Meat and some meat products are highly perishable due to their high-water content, pH, and high content of nutrients. Therefore, spoilage control in these products is one of the critical challenges in the food industry. On the other hand, the increasing widespread awareness about the undesirable effects of synthetic preservatives has promoted the breakthrough of the use of natural compounds or bio-preservation technology. Bio-preservation implies the application of microorganisms or their metabolites to extend the shelf life of food products. In this regard, according to the ancient and safe use of fermentation by lactic acid bacteria (LAB), their application in the bio-preservation of meat and meat products is gaining more attention.
- bio-preservation
- fermentation
- lactic acid bacteria
- meat products
- natural anti-microbial
1. Introduction
2. Fermented Meat Products and Their Health-Beneficial Properties
In the past, different techniques were used and developed for the preservation of meat and meat products, starting with adding some ingredients, such as salt and sugar to reduce microorganisms without an exact understanding of their preservative mechanisms. But today, the use of microorganisms in terms of fermentation of meat and meat products is known as an effective preservation method [5]. Microbial enzymatic activities during fermentation leads to various physicochemical and microbial changes based on the meat components (natural or added components) [26]. Fermentation can occur by two pathways: (1) the use of natural microflora of meat or (2) the use of starter cultures such as lactic acid bacteria and micrococci. Lactic acid bacteria breakdown the carbohydrates and micrococci reduce nitrates and nitrites to nitric oxide that leads to production of volatile and nonvolatile compounds and flavor and odor changes of the product [27]. Also, fermentation causes different health-beneficial properties in fermented meat products in comparison to non-fermented ones such as antioxidant, antimicrobial, anti-hypertensive, and antithrombotic [28,29][28][29]. Furthermore, some nutritional components are produced during fermentation that have the potential to prevent diabetes, cancers, and allergic sensitization [30]. More studies are needed to discover other health-beneficial potentials of these products and their exact mechanisms. Also, these products should be evaluated in terms of food safety.3. A Brief Overview on LAB
LAB are part of the natural microbial flora of fermented meats and the intestinal microbiota of humans. These aerotolerant bacteria are mainly non-sporing, Gram-positive, Catalase-negative, and have either a spherical-shaped or rod-shaped cell (Figure 1) [31]. LAB are microaerophilic organisms and preferably require anaerobic conditions for growth. They play an important role in food fermentations; in fact, LAB can ferment carbohydrates to high amounts of lactic acid as the final product (homofermentative bacteria); in addition