Earthworms and leeches are sentinel animals that represent the annelid phylum within terrestrial and freshwater ecosystems, respectively. One early stress signal in these organisms is related to innate immunity, but how nanomaterials affect it is poorly characterized. In this survey, we compare the latest literature on earthworm and leeches with examples of their molecular/cellular responses to inorganic (silver nanoparticles) and organic (carbon nanotubes) nanomaterials. A special focus is placed on the role of annelid immunocytes in the evolutionarily conserved antioxidant and immune mechanisms and protein corona formation and probable endocytosis pathways involved in nanomaterial uptake. Our summary helps to realize why these environmental sentinels are beneficial to study the potential detrimental effects of nanomaterials.
- nanoparticles
- innate immunity
- earthworms
- leeches
1. Introduction
Nanotechnology is an emerging field, which is claimed to be an ambassador in the new era of the industrial revolution
. Nanomaterials can have distinct physical properties and are also chemically more reactive than their bulk counterparts owing to their small sizes
. The number of engineered nanomaterials (ENMs) is steadily ascending; however, an additional increment is anticipated in the near future
. As a result of steady production and use, their deliberate or accidental discharge into the environment is of a particular concern for the health of the ecosystem and the human population
. In the environment, the toxicity of ENMs mainly depends upon their size distribution, chemical composition, surface charge, coating, shape, possible contaminants, and other physical properties
.
2. Risk Assessment of Silver Nanoparticles to Soil Invertebrates
Silver nanoparticles (AgNPs) in the size range of <100 nm are the most abundant metal-based ENMs and are increasingly being employed in industries, households, and commercial applications
. Despite their conceded antimicrobial attributes, several previous studies have reported on AgNPs’ “eco”-toxicity, which is frequently connected to the dissolved Ag ions (Ag
) released from AgNPs during the surface oxidation
. Nevertheless, it is also accepted that physico-chemical features of AgNPs have a substantial influence on ecotoxicity. For instance, previously it was confirmed that uncoated AgNPs are more toxic to soil-dwelling animals than their coated counterparts
. With the increment in Ag-based goods in our environment and daily life, it is fundamental to inquire about the possible toxicity of AgNPs and Ag
towards living organisms under multiple circumstances.
So far, bacteria, aquatic invertebrates, and soil-dwelling invertebrates have played prominent roles in nanotoxicology studies, since these organisms are among the first to encounter nanoparticles in our environment
. Several in vitro and in vivo studies (applying diverse cell lines and organisms) have dealt with the toxicity of AgNPs; however, the effects of particles on even closely-related species (in the sense of biochemical and immunological processes) are often debatable and ambiguous
. Furthermore, the use of AgNPs in different sizes and coatings makes these studies more complicated to compare. Among soil invertebrates, earthworms as key sentinel species are widely applied in soil nanotoxicity. They are often chronically exposed to contaminants via the epidermis and the gastrointestinal tract because of the ingested soil
. Earthworms possess relatively high heavy metal resistance and are also able to temporarily store and inactivate heavy metal ions; therefore, they are intensely impacted by soil contaminants
. Their responses towards metal exposures are evincible at several biological levels, from genetic to cellular
. During phylogenesis, differentiated mesodermal tissues and body (coelomic) cavities developed initially in these animals, and this progression makes earthworms more suitable toxicological models for cross-species comparisons with higher developed animals or vertebrates
.
To date, most results have emphasized the endpoints of AgNPs’ toxicity in earthworms to individuals’ physiological parameters for comprehending the mortality following chronic or acute exposures, cocoon production and reproduction, bioaccumulation, and occasionally growth or behavior
. During these in vivo examinations in diverse type of soils, ionic Ag (Ag
from AgNO
, used as a positive reference) was more toxic to earthworms than the NP counterpart. AgNPs also have noxious impacts on animals at higher concentrations
. Regarding in vivo studies we must consider the complexity of soil components and soil matrix properties with the soil type (e.g., organic matter, cations, moisture, water retention capacity, ionic strength, and fluctuating pH), because these factors may influence the behavior of NPs in soil (e.g., higher propensity to heteroagglomeration/homoagglomeration, change in morphology, and dissolution)
.
