Biomimetic cell-derived nanoparticles are attracting considerable interest due to their better biocompatibility and lower immunogenicity. Moreover, biomimetic cell-derived nanoparticles can achieve different preferred biological effects due to their inherent abundant source cell-relevant functions. This review summarizes the latest developments in biomimetic cell-derived nanoparticles for cancer immunotherapy, discusses the applications of each biomimetic system in cancer immunotherapy, and analyzes the challenges for clinical transformation.
Biomimetic cell-derived nanoparticles are attracting considerable interest due to their better biocompatibility and lower immunogenicity. Moreover, biomimetic cell-derived nanoparticles can achieve different preferred biological effects due to their inherent abundant source cell-relevant functions. This research summarizes the latest developments in biomimetic cell-derived nanoparticles for cancer immunotherapy, discusses the applications of each biomimetic system in cancer immunotherapy, and analyzes the challenges for clinical transformation.
- biomimetic nanoparticles
- cell-membrane cloaking
- cancer immunotherapy
1. Introduction
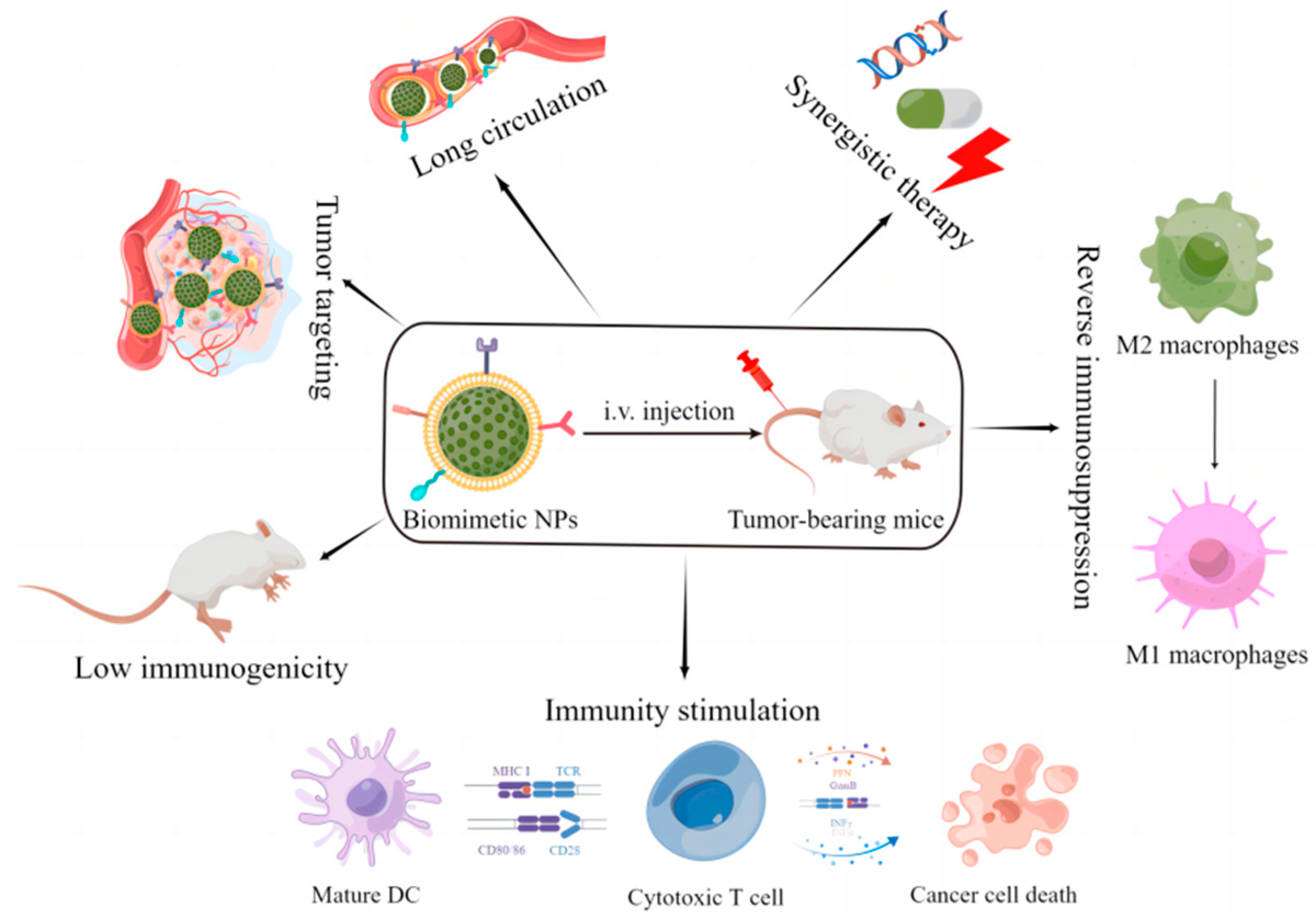
2. Biomimetic Cell-Derived Nanoparticles
2.1. Unique Function of Biomimetic Cell-Derived Nanoparticles
2.1.1. Erythrocyte Membranes
2.1.2. Immune Cell Membranes
2.1.3. Platelet Membranes
2.1.4. Cancer Cell Membranes
2.1.5. Stem Cell Membranes
2.1.6. Bacteria Membranes
2.1.7. Extracellular Vesicles
2.1.8. Hybrid Cell Membranes
2.2. Fabrication of Biomimetic Cell-Derived Nanoparticles
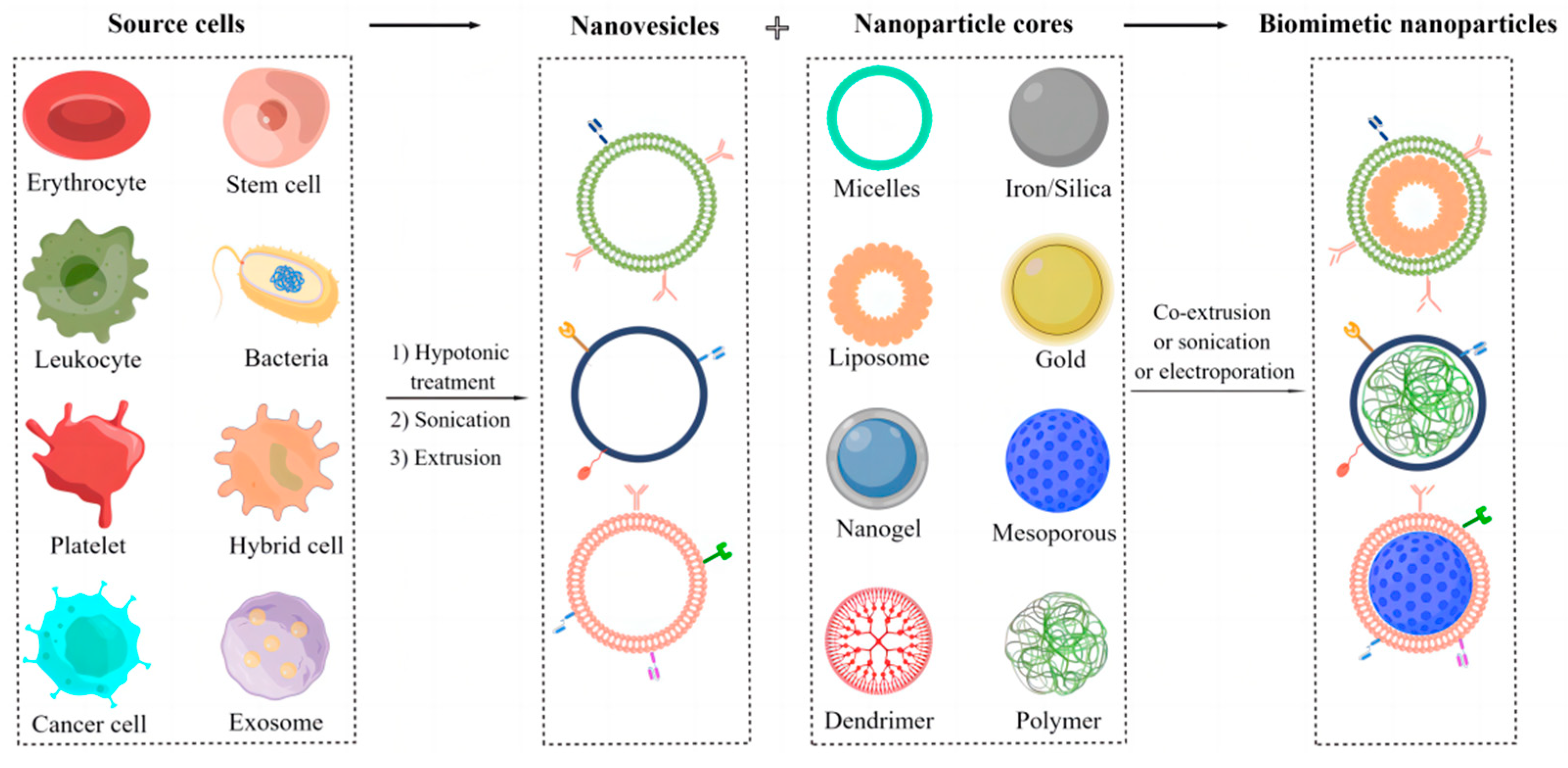
2.2.1. Isolation and Preparation of Parent Cell Membrane-Derived Vesicles
2.2.2. Fusion of Parent Cell Membrane-Derived Vesicles with Nanoparticle Cores
References
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337.
