Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by J. Emanuel Finet and Version 2 by Camila Xu.
Heart Failure (HF) is a clinical syndrome that is caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion. Metabolic exercise testing, also known as cardiopulmonary exercise testing, provides a comprehensive evaluation of the multisystem (i.e., neurological, respiratory, circulatory, and musculoskeletal) response to exercise performance.
- heart failure
- diagnosis
- prognosis
- cardiopulmonary exercise testing
1. Overview of Heart Failure
Heart Failure (HF) is a clinical syndrome that is caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion; it affects more than 6.7 million adults in the United States alone [1]. The calculated national cost of HF was USD 30.7 billion in 2012 [2]; due to the aging population, HF has become a growing health and financial burden to the United States and other developed countries [3][4][3,4].
2. Risk Prediction in Heart Failure
HF is a clinical syndrome with significant heterogeneity in presentation and severity. Serial risk-stratification and prognostication can guide management decisions, particularly in advanced HF, when progression toward advanced therapies or end-of-life care is warranted [5][6]. Each of the currently utilized prognostic markers carries its own set of challenges in acquisition, reproducibility, accuracy, and significance; some of them have been further discussed in this work (Figure 1). For example, LVEF is foundational for HF classification after clinical diagnosis and remains the primary parameter for inclusion in most modern HF clinical trials; however, it does not consistently correlate with symptoms or functional capacity [6][7]. Studies have also shown significant heterogeneity in the 6-minute walk test (6MWT) for the same New York Heart Association (NYHA) class [7][8][8,9].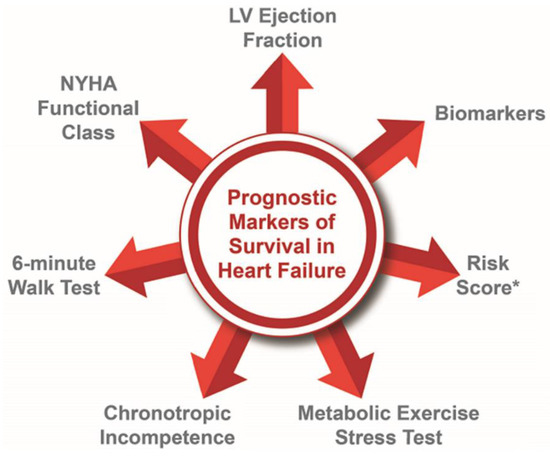
Figure 1. Prognostic Markers for Heart Failure. * Acute Decompensated Heart Failure National Registry-ADHERE [9][10]; AHA Get With The Guidelines Score [10][11]; Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity-CHARM Risk Score [11][12]; Controlled Rosuvastatin Multinational Trial in Heart Failure-CORONA Risk Score [12][13]; Enhanced Feedback for Effective Cardiac Treatment-EFFECT Risk Score [13][14]; Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness-ESCAPE Risk Model and Discharge Score [14][15]; Guiding Evidence-Based Therapy Using Biomarker Intensified Treatment-GUIDE-IT [15][16]; Heart Failure Survival Score [16][17]; Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training-HF-ACTION [17][18]; Meta-analysis Global Group in Chronic Heart Failure-MAGGIC [18][19]; Irbesartan in Heart Failure with Preserved Ejection Fraction-I-PRESERVE Score [19][20]; PARADIGM-HF [20][21]; Seattle Heart Failure Model [21][22]; Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial-TOPCAT [22][23].
