in cell–cell and cell–matrix interactions, and the cysteine-rich domain has cell adhesive and fusogenic properties [15]. The EGF-like domain, transmembrane domain, and cytoplasmic domain are pivotal for the signaling of ADAMs [13].
ADAMs are generally in inactive form, similarly to other metalloproteinases, and can be activated by proteolytic processing of the prodomain by different molecules (e.g., cytokines, proteinases, Ca2+ ionophores, protein kinase activators) generally located intracellularly in the Golgi system. Moreover, some ADAM members may be activated in different sites (in the ECM, such as ADAM-8 and ADAM-28) or in different modalities (auto-catalytic removal of prodomains or without the necessity of activation, such as ADAM-12, which is stored as already active in the cell and internally transferred to appropriate sites to act) [13].
Between the 1990s and the first decade of the 2000s, ADAMs were widely studied and several novel characteristics that went far beyond their role as fertilization proteins were discovered. In fact, over time, some important and specific features of the ADAM family have been extensively clarified [15]. ADAM proteins have sheddase activity; that is, the ability to cleave membrane proteins in order to release the extracellular domain (ectodomain shedding). This mechanism represents a post-translational modification that is important in regulating membrane shape and controlling the levels and functions of numerous membrane-bound proteins and acts in several physiological and pathological pathways related to growth factor signaling, cell adhesion, chronic inflammation, and apoptosis [51][52][53][52,53,54]. Moreover, the released ectodomain may, after shedding, influence distant targets, and this provides evidence of the paracrine and autocrine signaling activity of ADAMs [15][54][55][15,55,56].
Another important mechanism related to sheddase activity is so-called regulated intramembrane proteolysis (RIP), which, after the year 2000, was also studied in the ADAM family. In particular, after shedding the ectodomain due to ADAM activity, other proteases may synergistically act as the intramembrane cleavers of the intracellular part of the protein, and this intracellular stub may trigger intracellular signaling activity. ADAMs may mediate the RIP activity of several proteins involved in numerous biological processes [15][56][15,57].
ADAMs are involved in several physiological conditions related to embryogenesis, the cardiovascular system, tissue repair, and the human reproductive system (in particular, the uterus, testis, and epididymis). ADAMs are also involved in several diseases, such as rheumatoid arthritis, asthma, cardiovascular diseases (e.g., myocardial infarction, aneurysms, chronic venous disease, vascular ulcers), neurologic diseases (e.g., Guillain–Barrè syndrome, multiple sclerosis, Alzheimer’s disease), gynecological disease, and cancer (e.g., breast cancer). In particular, ADAMs have important roles in vascular biology controlling vascular smooth muscle cell (VSMC) functions, proliferation, migration, and apoptosis [2][3][13][15][57][2,3,13,15,58].
The disintegrin is important in cell–cell and cell–matrix interactions, and the cysteine-rich domain has cell adhesive and fusogenic properties [15]. The EGF-like domain, transmembrane domain, and cytoplasmic domain are pivotal for the signaling of ADAMs [13].
ADAMs are generally in inactive form, similarly to other metalloproteinases, and can be activated by proteolytic processing of the prodomain by different molecules (e.g., cytokines, proteinases, Ca2+ ionophores, protein kinase activators) generally located intracellularly in the Golgi system. Moreover, some ADAM members may be activated in different sites (in the ECM, such as ADAM-8 and ADAM-28) or in different modalities (auto-catalytic removal of prodomains or without the necessity of activation, such as ADAM-12, which is stored as already active in the cell and internally transferred to appropriate sites to act) [13].
Between the 1990s and the first decade of the 2000s, ADAMs were widely studied and several novel characteristics that went far beyond their role as fertilization proteins were discovered. In fact, over time, some important and specific features of the ADAM family have been extensively clarified [15]. ADAM proteins have sheddase activity; that is, the ability to cleave membrane proteins in order to release the extracellular domain (ectodomain shedding). This mechanism represents a post-translational modification that is important in regulating membrane shape and controlling the levels and functions of numerous membrane-bound proteins and acts in several physiological and pathological pathways related to growth factor signaling, cell adhesion, chronic inflammation, and apoptosis [51][52][53][52,53,54]. Moreover, the released ectodomain may, after shedding, influence distant targets, and this provides evidence of the paracrine and autocrine signaling activity of ADAMs [15][54][55][15,55,56].
Another important mechanism related to sheddase activity is so-called regulated intramembrane proteolysis (RIP), which, after the year 2000, was also studied in the ADAM family. In particular, after shedding the ectodomain due to ADAM activity, other proteases may synergistically act as the intramembrane cleavers of the intracellular part of the protein, and this intracellular stub may trigger intracellular signaling activity. ADAMs may mediate the RIP activity of several proteins involved in numerous biological processes [15][56][15,57].