3. Routes of AgNP Uptake in Invertebrates
The interaction of NPs and immune cells, be it through the protein corona or not, in general leads to uptake and/or activation of signaling cascades downstream of receptor ligation. Here we focus on the routes of NP uptake in invertebrates, for which some insights are available in the literature that may also be relevant in understanding the very process in annelids. According to the general classification for eukaryotes, there are two major endocytic pathways, such as phagocytosis and pinocytosis that can be distinguished based on the cell type-specificity and target entities that are taken up
[30].
Phagocytosis is involved in the immune function against large non-self elements of biological (bacteria and viruses) and chemical (including microparticles) origins and is performed mostly by professional immunocytes, such as macrophages, monocytes, dendritic cells, and neutrophils
[31]. Classically, the process starts with the recognition of opsonized particles, and proceeds to actin-driven ingestion and the forming of the phagolysosome
[30].
Macropinocytosis is another type of actin-dependent bulk engulfment, but it differs from phagocytosis in that it captures solutes in a non-specific manner. During macropinocytosis, membrane protrusions with the help of actin fuse around the solutes, forming 1 µm or bigger macropinosomes, which then merge with lysosomes.
Practically, all types of cells are capable of endocytosis, which takes place by at least four different pathways, including micropinocytosis, clathrin-mediated endocytosis (CME), caveolin-mediated endocytosis (CvME), and clathrin/caveolin-independent endocytosis
[30][32]. Briefly, internalization by CME occurs with the help of clathrin, which forms a basket-like structure at the cell membrane, typically around 150 nm in size
[30]. This process, just like phagocytosis, leads to the fusion with lysosomes forming endolysosomes
[32][33]. CvME is characterized by the formation of 50–80 nm flask-shaped membrane invaginations, the main component of which is caveolin, a cholesterol-binding protein. In contrast to abovementioned pathways, a formed caveosome does not contain enzymatic proteins, and the vesicle does not go through lysosomal degradation
[30][33]. Among the clathrin, caveolin-independent endocytic pathways, particularly well described is a mechanism involving 40–50 nm sized cholesterol-rich lipid “rafts”, which participate in sorting of intracellular molecules
[34]; however, these pathways are not reported to be extensively involved in NP uptake (
Figure 1)
[35].
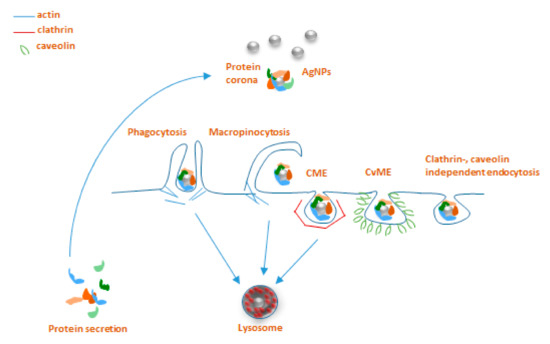
Main endocytosis pathways possibly involved in internalization of AgNPs: phagocytosis, macropinocytosis, clathrin-mediated endocytosis (CME), caveolin-mediated endocytosis (CvME), and clathrin/caveolin-independent endocytosis.
Very limited data are available about the involvement of particular endocytosis pathways for AgNP uptake in invertebrates, with practically no information on these mechanisms for earthworms. On the other hand, somewhat better unravelled AgNPs endocytosis mechanisms have been reported for other invertebrate species of nematodes, snails, and mussels. These studies applied various in vivo and in vitro approaches using pharmacological inhibitors originally developed for use in vertebrates. Endocytic mechanisms of the nematode
, one of the classical animal research models, are among the most deeply studied, compared to other invertebrates. Maurer et al.