2.1. Left Ventricular Ejection Fraction
Globally utilized as a fundamental clinical-trial inclusion criterion, LVEF is the default variable to initially classify HF syndrome; additionally, its prognostic role has been well-demonstrated [23][27]. At the present time, LVEF is most commonly measured by echocardiography or cardiac magnetic resonance imaging (MRI), and it is considered decisive in treatment-selection algorithms [24][28]. However, LVEF does not well represent the underlying pathophysiology of a specific disease process; moreover, heart failure mortality is not directly proportional across the LVEF spectrum [25][26][27][28][29,30,31,32]. Recent publications have highlighted the need for improved phenotyping among HFrEF patients, given that there is significant heterogeneity in clinical characteristics, outcomes, and responses to therapy [29][33]. As compared with peak aerobic exercise capacity (pVO2), LVEF has a modest correlation with hemodynamics, functional capacity, and overall prognosis [30][31][34,35]. Moreover, LVEF assessment by echocardiography has a high intra- and interobserver variability, with reported values of 8–21% and 6–13%, respectively [32][36]. Considering these many shortcomings, utilizing LVEF as the sole basis of prognostication provides an incomplete characterization of the HF syndrome, and it is prone to misguide medical decision-making when used in isolation [24][33][28,37].
2.2. New York Heart Association Functional Class
The New York Heart Association (NYHA) functional classification was first published in 1928 to help physicians communicate patients’ heart-failure symptoms in a shared language. NYHA functional class is widely incorporated in clinical studies, in society guidelines, and in clinical practice; however, patient and physician assessments of symptoms portend to unavoidable subjectivity [6][34][35][36][7,38,39,40]. It is often difficult to truly assess a patient’s functional capacity and how much heart failure contributes to such symptoms [37][38][39][40][41][41,42,43,44,45]. Similarly, Raphael et al. demonstrated that cardiologists have no consistent method of assessing NYHA class and that most research studies do not describe their methods for assigning NYHA class to study participants [39][43]. Significant heterogeneity of exercise aerobic capacity (pVO2) is also reported within all NYHA classes [6][7]. Despite these limitations, it is widely used as inclusion or exclusion criteria for therapy, as well as for prognostication and assessment of outcomes [42][43][44][46,47,48].
2.3. Six-Minute Walk Test
The 6-minute walk test (6MWT) is an objective, simple, and readily available test to determine functional capacity in heart failure. It is said to be a “poor man’s CPET”. Although it provides an absolute prognostic value, the results are affected by conditions unrelated to the patient’s cardiopulmonary status, such as age, sex, height, and weight, and do not account for physical conditioning [45][46][47][49,50,51]. Despite its inherent limitations, 6MWT does provide a more granular assessment of functional capacity, as compared to the NYHA class, and has been used in predicting outcomes in several conditions, including HF [6][48][49][50][7,52,53,54].
2.4. Chronotropic Incompetence
Chronotropic incompetence is defined as the inability to adequately increase heart rate (HR) commensurate to exercise aerobic capacity. The metabolic chronotropic index (MCI) relates HR reserve to the metabolic reserve at peak exercise (i.e., MCI = [(peak HR − rest HR)/(predicted peak HR − rest HR)]/[(peak VO2 − rest VO2)/(predicted peak VO2 − rest VO2)] [51][55]. The assessment of MCI can be confounded by commonly used cardiovascular medications, including beta-blockers, ivabradine, and other antiarrhythmic agents [52][56]; modified criteria have been described in determining MCI in this patient population [53][57]. Chronic atrial fibrillation and pacemaker dependency can also make the diagnosis of chronotropic incompetence more challenging [54][58]. Once diagnosed, this abnormal heart rate reserve is associated with reduced functional capacity, worse survival, and increased all-cause hospitalization in patients with HF [55][56][57][59,60,61].
2.5. Risk Score Models
Several risk score models have been used to prognosticate patients for chronic HFrEF, acute decompensated HFrEF, and HFpEF and are listed in Figure 1. Risk score models that incorporate metabolic exercise test parameters, including peak VO2, such as MECKI (Metabolic Exercise test data combined with Cardiac and Kidney Indexes) and HFSS (Heart Failure Survival Score), provide the most accurate HF risk prediction and are recommended when assessing patients for transplant listing [58][59][62,63]. These risk score models require the collection of multiple variables and may have certain variability in terms of prognostication; however, these risk score models in conjunction with other testing can help guide management decisions [60][64].