- Zeng, Y.; Li, S.; Zhang, S.; Wang, L.; Yuan, H.; Hu, F. Cell membrane coated-nanoparticles for cancer immunotherapy. Acta Pharm. Sin. B 2022, 12, 3233–3254.
- Peterson, C.; Denlinger, N.; Yang, Y. Recent Advances and Challenges in Cancer Immunotherapy. Cancers 2022, 14, 3972.
- Alhaj-Suliman, S.O.; Wafa, E.I.; Salem, A.K. Engineering nanosystems to overcome barriers to cancer diagnosis and treatment. Adv. Drug Deliv. Rev. 2022, 189, 114482.
- Yang, K.; Yang, Z.; Yu, G.; Nie, Z.; Wang, R.; Chen, X. Polyprodrug Nanomedicines: An Emerging Paradigm for Cancer Therapy. Adv. Mater. 2021, 34, e2107434.
- Hegde, M.; Naliyadhara, N.; Unnikrishnan, J.; Alqahtani, M.S.; Abbas, M.; Girisa, S.; Sethi, G.; Kunnumakkara, A.B. Nanoparticles in the diagnosis and treatment of cancer metastases: Current and future perspectives. Cancer Lett. 2023, 556, 216066.
- Shukla, A.; Maiti, P. Nanomedicine and versatile therapies for cancer treatment. MedComm 2022, 3, e163.
- Llop, J.; Lammers, T. Nanoparticles for Cancer Diagnosis, Radionuclide Therapy and Theranostics. ACS Nano 2021, 15, 16974–16981.
- Zhou, Y.; Dai, Z. New Strategies in the Design of Nanomedicines to Oppose Uptake by the Mononuclear Phagocyte System and Enhance Cancer Therapeutic Efficacy. Chem. Asian J. 2018, 13, 3333–3340.
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.H.; Sarhan, H.A.; Hussein, A.K.; et al. Polyethylene glycol (PEG): The nature, immunogenicity, and role in the hypersensitivity of PEGylated products. J. Control. Release 2022, 351, 215–230.
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079.
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175.
- Estape Senti, M.; de Jongh, C.A.; Dijkxhoorn, K.; Verhoef, J.J.F.; Szebeni, J.; Storm, G.; Hack, C.E.; Schiffelers, R.M.; Fens, M.H.; Boross, P. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J. Control. Release 2022, 341, 475–486.
- Hussain, Z.; Rahim, M.A.; Jan, N.; Shah, H.; Rawas-Qalaji, M.; Khan, S.; Sohail, M.; Thu, H.E.; Ramli, N.A.; Sarfraz, R.M.; et al. Cell membrane cloaked nanomedicines for bio-imaging and immunotherapy of cancer: Improved pharmacokinetics, cell internalization and anticancer efficacy. J. Control. Release 2021, 335, 130–157.
- Dhas, N.; Garcia, M.C.; Kudarha, R.; Pandey, A.; Nikam, A.N.; Gopalan, D.; Fernandes, G.; Soman, S.; Kulkarni, S.; Seetharam, R.N.; et al. Advancements in cell membrane camouflaged nanoparticles: A bioinspired platform for cancer therapy. J. Control. Release 2022, 346, 71–97.