investigated the uptake pathways for citrate‐coated 25 nm AgNPs using both pharmacological inhibitors and endocytosis‐deficient mutants. Interestingly, the CME inhibitor chlorpromazine almost entirely abolished the AgNP‐related toxicity, indicating the importance of CME in AgNPs uptake in nematodes. Moreover, the research also provided evidence for decreased sensitivity of endocytosis‐deficient mutants that accumulated fewer AgNPs than did the wild‐type nematodes. Khan and co‐workers
revealed, by exposing of estuarine snail
to 18 nm AgNPs in aqueous solution, that AgNPs uptake was largely affected by inhibition of CME by amantadine. Blocking CvME and the channel‐coupled H
pump also reduced the internalization, but only after 24h of exposure, indicating the possible differences in the uptake kinetics via different pathways. Phenylarsine oxide (non‐specific inhibitor of macropinocytosis) affected the AgNPs uptake after 6 h exposure, which might result from activation of macropinocytosis as a compensatory pathway when the other mechanisms are less active. Recent research on
mussel hemocytes discusses the connection between different endocytic pathways, AgNP cytotoxicity, and immunocyte counts
. Upon the blocking of CME by amantadine, small AgNPs (<50 nm) caused slight toxicity to hemocytes. A similar tendency was observed for AgNPs of a bigger size (>100 nm); when CvME was inhibited by nystatin, AgNPs caused only minor toxicity to those cells. This is controversial, considering the general opinion that 40–50 nm NPs are internalized by CvME, while bigger (100–120 nm) NPs are taken up by CME. Nevertheless, the endocytosis inhibitors changed the sensitivity of hemocytes towards the AgNPs, mainly showing a delayed effect in the onset of NP toxicity. Notably, none of them completely blocked AgNP uptake and related cytotoxic effects, suggesting that several uptake pathways participate in the process parallelly orby compensating for each other. Besides, in these studies, the AgNP content was measured in whole animal tissues and thus did not elucidate the role of invertebrate immunocytes in AgNP uptake, which are the first responders to foreign particles that infiltrate the body of the host organism. One of the limited studies on this topic investigated the transcriptomic changes of AgNP exposure in the earthworm
. It brought interesting results of upregulation of genes coding for endocytosis and functions of cell cilia. Earlier there was evidence that so‐called “ciliary pockets” in the invertebrates’ epithelial membranes are involved in CME
, which might indicate the involvement of this mechanism in earthworms as well. To conclude, the summarized studies give an understanding of evolutionarily conserved processes potentially participating in AgNP uptake in annelids. Nevertheless, the area needs further investigations to obtain the complete picture of the NP entry to the cell and the immune responses in invertebrates.
4. Carbon Nanotubes and Related Toxicological Risks
In the last few decades, the development of nanotechnologies led to the discovery and production of several new nanomaterials that have recently acquired numerous roles, especially in industry. Among them, carbon nanotubes (CNTs), which consist of concentric graphene tubes with diameters of 0.7 to 100nm, are classified as single‐walled (SWCNTs) and multiwalled (MWCNTs) carbon nanotubes and represent one of the most promising groups for industrial development, nanoelectronics, mechanical engineering, and biomedical applications
. Moreover, as their functions are constantly improving, these nanoparticles are also gaining importance in the biological field. Indeed, although their discovery produced both social and economic benefits, their manufacturing corresponds to a rapid expansion of their environmental discharge. In particular, due to their non‐biodegradable characteristics, their presence has been observed both in air and in soil, and often CNTs are also released into the surface water (such as rivers and lakes), posing potential risks to human and animal health. A number of studies recently demonstrated both the toxicity and the bio‐persistence of CNTs within tissues and cells
, and these elements are connected with their morphology, which is a crucial aspect for the development of human diseases, such as mesothelial injuries and carcinogenesis
. These features are very similar to those of asbestos fibers, showing asbestos‐like pathogenicity
. In fact, it has been observed that CNTs also induce frustrated phagocytosis
and cause the release of pro inflammatory cytokines and reactive oxygen species (ROS)
. They possess a very long half‐life in vivo and can affect the cellular functions after physically penetrating the biological barriers and causing chronic inflammation, which is often connected with cancer insurgence
. Interestingly, all these data point out that the immune system and ROS production represent sensitive physiological indicators following exposure to CNTs, even at low concentrations. In this context, the necessity to develop and optimize new approaches for investigating the effects of CNTs suggests the use of specific research models and the choice falls back on those normally employed in ecotoxicological studies.