2.6. Cardiac Biomarkers
Natriuretic peptides (NPs) are released by cardiac myocytes to maintain circulatory homeostasis. NPs are secreted in response to myocardial tension and increased intravascular volume [61][65]. N-terminal pro-Brain Natriuretic Peptide (NT-proBNP), which is more sensitive than Brain Natriuretic Peptide, has been consistently associated with increased risk for all-cause mortality and hospitalizations among HF patients regardless of clinical volume status [62][66]. Biomarkers that alter collagen turnover, cardiac fibrosis, and inflammation may also have diagnostic and predictive value in both HFpEF and HfrEF [63][67]. Galectin-3, CT-1, GDF-15, and sST2 have been assessed to be the best candidates for determining the early stage of HF development [64][65][66][67][68][68,69,70,71,72]. Impaired renal function based on the estimated glomerular filtration rate calculated from the simplified modification of diet in renal disease (MDRD) formula is independently associated with increased risk of all-cause death, cardiovascular death, and hospitalization for HF patients regardless of LVEF [69][73].
3. Overview of Metabolic Exercise Test
Due to the challenges in assessing HF severity with the parameters previously mentioned, there is a need for a more precise definition of hemodynamic involvement and refined prognostic assessment of HF [7][8]. Metabolic exercise testing (MET) provides a more objective and consistent way of assessing symptom and HF severity and is regarded as the gold-standard for the assessment of functional (aerobic) capacity [70][71][72][25,26,74].
MET allows for an integrated physiological assessment of the pulmonary, cardiovascular, muscular, and cellular oxidative systems [73][75]. The test allows clinicians to differentiate cardiac from pulmonary disorders, provide outcome prediction, and determine targeted therapies [74][75][76,77]. Its easy reproducibility and safe technique make it a suitable choice in the assessment for most patients with undifferentiated symptoms [76][77][78,79]. MET also reports variables incorporated in a standard electrocardiogram (ECG) exercise stress test. These include stress and recovery HR and blood pressure (BP), exercise time, exercise workload expressed as metabolic equivalent, patient-reported symptoms, and ECG changes, among others [78][80]. In conjunction with MET parameters described below, these variables obtained during a standardized exercise stress also confer prognostic significance. Severe findings, such as exercise-induced hypotension, abnormal heart rate recovery, decreased exercise duration, arrhythmias, and angina, all denote a higher risk of cardiac events [79][81]. Exercise aerobic capacity exhibits the strongest association with all-cause mortality and cardiac events [80][82].
Current consensus statements and guidelines provide clinical indications for the use of MET, including the assessment of unexplained dyspnea and exercise intolerance, timing of intervention for valvular or congenital heart disease, clinical-trial initiation, and grading severity and prognosticating established advanced cardiac or pulmonary disease [74][81][82][76,83,84].
3.1. Performing the Metabolic Exercise Test
The American Thoracic Society/American College of Chest Physicians guidelines on cardiopulmonary exercise testing (i.e., MET) describes full procedural and operational standards [83][85]. As part of MET preparation among patients with HF, it is common to instruct patients to take all of their standard-of-care medications prior to the test in order to best evaluate the typical medicated integrative physiological response to exercise [84][86]. Patients typically perform MET on a treadmill or upright cycle ergometer at an increasing workload until maximal exhaustion is achieved or other clinical-test-terminating indications are observed. A patient wears a nose clip and a mouthpiece or full facemask to ensure that continuous breath-to-breath gas exchange and ventilation measurements occur in real time, in a closed-system, for subsequent analysis [85][86][87,88]. The use of a cycle ergometer allows for the direct quantification of workload that increases in a ramp-slope or minute-to-minute pattern, whereas the use of a treadmill allows for an indirect estimation of workload according to incremental changes in the treadmill belt slope and speed [87][88][89,90]. The test is to be discontinued in the setting of exhaustion, severe arrhythmias, hypotension, angina, and/or severe symptoms, to name a few [79][87][81,89]. Documentation of the reason for termination and assessment of dyspnea and perceived effort is to be recorded using subjective scales, such as the modified Borg CR 10 scale [89][91].