to lactic acid, heterofermentative bacteria produce acetic acid, carbon dioxide, and ethanol, as by-products [32]. These organisms are acidophilus with the optimum acidic pH values of 5.5–6.2, but few can tolerate pH as low as 3.0 [33]. LAB are Generally Regarded As Safe (GRAS) according to the FDA and the European Food Safety Authority (EFSA) that have granted many LAB species Qualified Presumption of Safety status (QPS) [25,34,35][25][34][35]. Lactic acid bacteria possess considerable bioactive properties such as cholesterol reduction and antimicrobial properties, which has led to an increased interest in their effective role as preservatives in innovative food preservation technology, much more than their application in traditional fermentation [36,37,38][36][37][38]. The antibacterial activity of LAB strains has been proven in different studies [39]. It has been reported that different LAB strains secrete various compounds that inhibit bacterial growth such as diacetyl, phenyl-lactate, organic acids, hydroxy fatty acid, hydroxy phenyl-lactate, hydrogen peroxide, propionate, and cyclic dipeptides. These bacteria also secrete biosurfactants, bacteriocins (i.e., acidophilin, lactacin, bifidocin, helveticin, plantarim, pediocin, bulgaricin, diplococcin, and nisin), and bacteriocin-like inhibitory substances [40,41][40][41].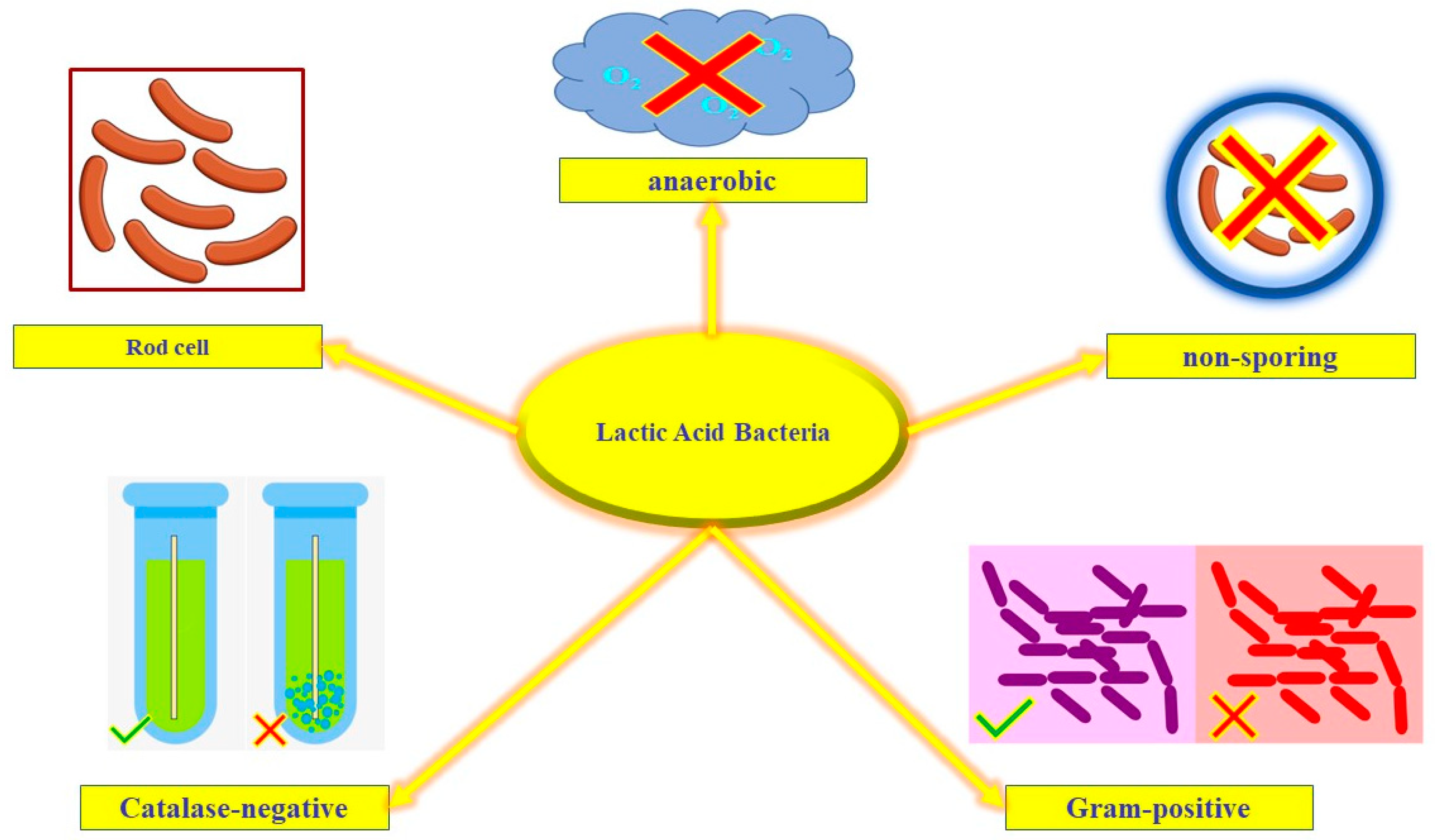
3.1. LAB Strains Involved in Fermented Meat Products
The technological characterization of LAB strains involved in the fermentation process of meat is essential to select the best strain to be utilized as starter cultures [42]. The genera Lactobacillus, Carnobacterium, Weissella, Pediococcus, Enterococcus, and Leuconostoc are the main LAB that play crucial role in the fermentation [42,43][42][43]. The list of some of GRAS LAB that are most commonly used in bio-preservation of meat and meat products are mentioned in Table 1. Members of the genus Lactobacillus are usually the dominant species in most fermented meat products, but in some slightly acidified sausages, both Enterococcus and Lactobacillus are present in similar amounts [43]; nevertheless, Lactobacillus plantarum and Lactobacillus curvatus are the most common LAB species in fermented sausages [26]. It has been reported that in many fermented sausages, Lactobacillus sakei has the most adaptability due to a higher maximum growth rate, higher final cell density, and a shorter lag phase [44,45][44][45]. It should be noted that in Southern European sausages, the most and least common species are Lactobacillus sakei and Pediococci spp., respectively [46]. Also, molds, such as Penicillium chrysogenum and Penicillium nalgiovense, are commonly used for sausage ripening in Southern Europe [47]. In artisan sausages from Southern Europe, a strain of Enterococcus faecium grows increasingly during the early stages of fermentation, producing a bacteriocin [48]. It has been reported that yeast genera, especially Debaryomyces hansenii, can be found in fermented meat products with appropriate organoleptic characteristics [49,50][49][50].Genus | Species | Genus | Species |