4.1. Carbon Nanotubes and Acquatic Invertebrates
Invertebrates attracted great interest thanks to the possibility of obtaining rapid and secure evaluations. The use of some species rapidly increased in the last decades, replacing vertebrates in many research fields. The benefits are related both to their anatomical and to their physiological characteristics that allow one to easily analyze many biological processes, and to their low complexity at the genetic, cellular, and molecular levels. In particular, the toxic effects of CNTs released in the ecosystems
, whose predicted environmental concentrations (PECs) in aqueous systems are projected to approximately be 0.001–1000 μg/L
, have been mostly investigated in aquatic invertebrates, especially in those from marine environments. It is well‐known that these animals coordinate fast and sensitive reactions, especially those associated with acute behavioural or developmental responses, enzymatic activity alterations, regenerative capacity, respiration rate, and biochemical performance, following exposure to CNTs. Indeed, exposure to carbon‐based nanomaterials causes in
inhibition of larval swimming and alterations in the enzyme activities in a concentration–dependent manner
. Moreover, MWCNTs induced negative effects on the regenerative capacity in the polychaete
. Additionally, higher MWCNT concentrations induce energy‐related responses characterized by higher values of electron transport system activity, glycogen, and protein concentrations in both the polychaete species
and
exposed to this contaminant. Furthermore, oxidative stress with higher lipid peroxidation, lower ratios between reduced and oxidized glutathione, and higher activity of antioxidant and biotransformation enzymes are detectable in those organisms exposed to MWCNTs
. A MWCNT concentration‐dependent effect is also evident in larval development in the two different populations (Mediterranean and Atlantic) of polychaete
. Surprisingly, an unexpected decrease in the effect, in terms of interference with the correct development of larvae, was observed at the highest exposure concentration of MWCNTs (9.00 mg/L). This apparent reduction in the toxic effect at high concentrations is probably due to the formation of large MWCNT aggregates that, through precipitating on the seabed by gravitational sedimentation
, interact less with the larvae. Sedimented MWCNT aggregates are, however, a cause of several toxic effects on benthic animals living on or in the seabed, such as adult polychaetes and mollusk bivalves. Indeed, studies performed on
clearly demonstrate that the accumulation of MWCNT aggregates provokes neurotoxicity, alters energy‐related biochemical processes, and activates antioxidant defenses and biotransformation mechanisms
. Moreover, the MWCNT aggregates sedimented by the water column have their capture by the gills of bivalves facilitated
. Indeed, due to the feeding by filtering, nanoparticles are trapped by gills of bivalves, flow into the gut and the digestive gland, and are translocated from the gut into the hemolymph accumulating inside the body. Large MWCNT aggregates, observed in the intestinal lumen, in the tubules of the digestive gland and gills of several species of bivalves (
), induce significant morphological organ damages, such as erosion and necrosis of the epithelium, increased vacuolization and apoptosis of the cells, and swelling of the connective tissue
. The contact of nanomaterials with the target tissues not only involves physical damages but also provokes oxidative stress in the cells, by directly inducing the production of ROS
. Moreover, evaluation of the hemolymph and circulating hemocytes reveals that MWCNTs may cause immunotoxicity in some bivalve species. Indeed, after 48 h exposure to MWCNTs, the total hemocyte count in the scallops
was significantly reduced while the average hemocyte granularity greatly increased. These effects could be caused not only by the death of hemocytes due to a negative influence of NPs but may also be related to mass migration of hemocytes from circulation towards other tissues for elimination of NPs. The increased hemocyte granularity is, on the other hand, probably connected with phagocytic clearance of NPs in the affected organs
.
References
- Hansen, S.F.; Michelson, E.S.; Kamper, A.; Borling, P.; Stuer‐Lauridsen, F.; Baun, A. Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicology 2008, 17, 438–447, doi:10.1007/s10646‐008‐0210‐4.
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 1–35, doi:10.1080/10590500802708267.
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical‐physical applications to nanomedicine. Molecules 2019, 25, 112, doi:10.3390/molecules25010112.
- Prajitha, N.; Athira, S.S.; Mohanan, P.V. Bio‐interactions and risks of engineered nanoparticles. Environ. Res. 2019, 172, 98–108, doi:10.1016/j.envres.2019.02.003.
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839, doi:10.1289/ehp.7339.
- Soni, D.; Naoghare, P.K.; Saravanadevi, S.; Pandey, R.A. Release, transport and toxicity of engineered nanoparticles. Rev. Environ. Contam. Toxicol. 2015, 234, 1–47, doi:10.1007/978‐3‐319‐10638‐0_1.