3.2. Variables Obtained in Metabolic Exercise Test
The RER refers to the respiratory exchange ratio and is calculated by dividing the carbon dioxide output (VCO2) variable by VO2. An RER ≥ 1.10 is used as one of the main MET features to indicate maximal patient physiological effort during MET [88][90]. For cardiovascular applications, both maximal and submaximal parameters have been described for clinical purposes. Maximal parameters include peak oxygen consumption (peak VO2), peak circulatory power (CP) (peak VO2 × peak systolic blood pressure), peak VO2 pulse (peak VO2/peak heart rate), and percentage of predicted peak VO2 (%PPVO2), among others [90][91][92][92,93,94]. Submaximal parameters include ventilatory efficiency (i.e., the slope of minute ventilation to CO2 production; also know as VE/VCO2 slope), VO2 at the ventilatory-derived anaerobic threshold (VO2@AT), oxygen uptake efficiency slope (slope of the relationship between peak VO2 and log minute ventilation; also known as OUES), end tidal pressure of CO2 (PEtCO2), and presence of oscillatory ventilation (EOV), among others [92][93][94][95][96][97][98][99][100][101][102][94,95,96,97,98,99,100,101,102,103,104]. Normal expected values for these variables are listed in Table 1, which, alongside the Wasserman–Hansen equations for predicting normal response levels, are generally accepted for use in modern cardiovascular applications [103][105]. However, because of various patient and data sampling factors that are associated with deriving predicted normal values for MET variables [104][106], some patient cases may require the referencing of other equation sets, such as those developed from the Fitness Registry and the Importance of Exercise National Database (FRIEND) registry [105][107].
Table 1.
MET components and normal values.
| Metabolic Exercise Variable | Normal Expected Range |
|---|---|
| Peak RER | 1.10–1.50 |
| Peak work-rate | >85% predicted peak Watts |
| Peak VO2 | >85% predicted peak VO2 |
| Rest VO2 | 2–5 mL/kg/min |
| VO2 at VAT | 40–75% predicted peak VO2 |
| CI | 0.80–1.30 |
| Peak VO2/HR | >85% predicted peak VO2/HR |
| VO2/WR slope | 8.5–12.5 mL/min/watts |
| Peak SpO2 | >95% |
| Peak VE | <85% predicted peak VE |
| Peak RR | <60 breaths/min |
| Peak VT | 1.5–3.0 liters |
| Peak PETCO2 | 35–41 mmHg |
| VE/VCO2 slope | <30 |
| EOV | None |
| Peak RPP | >18,966 mmHg × bpm |
| HGI | >1.06 bpm/mmHg |
| Circulatory Power | >3047 mmHg × mL/min/kg |
| OUES | >1.85 L/min |
| POUES | >0.88 L/min |
RER—respiratory exchange ratio; VO2—peak oxygen consumption; VAT—ventilatory anaerobic threshold; CI—chronotropic index; HR—heart rate; SpO2—oxygen saturation; VE—ventilation, RR—respiratory rate; VT—tidal volume; PETCO2—end-tidal pressure of carbon dioxide; EOV—exercise oscillatory ventilation; RPP—rate pressure product (heart rate × systolic blood pressure); HGI—hemodynamic gain index; OUES—oxygen uptake efficiency slope; POUES—oxygen uptake efficiency at peak exercise.