|---|
Product | Bio-Preservative Agent | Results | References | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Lactobacillus | Lactobacillus delbrueckii | Lacticaseibacillus | Lacticaseibacillus paracasei | |||||||||||||
Ground beef | L. reuteri or L. plantarum in combination with garlic extract | 1.4 and 1.5 log reduction of L. monocytogenes by using L. reuteri or L. plantarum in combination with 1% of garlic extract |
[116] |
[56] |
||||||||||||
Lactobacillus bulgaricus | Lacticaseibacillus rhamnosus | |||||||||||||||
Beef sausage | Bacteriocinogenic Enterococcus mundtii and 0.0075% ascorbic acid, 3% NaCl, 0.02% NaNO2, 0.75% glucose and 0.75% sucrose | >2 log cfu/g reduction of L. monocytogenes |
[117] |
[57] |
Lactobacillus gallinarum | Lacticaseibacillus casei | ||||||||||
Lactobacillus gasseri | ||||||||||||||||
Sliced beef | Bacteriocin from C. maltaromaticum combined with steam and chitosan | No synergistic effect 2 log reduction of S. typhimurium, E. coli and S. typhimurium |
[118] |
[58] |
||||||||||||
Minced beef meat | Pediococcus | Pediococcus acidilactici | ||||||||||||||
Mentha piperita essential oil with semipurified bacteriocin | Reduction in Enterobacteriaceae |
[119] |
[59] |
Lactobacillus lactis | ||||||||||||
Frozen ground beef patties | Pediococcus pentosaceus | |||||||||||||||
Bacteriocin-producing L. curvatus and L. lactis in combination with Na2EDTA | 1 log reduction of E. coli |
[120] |
[60] |
Lactobacillus helveticus | ||||||||||||
Fresh chicken meat burger | Pediococcus parvulus | |||||||||||||||
L. pseudomesenteroides combined with MAP (50% CO2 and 50% O2) | Reduction in L. monocytogenes and C. jejuni |
[115] |
[55] |
Lactobacillus reuteri | Leuconostoc | |||||||||||
Fresh pork sausage | Combination of essential oils, nisin, nitrite, and organic acid salts, encapsulated | Leuconostoc mesenteroides | ||||||||||||||
Reduction in L. monocytogenes |
[121] |
[61] |
Lactobacillus acidophilus | Leuconostoc citreum | ||||||||||||
Alheira paste | L. sakei and L. plantarum, vacuum packed or packed under MAP (20% CO2, 80% N2) | 2 log reduction in L. monocytogenes by L. sakei. No significant differences between vacuum or MAP |
[122] |
[62] |
Lactobacillus curvatus | Leuconostoc pseudomesenteroides | ||||||||||
Sliced lombo | Combination of Bacteriocin from P. acidilactici with HPP | Reduction in L. innocua |
[123] |
[63] |
Lactobacillus sakei | Leuconostoc carnosum | ||||||||||
Lactobacillusalivarius | ||||||||||||||||
Lactiplantibacillus | Lactiplantibacillus pentosus | Latilactobacillus | Latilactobacillus sakei | |||||||||||||
Lactiplantibacillus plantarum | Latilactobacillus curvatus | |||||||||||||||
Lactiplantibacillus brevis | Limosilactobacillus | Limosilactobacillus fermentum | ||||||||||||||
Lactiplantibacillus casei | Limosilactobacillus reuteri |