- Raza, F.; Zafar, H.; Zhang, S.; Kamal, Z.; Su, J.; Yuan, W.E.; Mingfeng, Q. Recent Advances in Cell Membrane-Derived Biomimetic Nanotechnology for Cancer Immunotherapy. Adv. Healthc. Mater. 2021, 10, e2002081.
- Zhang, F.; Mundaca-Uribe, R.; Askarinam, N.; Li, Z.; Gao, W.; Zhang, L.; Wang, J. Biomembrane-Functionalized Micromotors: Biocompatible Active Devices for Diverse Biomedical Applications. Adv. Mater. 2022, 34, e2107177.
- Chen, X.; Liu, B.; Tong, R.; Zhan, L.; Yin, X.; Luo, X.; Huang, Y.; Zhang, J.; He, W.; Wang, Y. Orchestration of biomimetic membrane coating and nanotherapeutics in personalized anticancer therapy. Biomater. Sci. 2021, 9, 590–625.
- Shen, M.; Wu, X.; Zhu, M.; Yi, X. Recent advances in biological membrane-based nanomaterials for cancer therapy. Biomater. Sci. 2022, 10, 5756–5785.
- Jha, A.; Nikam, A.N.; Kulkarni, S.; Mutalik, S.P.; Pandey, A.; Hegde, M.; Rao, B.S.S.; Mutalik, S. Biomimetic nanoarchitecturing: A disguised attack on cancer cells. J. Control. Release 2021, 329, 413–433.
- Zhao, J.; Ruan, J.; Lv, G.; Shan, Q.; Fan, Z.; Wang, H.; Du, Y.; Ling, L. Cell membrane-based biomimetic nanosystems for advanced drug delivery in cancer therapy: A comprehensive review. Colloids Surf. B Biointerfaces 2022, 215, 112503.
- Fang, R.H.; Gao, W.; Zhang, L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 2022, 20, 33–48.
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985.
- Liu, H.; Su, Y.Y.; Jiang, X.C.; Gao, J.Q. Cell membrane-coated nanoparticles: A novel multifunctional biomimetic drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 716–737.
- Chen, L.; Hong, W.; Ren, W.; Xu, T.; Qian, Z.; He, Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target. Ther. 2021, 6, 225.
- Zhang, M.; Cheng, S.; Jin, Y.; Zhang, N.; Wang, Y. Membrane engineering of cell membrane biomimetic nanoparticles for nanoscale therapeutics. Clin. Transl. Med. 2021, 11, e292.
- Krishnan, N.; Peng, F.X.; Mohapatra, A.; Fang, R.H.; Zhang, L. Genetically engineered cellular nanoparticles for biomedical applications. Biomaterials 2023, 296, 122065.
- Meng, Z.; Zhang, Y.; Zhou, X.; Ji, J.; Liu, Z. Nanovaccines with cell-derived components for cancer immunotherapy. Adv. Drug Deliv. Rev. 2022, 182, 114107.
- Shi, Y.; Qian, H.; Rao, P.; Mu, D.; Liu, Y.; Liu, G.; Lin, Z. Bioinspired membrane-based nanomodulators for immunotherapy of autoimmune and infectious diseases. Acta Pharm. Sin. B 2022, 12, 1126–1147.
- Wu, Z.; Zhang, H.; Yan, J.; Wei, Y.; Su, J. Engineered biomembrane-derived nanoparticles for nanoscale theranostics. Theranostics 2023, 13, 20–39.
- Shi, Y.; Lu, A.; Wang, X.; Belhadj, Z.; Wang, J.; Zhang, Q. A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives. Acta Pharm. Sin. B 2021, 11, 2396–2415.
- Castro, F.; Martins, C.; Silveira, M.J.; Moura, R.P.; Pereira, C.L.; Sarmento, B. Advances on erythrocyte-mimicking nanovehicles to overcome barriers in biological microenvironments. Adv. Drug Deliv. Rev. 2021, 170, 312–339.
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689.
- Bidkar, A.P.; Sanpui, P.; Ghosh, S.S. Transferrin-Conjugated Red Blood Cell Membrane-Coated Poly(lactic-co-glycolic acid) Nanoparticles for the Delivery of Doxorubicin and Methylene Blue. ACS Appl. Nano Mater. 2020, 3, 3807–3819.