4. Metabolic Exercise Test in Patients with Heart Failure
4.1. VO2
Peak VO2 (pVO2) pertains to the maximum consumption of oxygen during exercise. It is the most objective method to assess functional aerobic capacity [81][83]. Peak VO2 is determined by the interdependent actions of pulmonary respiration, oxygen diffusion at the alveolocapillary membrane, oxygen transport by the cardiovascular system, oxygen diffusion at peripheral capillary beds, and oxygen utilization via the Krebs cycle and the electron transport chain. When there is a reduction in muscle oxygen supply, together with increasing demands for oxygen, this can be a major cause for an inability to achieve a normal pVO2 based on age, gender, and weight [74][106][76,108]. Among patients with HF, low O2 delivery can serve as the primary rate-limiting step which may allow for VO2 to be interpreted as a surrogate marker of cardiovascular capacity [107][109]. Cutoff values for normal pVO2 are dependent on patient age, sex, and body mass index, with the consideration of using lean body mass for obese individuals [108][110].
Weber et al. introduced the classification of patients with HFrEF based on a pVO2 ranging from A (pVO2 > 20 mL/kg/min) to B (pVO2 15–20 mL/kg/min) to C (pVO2 10–15 mL/kg/min) to D (pVO2 < 10 mL/kg/min) [109][111]. Mancini et al. subsequently published a landmark study illustrating that, among patients with HFrEF, the established pVO2 “abnormality” cutoff of <14 mL/kg/min was associated with significantly lower 1-year survival as compared to patients who underwent orthotopic heart transplantation (OHT) [30][34]. Similar observations were subsequently reported among patients on chronic beta-blocker therapy, suggesting a cutoff of <12 mL/kg/min [110][111][112,113]. Other groups have consistently reported data supporting the view that pVO2 is a strong univariate predictor of mortality in heart-failure patients [112][114]. An extremely low pVO2 (i.e., <10 mL/kg/min), when coupled with an abnormal exercise cardiac output response, has been shown to convey the worst prognosis in the HF population [31][35].
In the HF-ACTION trial, pVO2, percent predicted pVO2, and exercise duration all demonstrated the highest ability to predict mortality in HfrEF [113][115]. Similar observations have been reported in patients with HfpEF [114][115][116,117]. Additionally, in a secondary analysis of the HF-ACTION trial data, a modest increase in pVO2 over 3 months was associated with a lower composite rate of all-cause mortality and hospitalization even with the use of optimal medical and device therapy in HfrEF [116][118]. The effect of cardiac resynchronization therapy on pVO2 was investigated in the COMPANION trial sub-study, with the results showing no significant improvement in pVO2 at 6 months compared to those on guideline-directed medical therapy [117][119].
Other measurements which incorporate VO2 can also demonstrate prognostic power in heart-failure patients, even in submaximal aerobic efforts (RER < 1.1). The absolute VO2 response at the ventilatory-derived anaerobic threshold is a useful submaximal exercise prognostic marker; a value of <11 mL/kg/min is associated with higher mortality among HFrEF patients [118][120]. Flattening of the VO2/HR slope has also been recognized as an indication of inability to augment stroke volume in response to increasing metabolic demand [119][121]. In repeated MET testing, a >6% pVO2 reduction has been deemed to be high risk [82][84].
Nadruz et al. published data that pVO2 was independently associated with the total number of HF hospitalizations in all LVEF categories. He also reported that the pVO2 and VE/VCO2 slope provided an independent and incremental prognostic value for all-cause death, LVAD implantation, or heart transplant [37][41]. These variables also predicted incident HF hospitalization in HFpEF patients. However, when studied in HFmrEF patients, outcomes were only intermediate [37][41]. Among HFpEF patients, the percentage-predicted pVO2 has been identified as in important prognostic marker [120][122]. The ESC position paper uses a percentage-predicted pVO2 cutoff of ≥50% as a marker for very low-risk patients [82][84].