3.2. Bio-Preservation of Meat and Meat Products by LAB and Their Metabolites
3.3. LAB or Their Metabolites as a Part of Hurdle Technology
Hurdle technology refers to the combination of different preservative factors such as water activity (aw), temperature, redox potential (Eh), and novel preservative techniques, such as gas packaging, natural extracts, essential oils, and bacteriocins, to create more selective and efficient defensive systems to overcome pathogenic and spoilage microorganisms [69][52]. So, LAB and their metabolites can be used as a part of hurdles technology that act synergistically and inhibit food spoilage in combination with other preservative agents. In this way, less intensities of technological treatment and/or doses of preservative agents are required [113][53]. In the application of different antimicrobial agents, it is very important to select the best combination, so that desirable preservative effects are achieved. In this regard, it has been reported that the addition of chelating agents makes the outer membranes of Gram-negative bacteria more permeable and sensitive to the hydrophobic peptides such as bacteriocins [114][Goat meat emulsion | ||||||||||
Combination of Pediocin from | ||||||||||
P. pentosaceus | ||||||||||
and | Murraya koenigii berries extract | Reduction in L. innocua |
[124] |
[64] |
||||||
Ready-to-eat porkham | Bacteriocin-like inhibitory substances (BLIS) from P. pentosaceus and nisin | Inhibition of growth of L. seeligeri |
[76] |
[65] |
3.4. Kinetics Models for Microbial Inactivation
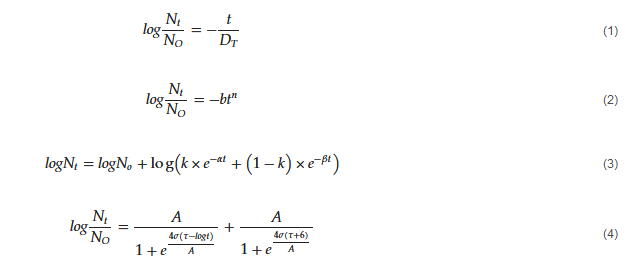
References
- Bohrer, B.M. Nutrient Density and Nutritional Value of Meat Products and Non-Meat Foods High in Protein. Trends Food Sci. Technol. 2017, 65, 103–112.
- Gao, X.; Li, C.; He, R.; Zhang, Y.; Wang, B.; Zhang, Z.-H.; Ho, C.-T. Research Advances on Biogenic Amines in Traditional Fermented Foods: Emphasis on Formation Mechanism, Detection and Control Methods. Food Chem. 2022, 405, 134911.
- Favaro, L.; Todorov, S.D. Bacteriocinogenic LAB Strains for Fermented Meat Preservation: Perspectives, Challenges, and Limitations. Probiotics Antimicrob. Proteins 2017, 9, 444–458.
- Laukkanen-Ninios, R.; Fredriksson-Ahomaa, M.; Korkeala, H. Enteropathogenic Yersinia in the Pork Production Chain: Challenges for Control. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1165–1191.
- Ashaolu, T.J.; Khalifa, I.; Mesak, M.A.; Lorenzo, J.M.; Farag, M.A. A Comprehensive Review of the Role of Microorganisms on Texture Change, Flavor and Biogenic Amines Formation in Fermented Meat with Their Action Mechanisms and Safety. Crit. Rev. Food Sci. Nutr. 2021, 20, 1–18.
- Deak, T. Thermal Treatment. In Food Safety Manag.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 423–442.
- Buncic, S.; Nychas, G.-J.; Lee, M.R.F.; Koutsoumanis, K.; Hébraud, M.; Desvaux, M.; Chorianopoulos, N.; Bolton, D.; Blagojevic, B.; Antic, D. Microbial Pathogen Control in the Beef Chain: Recent Research Advances. Meat Sci. 2014, 97, 288–297.