ADAMs are involved in several physiological conditions related to embryogenesis, the cardiovascular system, tissue repair, and the human reproductive system (in particular, the uterus, testis, and epididymis). ADAMs are also involved in several diseases, such as rheumatoid arthritis, asthma, cardiovascular diseases (e.g., myocardial infarction, aneurysms, chronic venous disease, vascular ulcers), neurologic diseases (e.g., Guillain–Barrè syndrome, multiple sclerosis, Alzheimer’s disease), gynecological disease, and cancer (e.g., breast cancer). In particular, ADAMs have important roles in vascular biology controlling vascular smooth muscle cell (VSMC) functions, proliferation, migration, and apoptosis [2][3][13][15][57][2,3,13,15,58].
 At present, 20 ADAMTS members are known. They are secreted metalloproteinases, and most of their targets are represented by ECM components. Similarly to ADAMs, they have several physiological roles in the same biological system (reproductive, cardiovascular, etc.) and particular functions in vascular biology where they are able to control several pathways and process. ADAMTSs, similarly to ADAMs, may act as sheddases and can activate several cell surface proteins [13][59][13,60]. ADAMTs are involved in the same diseases in which ADAMS are involved, but they have a particular tropism towards cardiovascular disease (heart valve disease, coronary artery disease, aneurysms, chronic venous disease, hypertension) [60][61]. In this family, ADAMTS-13 is one of the most studied members. ADAMTS-13 controls hemostasis and thrombosis pathways and the dysregulation of this MP may lead both to bleeding and thrombosis. Moreover, ADAMTS-13 is considered an important factor related to several diseases characterized by vascular inflammation and thrombosis, such as cancer, vascular diseases, infections, neurological diseases, and liver diseases [61][62]. Furthermore, low ADAMTS-13 levels seem to increase the risk of ischemic stroke [60][62][61,63]. Several members of this family are also pivotal in the development of the heart and vessels. In particular, ADAMTS-7 is related to coronary artery disease (CAD) and may represent a potential therapeutic target, and ADAMTS-9 and ADAMTS-19 are associated with heart valve disease [62][63]. ADAMTS-1, ADAMTS-4, and ADAMTS-7 are also involved in chronic venous disease (CVD) [3].
At present, 20 ADAMTS members are known. They are secreted metalloproteinases, and most of their targets are represented by ECM components. Similarly to ADAMs, they have several physiological roles in the same biological system (reproductive, cardiovascular, etc.) and particular functions in vascular biology where they are able to control several pathways and process. ADAMTSs, similarly to ADAMs, may act as sheddases and can activate several cell surface proteins [13][59][13,60]. ADAMTs are involved in the same diseases in which ADAMS are involved, but they have a particular tropism towards cardiovascular disease (heart valve disease, coronary artery disease, aneurysms, chronic venous disease, hypertension) [60][61]. In this family, ADAMTS-13 is one of the most studied members. ADAMTS-13 controls hemostasis and thrombosis pathways and the dysregulation of this MP may lead both to bleeding and thrombosis. Moreover, ADAMTS-13 is considered an important factor related to several diseases characterized by vascular inflammation and thrombosis, such as cancer, vascular diseases, infections, neurological diseases, and liver diseases [61][62]. Furthermore, low ADAMTS-13 levels seem to increase the risk of ischemic stroke [60][62][61,63]. Several members of this family are also pivotal in the development of the heart and vessels. In particular, ADAMTS-7 is related to coronary artery disease (CAD) and may represent a potential therapeutic target, and ADAMTS-9 and ADAMTS-19 are associated with heart valve disease [62][63]. ADAMTS-1, ADAMTS-4, and ADAMTS-7 are also involved in chronic venous disease (CVD) [3].
3. The “A Disintegrin and Metalloprotease” (ADAM) Family
At present, in humans, there are 21 ADAM family members. Among them, 13 are catalytically active (ADAM-8, ADAM-9, ADAM-10, ADAM-12, ADAM-15, ADAM-17, ADAM-19, ADAM-20, ADAM-21, ADAM-28, ADAM-30, ADAM-33, and ADAM-DEC1) and 8 seem to be catalytically inactive [50][51]. ADAMs are structurally similar to MMPs but they have a disintegrin, a cysteine-rich domain, and an epidermal growth factor-like (EGF-like) domain (Figure 2).
Figure 2. Schematic representation of ADAMs. ADAM: a disintegrin and metalloprotease; EGF-like: epidermal growth factor-like; Zn2+: Zinc ion.
4. The “A Disintegrin and Metalloprotease with Thrombospondin Motifs” (ADAMTS) Family
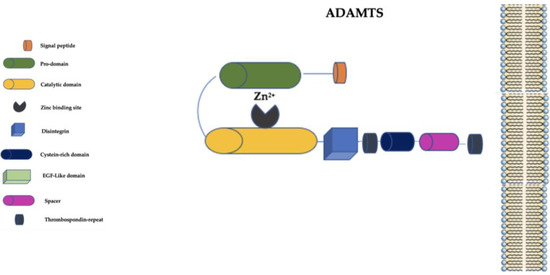
The ADAMTS family is distinguished from the ADAM family primarily by the presence of a thrombospondin-repeat domain and the lack of EGF-like transmembrane and cytoplasmic domains (Figure 3) [58][59].
Figure 3. Schematic representation of ADAMTS family. ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs; EGF-Like: epidermal growth factor-like; Zn2+: Zinc ion.