4.2. VE/VCO2 Slope
A high VE/VCO2 slope indicates the presence of ventilatory inefficiency, as indicated by a disproportionately high rate of rise in VE relative to that of VCO2, and the coincidence with a sharp fall in arterial CO2 associated with hypocapnia [73][106][75,108]. The increased VE/VCO2 during exercise in HF is attributed to the combination of hyperventilation and increased dead space ventilation [121][123]. The increased ventilatory drive can also be attributed to the increased activation of mechano-sensitive j-receptors in response to the abnormal distension of pulmonary vasculature that occurs in pulmonary congestion [122][124]. A low cardiac index can also be associated with a high VE/VCO2 slope due to the presence of a high V/Q mismatch due to decreased transpulmonary flow [121][123][123,125].
An elevated VE/VCO2 slope is associated with an increased pulmonary vascular resistance and inversely associated with a right ventricular ejection fraction in HfrEF [124][126]. A VE/VCO2 slope > 34, and much more when greater than 45, indicates higher-risk heart-failure patients and predicts worse prognosis in both HFrEF and HFpEF patients [95][125][126][127][97,127,128,129]. The VE/VCO2 slope has also been found to be independently associated with the total number of HF hospitalizations in the HFrEF population [37][41]. A comparison with invasive hemodynamics showed how the VE/VCO2 slope obtained during submaximal exercise closely reflects cardiac output, pulmonary artery pressure, and pulmonary capillary wedge pressure [128][130].
Several studies have suggested a greater value of the VE/VCO2 slope over pVO2 for HF prognosis [95][125][126][97,127,128]. The VE/VCO2 slope provides a better assessment of the complex interplay of pulmonary, cardiac, and peripheral manifestations of heart failure [129][131]. This parameter is also less likely to fluctuate and is less dependent on the patient’s level of effort as compared to pVO2 [130][132]. Among the submaximal exercise parameters included in the REVIVAL study analysis, the VE/VCO2 slope is the most reliable and strongest MET predictor of poor outcome within 1 year (durable mechanical circulatory support (MCS) implantation, OHT, or mortality) [131][133].
4.3. Exercise Oscillatory Ventilation
Exercise oscillatory ventilation (EOV) is a form of irregularly irregular cyclic variation of minute ventilation during exercise [132][134]. EOV can be seen in HF patients regardless of LVEF and indicates a poor prognosis [102][132][133][104,134,135]. EOV is thought to arise from the instability of the feedback systems controlling ventilation similar to Cheyne–Stokes breathing. This can be due to increased circulation time due to a reduced cardiac index, increased chemosensitivity to PaCO2, or baroreflex impairment [134][136]. EOV can be indicative of autonomic dysregulation and the presence of high chronic levels of sympatho-excitation. Patients with EOV have been found to have clinical characteristics and exercise ventilatory responses consistent with more advanced HF compared to patients with similar LV function [101][103].
4.4. Circulatory Power
Circulatory power (CP) was introduced by Cohen-Solal et al. and is calculated as the product of pVO2 and SBP, thereby estimating total work [91][93]. This formula utilizes pVO2 as a surrogate for cardiac output and systolic blood pressure as a surrogate for MAP. A circulatory power < 3047 mmHg/mL/min/kg was associated with a lower 1-year survival among patients with HfrEF [91][93]. In the REVIVAL study cohort, CP was found to be the maximal parameter that was most strongly associated with MCS implantation, OHT, or death at 1 year [131][133]. Circulatory power also had a greater discriminative capacity to predict the combined endpoint of MCS, OHT, or death based the on receiver-operating-characteristic analysis when compared to O2 pulse and pVO2 [5][6].