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich‐Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size‐dependent generation of reactive oxygen species. J. Phys. Chem. B. 2008, 112, 13608–13619, doi:10.1021/jp712087m.
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F.; Rejesk, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein, J. Nanotechnol. 2015, 6, 1769–1780, doi:10.3762/bjnano.6.181.
- Ahmed, K.B.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. In Vitro 2017, 38, 179–192, doi:10.1016/j.tiv.2016.10.012.
- Du, J.; Tang, J.; Xu, S.; Ge, J.; Dong, Y.; Li, H.; Jin, M. A review on silver nanoparticles‐induced ecotoxicity and the underlying toxicity mechanisms. Regul. Toxicol. Pharmacol. 2018, 98, 231–239, doi:10.1016/j.yrtph.2018.08.003.
- Shoults‐Wilson, W.A.; Reinsch, B.C.; Tsyusko, O.V.; Bertsch, P.M.; Lowry, G.V.; Unrine, J.M. Effect of silver nanoparticle surface coating on bioaccumulation and reproductive toxicity in earthworms (Eisenia fetida). Nanotoxicology 2011, 5, 432–444, doi:10.3109/17435390.2010.537382.
- Hayashi, Y.; Engelmann, P.; Foldbjerg, R.; Szabó, M.; Somogyi, I.; Pollák, E.; Molnár, L.; Autrup, H.; Sutherland, D.S.; Scott‐Fordsmand, J.; et al. Earthworms and humans in vitro: Characterizing evolutionarily conserved stress and immune responses to silver nanoparticles. Environ. Sci. Technol. 2012, 46, 4166–4173, doi:10.1021/es3000905.
- Zhang, C.; Hu, Z.; Deng, B. Silver nanoparticles in aquatic environments: Physiochemical behavior and antimicrobial mechanisms. Water Res. 2016, 88, 403–427, doi:10.1016/j.watres.2015.10.025.
- Rajala, J.E.; Vehniäinen, E.R.; Väisänen, A.; Kukkonen, J.V.K. Toxicity of silver nanoparticles to Lumbriculus variegatus is a function of dissolved silver and promoted by low sediment pH. Environ. Toxicol. Chem. 2018,37, 1889–1897, doi:10.1002/etc.4136.5624.
- Tang, S.; Zheng, J. Antibacterial activity of silver nanoparticles: Structural effects. Adv. Healthc. Mater. 2018,7, e1701503, doi:10.1002/adhm.201701503.
- Shoults‐Wilson, W.A.; Zhurbich, O.I.; McNear, D.H.; Tsyusko, O.V.; Bertsch, P.M.; Unrine, J.M. Evidence for avoidance of Ag nanoparticles by earthworms (Eisenia fetida). Ecotoxicology 2011, 20, 385–396, doi:10.1007/s10646‐010‐0590‐0.
- Makama, S.; Piella, J.; Undas, A.; Dimmers, W.J.; Peters, R.; Puntes, V.F.; van den Brink, N.W. Properties of silver nanoparticles influencing their uptake in and toxicity to the earthworm Lumbricus rubellus following exposure in soil. Environ. Pollut. 2016, 218, 870–878, doi:10.1016/j.envpol.2016.08.016.
- Exbrayat, J.M.; Moudilou, E.N.; Lapied, E. Harmful effects of nanoparticles on animals. J. Nanobiotechnol. 2015, 2015, 1–10.
- Kim, S.; Ryu, D.Y. Silver nanoparticle‐induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J. Appl. Toxicol. 2013, 33, 78–89, doi:10.1002/jat.2792.
- Tao, C. Antimicrobial activity and toxicity of gold nanoparticles: Research progress, challenges and prospects. Lett. Appl. Microbiol. 2018, 67, 537–543, doi:10.1111/lam.13082.
- Crane, J.K. Metal nanoparticles in infection and immunity. Immunol. Invest. 2020, 1–14, doi:10.1080/08820139.2020.1776724.