- Kaveh, S.; Mahoonak, A.S.; Ghorbani, M.; Jafari, S.M. Fenugreek Seed (Trigonella Foenum Graecum) Protein Hydrolysate Loaded in Nanosized Liposomes: Characteristic, Storage Stability, Controlled Release and Retention of Antioxidant Activity. Ind. Crops Prod. 2022, 182, 114908.
- Radi, M.; Shadikhah, S.; Sayadi, M.; Kaveh, S.; Amiri, S.; Bagheri, F. Effect of Thymus Vulgaris Essential Oil-Loaded Nanostructured Lipid Carriers in Alginate-Based Edible Coating on the Postharvest Quality of Tangerine Fruit. Food Bioprocess Technol. 2023, 16, 185–198.
- Duarte, M.; de Fátima Carrijo, K. Quantificação Do Teor de Nitrito de Sódio Residual Em Linguiças Cozidas Tipo Calabresa Comercializadas No Sul Do Estado Do Rio de Janeiro, Brasil. Enciclopédia Biosf. 2014, 10, 1606–1615.
- Eskandari, M.H.; Hosseinpour, S.; Mesbahi, G.; Shekarforoush, S. New Composite Nitrite-free and Low-nitrite Meat-curing Systems Using Natural Colorants. Food Sci. Nutr. 2013, 1, 392–401.
- Bhattacharya, D.; Nanda, P.K.; Pateiro, M.; Lorenzo, J.M.; Dhar, P.; Das, A.K. Lactic Acid Bacteria and Bacteriocins: Novel Biotechnological Approach for Biopreservation of Meat and Meat Products. Microorganisms 2022, 10, 2058.
- Flores, M.; Toldrá, F. Chemistry, Safety, and Regulatory Considerations in the Use of Nitrite and Nitrate from Natural Origin in Meat Products-Invited Review. Meat Sci. 2021, 171, 108272.
- Hernández-Aquino, S.; Miranda-Romero, L.A.; Fujikawa, H.; Maldonado-Simán, E.D.E.J.; Alarcon-Zuniga, B. Antibacterial Activity of Lactic Acid Bacteria to Improve Shelf Life of Raw Meat. Biocontrol Sci. 2019, 24, 185–192.
- Jafarpour, D.; Hashemi, S.M.B. Pure and Co-Fermentation of Quinoa Seeds by Limosilactobacillus Fermentum and Lacticaseibacillus Rhamnosus: Bioactive Content, Antidiabetic and Antioxidant Activities. Fermentation 2023, 9, 80.
- Kaveh, S.; Gholamhosseinpour, A.; Mohammad, S.; Hashemi, B.; Jafarpour, D.; Castagnini, J.M. Original Article Recent Advances in Ultrasound Application in Fermented and Non-Fermented Dairy Products: Antibacterial and Bioactive Properties. Int. J. Food Sci. Technol. 2023, 58, 3591–3607.
- Rezazadeh-Bari, M.; Najafi-Darmian, Y.; Alizadeh, M.; Amiri, S. Numerical Optimization of Probiotic Ayran Production Based on Whey Containing Transglutaminase and Aloe Vera Gel. J. Food Sci. Technol. 2019, 56, 3502–3512.
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An Overview of Natural Antimicrobials Role in Food. Eur. J. Med. Chem. 2018, 143, 922–935.
- Singh, V.P. Recent Approaches in Food Bio-Preservation-a Review. Open Vet. J. 2018, 8, 104–111.
- Radosavljević, M.; Lević, S.; Pejin, J.; Mojović, L.; Nedović, V. Encapsulation Technology of Lactic Acid Bacteria in Food Fermentation. In Lactic Acid Bacteria in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 319–347.
- Register, F. Nisin Preparation: Affirmation of GRAS Status as a Direct Human Food Ingredient. Fed. Regist. 1988, 54, 11247–11251.