- Choi, M.J.; Lee, Y.K.; Choi, K.C.; Lee, D.H.; Jeong, H.Y.; Kang, S.J.; Kim, M.W.; You, Y.M.; Im, C.S.; Lee, T.S.; et al. Tumor-Targeted Erythrocyte Membrane Nanoparticles for Theranostics of Triple-Negative Breast Cancer. Pharmaceutics 2023, 15, 350.
- Long, Y.; Wang, Z.; Fan, J.; Yuan, L.; Tong, C.; Zhao, Y.; Liu, B. A hybrid membrane coating nanodrug system against gastric cancer via the VEGFR2/STAT3 signaling pathway. J. Mater. Chem. B 2021, 9, 3838–3855.
- Huo, Y.Y.; Song, X.; Zhang, W.X.; Zhou, Z.L.; Lv, Q.Y.; Cui, H.F. Thermosensitive Biomimetic Hybrid Membrane Camouflaged Hollow Gold Nanoparticles for NIR-Responsive Mild-Hyperthermia Chemo-/Photothermal Combined Tumor Therapy. ACS Appl. Bio Mater. 2022, 5, 5113–5125.
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867.
- Li, R.; He, Y.; Zhang, S.; Qin, J.; Wang, J. Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharm. Sin. B 2018, 8, 14–22.
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M.A. Immune Cell Membrane-Coated Biomimetic Nanoparticles for Targeted Cancer Therapy. Small 2021, 17, e2006484.
- Cheng, S.; Xu, C.; Jin, Y.; Li, Y.; Zhong, C.; Ma, J.; Yang, J.; Zhang, N.; Li, Y.; Wang, C.; et al. Artificial Mini Dendritic Cells Boost T Cell-Based Immunotherapy for Ovarian Cancer. Adv. Sci. 2020, 7, 1903301.
- Xu, C.; Jiang, Y.; Han, Y.; Pu, K.; Zhang, R. A Polymer Multicellular Nanoengager for Synergistic NIR-II Photothermal Immunotherapy. Adv. Mater. 2021, 33, e2008061.
- Gong, P.; Wang, Y.; Zhang, P.; Yang, Z.; Deng, W.; Sun, Z.; Yang, M.; Li, X.; Ma, G.; Deng, G.; et al. Immunocyte Membrane-Coated Nanoparticles for Cancer Immunotherapy. Cancers 2020, 13, 77.
- Khatoon, N.; Zhang, Z.; Zhou, C.; Chu, M. Macrophage membrane coated nanoparticles: A biomimetic approach for enhanced and targeted delivery. Biomater. Sci. 2022, 10, 1193–1208.
- Gaber, T.; Chen, Y.; Krauss, P.L.; Buttgereit, F. Metabolism of T Lymphocytes in Health and Disease. Int. Rev. Cell Mol. Biol. 2019, 342, 95–148.
- Notarbartolo, S.; Abrignani, S. Human T lymphocytes at tumor sites. Semin. Immunopathol. 2022, 44, 883–901.
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell Physiol. 2019, 234, 8509–8521.
- Yaman, S.; Ramachandramoorthy, H.; Oter, G.; Zhukova, D.; Nguyen, T.; Sabnani, M.K.; Weidanz, J.A.; Nguyen, K.T. Melanoma Peptide MHC Specific TCR Expressing T-Cell Membrane Camouflaged PLGA Nanoparticles for Treatment of Melanoma Skin Cancer. Front. Bioeng. Biotechnol. 2020, 8, 943.
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gogenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367.
- Chen, B.Q.; Zhao, Y.; Zhang, Y.; Pan, Y.J.; Xia, H.Y.; Kankala, R.K.; Wang, S.B.; Liu, G.; Chen, A.Z. Immune-regulating camouflaged nanoplatforms: A promising strategy to improve cancer nano-immunotherapy. Bioact. Mater. 2023, 21, 1–19.
- Han, B.; Mao, F.Y.; Zhao, Y.L.; Lv, Y.P.; Teng, Y.S.; Duan, M.; Chen, W.; Cheng, P.; Wang, T.T.; Liang, Z.Y.; et al. Altered NKp30, NKp46, NKG2D, and DNAM-1 Expression on Circulating NK Cells Is Associated with Tumor Progression in Human Gastric Cancer. J. Immunol. Res. 2018, 2018, 6248590.