- Scott‐Fordsmand, J.J.; Krogh, P.H.; Schaefer, M.; Johansen, A. The toxicity testing of double‐walled nanotubes‐contaminated food to Eisenia veneta earthworms. Ecotoxicol. Environ. Saf. 2008, 71, 616–619, doi:10.1016/j.ecoenv.2008.04.011.
- Lapied, E.; Moudilou, E.; Exbrayat, J.M.; Oughton, D.H.; Joner, E.J. Silver nanoparticle exposure causes apoptotic response in the earthworm Lumbricus terrestris (Oligochaeta). Nanomedicine 2010, 5, 975-984, doi:10.2217/nnm.10.58.
- Demuynck, S.; Grumiaux, F.; Mottier, V.; Schikorski, D.; Lemière, S.; Leprêtre, A. Cd/Zn exposure interactions on metallothionein response in Eisenia fetida (Annelida, Oligochaeta). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 658–668, doi:10.1016/j.cbpc.2007.03.001.
- Irizar, A.; Rodríguez, M.P.; Izquierdo, A.; Cancio, I.; Marigómez, I.; Soto, M. Effects of soil organic matter content on cadmium toxicity in Eisenia fetida: Implications for the use of biomarkers and standard toxicity tests. Arch. Environ. Contam. Toxicol. 2015, 68, 181–192, doi:10.1007/s00244‐014‐0060‐4.
- Calisi, A.; Lionetto, M.G.; Schettino, T. Pollutant‐induced alterations of granulocyte morphology in the earthworm Eisenia foetida. Ecotoxicol. Environ. Saf. 2009, 72, 1369–1377, doi:10.1016/j.ecoenv.2009.03.010.
- Bodó, K.; Hayashi, Y.; Gerencsér, G.; László, Z.; Kéri, A.; Galbács, G.; Telek, E.; Mészáros, M.; Deli, A.M.; Kokhanyuk, B.; et al.; Engelmann, P. Species‐specific sensitivity of Eisenia earthworms towards nobel metal nanoparticles: A multiparametric in vitro study. Environ. Sci. Nano. 2020, doi:10.1039/C9EN0105E.
- Gomes, S.I.L.; Soares, A.M.V.M.; Scott‐Fordsmand, J.J.; Amorim, M.J.B. Mechanisms of response to silver nanoparticles on Enchytraeus albidus (Oligochaeta): Survival, reproduction and gene expression profile. J. Hazard. Mater. 2013, 254‐255, 336–344, doi:10.1016/j.jhazmat.2013.04.005.
- Velicogna, J.R.; Ritchie, E.E.; Scroggins, R.P.; Princz, J.I. A comparison of the effects of silver nanoparticles and silver nitrate on a suite of soil dwelling organisms in two field soils. Nanotoxicology 2016, 10, 1144–1151, doi:10.1080/17435390.2016.1181807.
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44, doi:10/1038/nature01451.
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623, doi:10.1146/annurev.immunol.17.1.593.
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896, doi:10.1007/s00018‐009‐0053‐z.
- Bareford, L.M.; Swaan, P.W. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 748–758, doi:10.1016/j.addr.2007.06.008
- Edidin, M. Shrinking patches and slippery rafts: Scales of domains in the plasma membrane. Trends Cell Biol. 2001, 11, 492–496, doi:10.1016/s0962‐89.
- Manzanares, D.; Ceña, V. Endocytosis: The nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics 2020, 12, 371, doi:10.3390/pharmaceutics12040371
- Maurer, L.L.; Yang, X.; Schindler, A.J.; Taggart, R.K.; Jiang, C.; Hsu‐Kim, H.; Sherwood, D.R.; Meyer, J.N. Intracellular trafficking pathways in silver nanoparticle uptake and toxicity in Caenorhabditis elegans. Nanotoxicology 2016, 10, 831–835, doi:10.3109/17435390.2015.1110759
- Khan, F.R.; Misra, S.K.; Bury, N.R.; Smith, B.D.; Rainbow, P.S.; Luoma, S.N.; Valsami‐Jones, E. Inhibition of potential uptake pathways for silver nanoparticles in the estuarine snail Peringia ulvae. Nanotoxicology 2015, 9, 493–501, doi:10.3109/17435390.2014.948519
- Bouallegui, Y.; Younes, R.B.; Turki, F.; Oueslati, R. Impact of exposure time, particle size and uptake pathway on silver nanoparticle effects on circulating immune cells in Mytilus galloprovincialis. J. Immunotoxicol. 2017, 14, 116–124, doi:10.1080/1547691X.2017.1335810.