4.5. VO2/WR Slope and VO2/HR
The VO2/WR slope (also known as aerobic efficiency) describes the relationship between the oxygen consumption of working muscles to the degree of work generated by them throughout the exercise test [87][89]. In normal conditions, it increases linearly, as a progressive workload demands progressive oxygen consumption; however, in HF patients, the pattern is a shallow downward shift that is suggestive of VO2 flattening for a given work-rate due to the inability of the heart to match the oxygen delivery required by the workload [73][75]. As it relates to this variable, VO2/HR is a surrogate stroke volume, also called pVO2 pulse. This surrogate is based on the Fick assumption that the pVO2 is a surrogate of the peak cardiac output, assuming a “normal” mitochondrial function and O2 extraction by the working skeletal muscle [135][137]. For both VO2/WR and VO2/HR, a shallower slope indicates cardiovascular dysfunction [136][138].
4.6. Ventilatory Power
Ventilatory power is an index derived from combining the VE/VCO2 slope with systolic blood pressure [129][131]. Forman et al. demonstrated that ventilatory power is a stronger predictor of cardiac events than the pVO2 and VE/VCO2 slope. When analyzed with circulatory power, the prognostic discrimination of ventilatory power is synergistic. A value of <3.5 mmHg was associated with worse prognosis [129][131].
4.7. Hemodynamic Gain Index
The hemodynamic gain index (HGI) is calculated using the formula [(SBPpeak × HRpeak) − (SBPrest × HRrest)]/(SBPrest × HRrest) and was first described by Vainshelboim et al. Initial studies identified the association between a lower HGI and a greater risk of all-cause mortality [137][138][139,140]. Further studies have elucidated that a lower HGI was independently associated with all-cause mortality regardless of sex, BMI, and LVEF [139][141]. HGI provides an integrated measurement of maximal hemodynamic response during exercise. Because it is derived from the relative gain of the rate-pressure product, it reflects cardiovascular function and myocardial oxygen consumption [81][140][83,142]. A lower HGI may be suggestive of impaired cardiac functioning, vascular compliance, and vascular performance [139][141].
4.8. Metabolic Exercise Test in Patients with Mechanical Circulatory Support and Cardiac Transplantation
4.8.1. Orthotopic Heart Transplantation
Data from Mancini et al. proposed a pVO2 < 14 mL/kg/min as the cutoff for cardiac transplantation eligibility in patients who are intolerant to beta-blockers, and a threshold of <10 mL/kg/min for those HFrEF patients on chronic betablocker therapy [30][34]. Current ISHLT recommendations, however, conversely recommend a pVO2 < 12 mL/kg/min cutoff in the presence of chronic betablockers, and a percent-predicted pVO2 < 50% in patients <50 years old and women [141][143]. Moreover, in obese patients (i.e., BMI > 30 kg/m2), adjusting the pVO2 to lean body mass may be considered; a lean-body-mass-adjusted pVO2 < 19 mL/kg/min can serve as a suitable threshold for cardiac transplantation eligibility [141][143]. Although there is evidence suggesting the VE/VCO2 slope as a stronger prognostic marker than pVO2 in the HFrEF population, current ACC/AHA guidelines recognize pVO2 as the sole marker of impaired aerobic capacity, enough to trigger heart-transplant consideration [74][95][76,97]. Conversely, the ISHLT recommends solely the use of the VE/VCO2 slope in the setting of submaximal effort (RER < 1.05) [74][142][143][76,144,145].
MET parameters post-transplantation demonstrate improvement compared to pre-transplant values; however, they may continue to remain abnormal when compared with age-matched healthy individuals, likely due to circulatory deconditioning and impaired cardiac denervation, limiting acute exercise response [144][145][146,147]. Several studies have been inconclusive regarding the prognostic value to MET after cardiac transplantation [146][147][148][148,149,150]. Iglesias et al. identified pVO2, oxygen pulse, and percent predicted pVO2 as being independently associated with hospitalizations after cardiac transplantation [149][151].