- Qiu, Y.; Lei, P.; Zhang, Y.; Sha, Y.; Zhan, Y.; Xu, Z.; Li, S.; Xu, H.; Ouyang, P. Recent Advances in Bio-Based Multi-Products of Agricultural Jerusalem Artichoke Resources. Biotechnol. Biofuels 2018, 11, 1–15.
- Venegas-Ortega, M.G.; Flores-Gallegos, A.C.; Martínez-Hernández, J.L.; Aguilar, C.N.; Nevárez-Moorillón, G. V Production of Bioactive Peptides from Lactic Acid Bacteria: A Sustainable Approach for Healthier Foods. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1039–1051.
- Ananou, S.; Maqueda, M.; Martínez-Bueno, M.; Valdivia, E. Biopreservation, an Ecological Approach to Improve the Safety and Shelf-Life of Foods. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 1, 475–487.
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordónez, A. Application of Lactic Acid Bacteria for the Biopreservation of Meat Products: A Systematic Review. Meat Sci. 2022, 183, 108661.
- Ojha, K.S.; Kerry, J.P.; Duffy, G.; Beresford, T.; Tiwari, B.K. Technological Advances for Enhancing Quality and Safety of Fermented Meat Products. Trends Food Sci. Technol. 2015, 44, 105–116.
- Lorenzo, J.M.; Bedia, M.; Bañón, S. Relationship between Flavour Deterioration and the Volatile Compound Profile of Semi-Ripened Sausage. Meat Sci. 2013, 93, 614–620.
- Ashaolu, T.J.; Reale, A. A Holistic Review on Euro-Asian Lactic Acid Bacteria Fermented Cereals and Vegetables. Microorganisms 2020, 8, 1176.
- Xing, L.; Liu, R.; Cao, S.; Zhang, W.; Guanghong, Z. Meat Protein Based Bioactive Peptides and Their Potential Functional Activity: A Review. Int. J. Food Sci. Technol. 2019, 54, 1956–1966.
- Ashaolu, T.J. Safety and Quality of Bacterially Fermented Functional Foods and Beverages: A Mini Review. Food Qual. Saf. 2020, 4, 123–127.
- Strafella, S.; Simpson, D.J.; Yaghoubi Khanghahi, M.; De Angelis, M.; Gänzle, M.; Minervini, F.; Crecchio, C. Comparative Genomics and in Vitro Plant Growth Promotion and Biocontrol Traits of Lactic Acid Bacteria from the Wheat Rhizosphere. Microorganisms 2020, 9, 78.
- Ribeiro, S.C.; Coelho, M.C.; Silva, C.C.G. A Rapid Screening Method to Evaluate Acidifying Activity by Lactic Acid Bacteria. J. Microbiol. Methods 2021, 185, 106227.
- Khalid, K. An Overview of Lactic Acid Bacteria. Int. J. Biosci. 2011, 1, 1–13.
- BIOHAZ; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F. Scientific Opinion on the Update of the List of QPS-recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA (2017–2019). EFSA J. 2020, 18, e05966.
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858.
- Saranraj, P.; Naidu, M.A.; Sivasakthivelan, P. Lactic Acid Bacteria and Its Antimicrobial Properties: A Review. Int. J. Pharm. Biol. Arch. 2013, 4, 1124–1133.
- Hashemi, S.M.B.; Abedi, E.; Kaveh, S.; Mousavifard, M. Hypocholesterolemic, Antidiabetic and Bioactive Properties of Ultrasound-Stimulated Exopolysaccharide Produced by Lactiplantibacillus Plantarum Strains. Bioact. Carbohydr. Diet. Fibre 2022, 28, 100334.
- Walhe, R.A.; Diwanay, S.S.; Patole, M.S.; Sayyed, R.Z.; Al-Shwaiman, H.A.; Alkhulaifi, M.M.; Elgorban, A.M.; Danish, S.; Datta, R. Cholesterol Reduction and Vitamin B12 Production Study on Enterococcus Faecium and Lactobacillus Pentosus Isolated from Yoghurt. Sustainability 2021, 13, 5853.