- Bouallegui, Y.; Ben Younes, R.; Turki, F.; Mezni, A.; Oueslati, R. Effect of exposure time, particle size and uptake pathways in immune cell lysosomal cytotoxicity of mussels exposed to silver nanoparticles. Drug Chem. Toxicol. 2018, 41, 169–174, doi:10.1080/01480545.2017.1329317
- Novo, M.; Lahive, E.; Diez‐Ortiz, M.; Matzke, M.; Morgan, A.J.; Spurgeon, D.J.; Svendsen, C.; Kille, P. Different routes, same pathways: Molecular mechanisms under silver ion and nanoparticle exposures in the soil sentinel Eisenia fetida. Environ. Pollut. 2015, 205, 385–393, doi:10.1016/j.envpol.2015.07.010
- Benmerah, A. The ciliary pocket. Curr. Opin. Cell Biol. 2013, 25, 78–84, doi:10.1016/j.ceb.2012.10.011
- Mauter, M.S.; Eimelech, M.; Osuji, C.O. Nanocomposites of vertically aligned single‐walled carbon nanotubes by magnetic alignment and polymerization of a lyotropic precursor. ACS Nano 2010, 4, 6651-6658, doi:10.1021/nn102047j.
- Serpell, C.J.; Kostarelos, K.; Davis, B.G. Can carbon nanotubes deliver on their promise in biology; Harnessing unique properties for unparalleled applications. ACS Cent. Sci. 2016, 2, 190–200, doi:10.1021/acscentralsci.6b00005
- Saria, R.; Mouchet, F.; Perrault, A.; Flahaut, E.; Laplanche, C.; Boutonnet, C.; Pinelli, E.; Gauthier, L. Short term exposure to multi‐walled carbon nanotubes induce oxidative stress and DNA damage in Xenopus laevis tadpoles. Ecotox. Environ. Saf. 2014, 107, 22–29, doi:10.1016/j.ecoenv.2014.05.010.
- Pereira, M.M.; Mouton, L.; Yéprémian, C.; Couté, A.; Lo, J.; Marconcini, J.M.; Ladeira, L.O.; Raposo, N.R.; Brandão, H.M.; Brayner, R. Ecotoxicological effects of carbon nanotubes and cellulose nanofibers in Chlorella vulgaris. J. Nanobiotechnol. 2014, 12, 15, doi:10.1186/1477‐3155‐12‐15.
- Velseboer, I.; Peeters, E.T.; Koelmans, A.A. Multiwalled carbon nanotubes at environmentally relevant concentrations affect the composition of benthic communities. Environ. Sci. Technol. 2013, 47, 7475 7482, doi:10.1021/es400777j.
- Funahashi, S.; Okazaki, Y.; Ito, D.; Asakawa, A.; Nagai, H.; Tajima, M.; Toyokuni, S. Asbestos and multiwalled carbon nanotubes generate distinct oxidative responses in inflammatory cells. J. Clin. Biochem. Nutr. 2015, 56, 111–117, doi:10.3164/jcbn.14‐92.
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.; Seaton, A.; Stone, V.; Brown, S.; Macnee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos‐like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428, doi:10.1038/nnano.2008.111.
- Murphy, F.A.; Schinwald, A.; Poland, C.A.; Donaldson, K. The mechanism of pleural inflammation by long carbon nanotubes: Interaction of long fibres with macrophages stimulates them to amplify proinflammatory responses in mesothelial cells. Part. Fibre Toxicol. 2012, 9, 8, doi:10.1186/1743‐8977‐9‐8
- Dörger, M.; Münzing, S.; Allmeling, A.M.; Messmer, K.; Krombach, F. Differential responses of rat alveolar and peritoneal macrophages to man‐made vitreous fibers in vitro. Environ. Res. 2001, 85, 207–214, doi:10.1006/enrs.2001.4234
- Boyles, M.S.; Young, L.; Brown, D.M.; MacCalman, L.; Cowie, H.; Moisala, A.; Smail, F.; Smith, P.J.; Proudfoot, L.; Windle, A.H.; et al. Multi‐walled carbon nanotube induced frustrated phagocytosis, cytotoxicity and pro-inflammatory conditions in macrophages are length dependent and greater than that of asbestos. Toxicol. Vitro 2015, 29, 1513–1528, doi:10.1016/j.tiv.2015.06.012
- Petersen, E.J.; Zhang, L.; Mattison, N.T.; OʹCarroll, D.M.; Whelton, A.J.; Uddin, N.; Nguyen, T.; Huang, Q.; Henry, T.B.; Holbrook, R.D.; et al. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ. Sci. Technol. 2011, 45, 9837–9856, doi:10.1021/es201579y.