4.8.2. Durable Mechanical Circulatory Support
Several MET parameters have been described to predict outcomes after implantation of durable mechanical circulatory support devices (LVADs). Peak VO2 and OUES were associated with 1-, 3-, and 5-year mortality after LVAD implantation. Also, the VE/VCO2 minimum was associated with 3- and 5-year mortality, while the VE/VCO2 slope was also strongly correlated with 5-year mortality [107][109]. These pre-implantation MET parameters seem to consistently indicate a suitable long-term survival correlation; however, the association with short-term post-implantation outcomes has been shown to be less consistent and weaker [107][109]. Grinstein et al. additionally reported the association between pre-implantation VE/VCO2 slope and right ventricular failure and mortality following LVAD implantation [150][152].
The effect of LVAD implantation on VO2 is uncertain, as studies have demonstrated inconsistent results [151][152][153][153,154,155]. Studies by Mirza et al. and Moss et al., among others, have reported that LVAD-supported patients continued to have abnormal pVO2 and impaired exercise capacity, with similar findings even after adjusting for pump speed [154][155][156,157]. Similarly, the pVO2 remains significantly impaired when compared with patients who underwent heart transplantation [156][157][158,159]; this could be, in part, explained by the unsupported right ventricle (RV), which is also a determinant in circulatory sufficiency. The association between LV function recovery and pVO2 in LVAD patients has also shown mixed results [158][159][160][161][160,161,162,163]. In this population, pVO2 is an independent predictor of 3- and 5-year mortality, while the VE/VCO2 maximum was an independent predictor of 3-year mortality [107][109]. Although very few patients undergo LVAD explantation, the incorporation of pVO2 and other MET parameters can be considered in determining the timing of device explantation [162][164]. Future studies assessing the utility of MET parameters in listing LVAD patients for transplantation should also be considered [154][156].
4.8.3. Invasive Metabolic Exercise Test
The invasive MET (iMET) incorporates the use of radial and pulmonary catheters for simultaneous hemodynamic monitoring. With the hemodynamic data provided by iMET, it is possible to dissect the oxygen delivery from the oxygen extraction component from the aerobic capacity (i.e., VO2), evaluating in isolation the cardiovascular and mitochondrial functions of the skeletal muscle, sequentially. It is essential for the diagnosis of mitochondrial myopathies and helpful in the evaluation and diagnosis of other conditions of undifferentiated dyspnea, such as exercise-induced pulmonary arterial hypertension (PAH), exercise-induced HFpEF, preload-dependent limitations to cardiac output, chronic thromboembolic disease (CTED), and chronic thromboembolic pulmonary hypertension (CTEPH), among others [163][164][165][165,166,167]. Among LVAD patients, iMET allows to assess the ability of the right ventricle (RV) to augment pulmonary artery pressure (PAP) and the right ventricular stroke work index. A PAP plateau at exercise indicates the RV’s inability to accommodate increased blood flow, thereby leading to a poor prognosis in these patients [166][168]. iMET has a role in prognosticating not only LVAD patients but also PAH patients because it can quantify functional limitations to exercise [167][169]. Issues with invasive MET include its inherent vascular access complications and technical inaccuracies in reliably obtaining pulmonary hemodynamic tracings despite averaging repeated measurements [73][75].
4.8.4. Metabolic Exercise Test as Clinical Endpoints
MET has been used as an endpoint for clinical trials because it allows for the objective measurement of functional capacity at both maximal and submaximal exertion. However, MET incurs a greater overall cost than a submaximal functional or health status assessment and requires expertise in implementation and interpretation. The Heart Failure Collaboratory, and the Academic Research Consortium consensus document indicates that the minimal clinically important difference for maximal VO2 on MET has not been validated. Meaningful difference in peak VO2 has been reported as a 6% change or 1 mL/kg/min but is based on repeat test variability and lack of clinical relevance [168][170]. Overall, various MET parameters have significant prognostic value in assessing mortality in HF patients regardless of LVEF. Parameters associated with a 1-year mortality risk are summarized in Figure 2.


Figure 2.
Metabolic Exercise Test Component Threshold.