- Al-Dhabi, N.A.; Esmail, G.A.; Valan Arasu, M. Co-Fermentation of Food Waste and Municipal Sludge from the Saudi Arabian Environment to Improve Lactic Acid Production by Lactobacillus Rhamnosus AW3 Isolated from Date Processing Waste. Sustainability 2020, 12, 6899.
- Lee, N.-K.; Paik, H.-D. Prophylactic Effects of Probiotics on Respiratory Viruses Including COVID-19: A Review. Food Sci. Biotechnol. 2021, 30, 773–781.
- Mei, J.; Ma, X.; Xie, J. Review on Natural Preservatives for Extending Fish Shelf Life. Foods 2019, 8, 490.
- Hugo, C.J.; Hugo, A. Current Trends in Natural Preservatives for Fresh Sausage Products. Trends Food Sci. Technol. 2015, 45, 12–23.
- Ammor, S.; Dufour, E.; Zagorec, M.; Chaillou, S.; Chevallier, I. Characterization and Selection of Lactobacillus Sakei Strains Isolated from Traditional Dry Sausage for Their Potential Use as Starter Cultures. Food Microbiol. 2005, 22, 529–538.
- Hugas, M.; Garriga, M.; Aymerich, M.T. Functionalty of Enterococci in Meat Products. Int. J. Food Microbiol. 2003, 88, 223–233.
- Toldrá, F.; Hui, Y.H. Dry-Fermented Sausages and Ripened Meats: An Overview. In Handbook of fermented meat and poultry; Toldrá, F., Hui, Y.H., Astiasarán, I., Sebranek, J.G., Talon, R., Eds.; Wiley Online Library: New York, NY, USA, 2014; pp. 1–6.
- Franz, C.M.A.P.; Stiles, M.E.; Schleifer, K.H.; Holzapfel, W.H. Enterococci in Foods—A Conundrum for Food Safety. Int. J. Food Microbiol. 2003, 88, 105–122.
- Leroy, F.; Verluyten, J.; De Vuyst, L. Functional Meat Starter Cultures for Improved Sausage Fermentation. Int. J. Food Microbiol. 2006, 106, 270–285.
- Todorov, S.D.; Favaro, L.; Gibbs, P.; Vaz-Velho, M. Enterococcus Faecium Isolated from Lombo, a Portuguese Traditional Meat Product: Characterisation of Antibacterial Compounds and Factors Affecting Bacteriocin Production. Benef. Microbes 2012, 3, 319–330.
- Andrade, M.J.; Córdoba, J.J.; Casado, E.M.; Córdoba, M.G.; Rodríguez, M. Effect of Selected Strains of Debaryomyces Hansenii on the Volatile Compound Production of Dry Fermented Sausage “Salchichón”. Meat Sci. 2010, 85, 256–264.
- Cano-García, L.; Flores, M.; Belloch, C. Molecular Characterization and Aromatic Potential of Debaryomyces Hansenii Strains Isolated from Naturally Fermented Sausages. Food Res. Int. 2013, 52, 42–49.
- Egan, K.; Field, D.; Rea, M.C.; Ross, R.P.; Hill, C.; Cotter, P.D. Bacteriocins: Novel Solutions to Age Old Spore-Related Problems? Front. Microbiol. 2016, 7, 461.
- Castellano, P.; Belfiore, C.; Fadda, S.; Vignolo, G. A Review of Bacteriocinogenic Lactic Acid Bacteria Used as Bioprotective Cultures in Fresh Meat Produced in Argentina. Meat Sci. 2008, 79, 483–499.
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N. Ben Bacteriocin-Based Strategies for Food Biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70.
- Belfiore, C.; Castellano, P.; Vignolo, G. Reduction of Escherichia Coli Population Following Treatment with Bacteriocins from Lactic Acid Bacteria and Chelators. Food Microbiol. 2007, 24, 223–229.
- Melero, B.; Diez, A.M.; Rajkovic, A.; Jaime, I.; Rovira, J. Behaviour of Non-Stressed and Stressed Listeria Monocytogenes and Campylobacter Jejuni Cells on Fresh Chicken Burger Meat Packaged under Modified Atmosphere and Inoculated with Protective Culture. Int. J. Food Microbiol. 2012, 158, 107–112.