- Zhang, P.; Selck, H.; Tanga, S.R.; Pang, C.; Zhao, B. Bioaccumulation and effects of sediment‐associated gold‐ and graphene oxide nanoparticles on Tubifex tubifex. J. Environ. Sci. 2017, 51, 138–145, doi:10.1016/j.jes.2016.08.015
- Mesarič, T.; Gambardella, C.; Milivojević, T.; Faimali, M.; Drobne, D.; Falugi, C.; Makovec, D.; Jemec, A.; Sepčić, K. High surface adsorption properties of carbon‐based nanomaterials are responsible for mortality, swimming inhibition, and biochemical responses in Artemia salina larvae. Aquat. Toxicol. 2015, 163, 121–129, doi:10.1016/j.aquatox.2015.03.014
- De Marchi, L.; Neto, V.; Pretti, C.; Figueira, E.; Chiellini, F.; Morelli, A.; Soares, A.; Freitas, R. The influence of salinity on the effects of Multi‐walled carbon nanotubes on polychaetes. Sci. Rep. 2018, 8, 8571, doi:10.1038/s41598‐018‐26729‐2
- Oliva, M.; De Marchi, L.; Vieira S.M.; Pires, A.; Cuccaro, A.; Baratti, M.; Chiellini, F.; Morelli, A.; Freitas, R.; Pretti, C. Atlantic and Mediterranean populations of the widespread serpulid Ficopomatus enigmaticus: Developmental responses to carbon nanotubes. Mar. Pollut. Bull. 2020, 156, 111265, doi:10.1016/j.marpolbul.2020.111265
- Saleh, N.B.; Pfefferle, L.D.; Elimelech, M. Aggregation kinetics of multi‐walled carbon nanotubes in aquatic systems: Measurements and environmental implications. Environ. Sci. Technol. 2008, 42, 7963 7969, doi:10.1021/es801251c.
- Babakhani, P. The impact of nanoparticle aggregation on their size exclusion during transport in porous media: One‐and three‐dimensional modelling investigations. Sci. Rep. 2019, 9, 1–12, doi:10.1038/s41598 019‐50493‐6.
- Ward, J.E.; Kach, D.J. Marine aggregates facilitate ingestion of nanoparticles by suspension feeding bivalves. Mar. Environ. Res. 2009, 68, 137–142, doi:10.1016/j.marenvres.2009.05.002
- Anisimova, A.A.; Chaika, V.V.; Kuznetsov, V.L.; Golokhvast, K.S. Study of the influence of multiwalled carbon nanotubes (12–14 nm) on the main target tissues of the bivalve Modiolus modiolus. Nanotechnol. Russia. 2015, 10, 278–287, doi:10.1134/S1995078015020020.
- Anisimova, A.; Lukyanova, O.; Chaika, V.; Kalitnik, A.; Danilenko, S.; Kuznetsov, V.; Golokhvast, K. S. Short‐time effect of multi‐walled carbon nanotubes on some histological and biochemical parameters in marine bivalves Crenomytilus grayanus (Dunker, 1853) and Swiftopecten swifti (Bernardi, 1858). Nano Hybr. Comp. 2016, 13, 225–231, doi:10.4028/www.scientific.net/NHC.13.225
- Ringwood, A.H.; Levi Polyachenko, N.; Carroll, D.L. Fullerene exposures with oysters: Embryonic, adult, and cellular responses. Environ. Sci. Technol. 2009, 43, 7136–7141, doi:10.1021/es900621j.