- Khalili Sadaghiani, S.; Aliakbarlu, J.; Tajik, H.; Mahmoudian, A. Anti-listeria Activity and Shelf Life Extension Effects of Lactobacillus along with Garlic Extract in Ground Beef. J. Food Saf. 2019, 39, e12709.
- Orihuel, A.; Bonacina, J.; Vildoza, M.J.; Bru, E.; Vignolo, G.; Saavedra, L.; Fadda, S. Biocontrol of Listeria Monocytogenes in a Meat Model Using a Combination of a Bacteriocinogenic Strain with Curing Additives. Food Res. Int. 2018, 107, 289–296.
- Hu, Z.Y.; Balay, D.; Hu, Y.; McMullen, L.M.; Gänzle, M.G. Effect of Chitosan, and Bacteriocin–Producing Carnobacterium Maltaromaticum on Survival of Escherichia Coli and Salmonella Typhimurium on Beef. Int. J. Food Microbiol. 2019, 290, 68–75.
- Smaoui, S.; Hsouna, A.B.; Lahmar, A.; Ennouri, K.; Mtibaa-Chakchouk, A.; Sellem, I.; Najah, S.; Bouaziz, M.; Mellouli, L. Bio-Preservative Effect of the Essential Oil of the Endemic Mentha Piperita Used Alone and in Combination with BacTN635 in Stored Minced Beef Meat. Meat Sci. 2016, 117, 196–204.
- Castellano, P.; Belfiore, C.; Vignolo, G. Combination of Bioprotective Cultures with EDTA to Reduce Escherichia Coli O157: H7 in Frozen Ground-Beef Patties. Food Control 2011, 22, 1461–1465.
- Ghabraie, M.; Vu, K.D.; Huq, T.; Khan, A.; Lacroix, M. Antilisterial Effects of Antibacterial Formulations Containing Essential Oils, Nisin, Nitrite and Organic Acid Salts in a Sausage Model. J. Food Sci. Technol. 2016, 53, 2625–2633.
- Vaz-Velho, M.; Jácomea, S.; Noronhab, L.; Todorovc, S.; Fonsecaa, S.; Pinheiro, R.; Moraisb, A.; Silvab, J.; Teixeirab, P. Comparison of Antilisterial Effects of Two Strains of Lactic Acid Bacteria during Processing and Storage of a Portuguese Salami-like Product “Alheira”. Chem. Eng. 2013, 32, 1807–1812.
- Castro, S.M.; Silva, J.; Casquete, R.; Queirós, R.; Saraiva, J.A.; Teixeira, P. Combined Effect of Pediocin BacHA-6111-2 and High Hydrostatic Pressure to Control Listeria Innocua in Fermented Meat Sausage. Int. Food Res. J. 2018, 25, 553–560.
- Kumar, Y.; Kaur, K.; Shahi, A.K.; Kairam, N.; Tyagi, S.K. Antilisterial, Antimicrobial and Antioxidant Effects of Pediocin and Murraya Koenigii Berry Extract in Refrigerated Goat Meat Emulsion. LWT-Food Sci. Technol. 2017, 79, 135–144.
- de Azevedo, P.O.S.; Mendonça, C.M.N.; Seibert, L.; Domínguez, J.M.; Converti, A.; Gierus, M.; Oliveira, R.P.S. Bacteriocin-like Inhibitory Substance of Pediococcus Pentosaceus as a Biopreservative for Listeria Sp. Control in Ready-to-Eat Pork Ham. Braz. J. Microbiol. 2020, 51, 949–956.
- Esua, O.J.; Sun, D.-W.; Ajani, C.K.; Cheng, J.-H.; Keener, K.M. Modelling of inactivation kinetics of Escherichia coli and Listeria monocytogenes on grass carp treated by combining ultrasound with plasma functionalized buffer. Ultrason. Sonochem. 2022, 88, 106086.