- Jain, N.; Shahrukh, S.; Famta, P.; Shah, S.; Vambhurkar, G.; Khatri, D.K.; Singh, S.B.; Srivastava, S. Immune cell-camouflaged surface-engineered nanotherapeutics for cancer management. Acta Biomater. 2023, 155, 57–79.
- Han, H.; Bartolo, R.; Li, J.; Shahbazi, M.A.; Santos, H.A. Biomimetic platelet membrane-coated nanoparticles for targeted therapy. Eur. J. Pharm. Biopharm. 2022, 172, 1–15.
- Hu, C.M.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121.
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.N.; Gu, Z. Anticancer Platelet-Mimicking Nanovehicles. Adv. Mater. 2015, 27, 7043–7050.
- Fabricius, H.A.; Starzonek, S.; Lange, T. The Role of Platelet Cell Surface P-Selectin for the Direct Platelet-Tumor Cell Contact During Metastasis Formation in Human Tumors. Front. Oncol. 2021, 11, 642761.
- Chen, Z.; Zhao, P.; Luo, Z.; Zheng, M.; Tian, H.; Gong, P.; Gao, G.; Pan, H.; Liu, L.; Ma, A.; et al. Cancer Cell Membrane-Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. ACS Nano 2016, 10, 10049–10057.
- Harris, J.C.; Scully, M.A.; Day, E.S. Cancer Cell Membrane-Coated Nanoparticles for Cancer Management. Cancers 2019, 11, 1836.
- Tian, H.; Luo, Z.; Liu, L.; Zheng, M.; Chen, Z.; Ma, A.; Liang, R.; Han, Z.; Lu, C.; Cai, L. Cancer Cell Membrane-Biomimetic Oxygen Nanocarrier for Breaking Hypoxia-Induced Chemoresistance. Adv. Funct. Mater. 2017, 27, 1703197.
- Zhu, J.Y.; Zheng, D.W.; Zhang, M.K.; Yu, W.Y.; Qiu, W.X.; Hu, J.J.; Feng, J.; Zhang, X.Z. Preferential Cancer Cell Self-Recognition and Tumor Self-Targeting by Coating Nanoparticles with Homotypic Cancer Cell Membranes. Nano Lett. 2016, 16, 5895–5901.
- Sun, H.; Su, J.; Meng, Q.; Yin, Q.; Chen, L.; Gu, W.; Zhang, P.; Zhang, Z.; Yu, H.; Wang, S.; et al. Cancer-Cell-Biomimetic Nanoparticles for Targeted Therapy of Homotypic Tumors. Adv. Mater. 2016, 28, 9581–9588.
- Pei, X.; Pan, X.; Xu, X.; Xu, X.; Huang, H.; Wu, Z.; Qi, X. 4T1 cell membrane fragment reunited PAMAM polymer units disguised as tumor cell clusters for tumor homotypic targeting and anti-metastasis treatment. Biomater. Sci. 2021, 9, 1325–1333.
- Yue, X.S.; Murakami, Y.; Tamai, T.; Nagaoka, M.; Cho, C.S.; Ito, Y.; Akaike, T. A fusion protein N-cadherin-Fc as an artificial extracellular matrix surface for maintenance of stem cell features. Biomaterials 2010, 31, 5287–5296.
- Iurisci, I.; Cumashi, A.; Sherman, A.A.; Tsvetkov, Y.E.; Tinari, N.; Piccolo, E.; D’Egidio, M.; Adamo, V.; Natoli, C.; Rabinovich, G.A.; et al. Synthetic inhibitors of galectin-1 and -3 selectively modulate homotypic cell aggregation and tumor cell apoptosis. Anticancer Res. 2009, 29, 403–410.
- Osta, W.A.; Chen, Y.; Mikhitarian, K.; Mitas, M.; Salem, M.; Hannun, Y.A.; Cole, D.J.; Gillanders, W.E. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004, 64, 5818–5824.
- Wu, H.H.; Zhou, Y.; Tabata, Y.; Gao, J.Q. Mesenchymal stem cell-based drug delivery strategy: From cells to biomimetic. J. Control. Release 2019, 294, 102–113.
- Khosravi, N.; Pishavar, E.; Baradaran, B.; Oroojalian, F.; Mokhtarzadeh, A. Stem cell membrane, stem cell-derived exosomes and hybrid stem cell camouflaged nanoparticles: A promising biomimetic nanoplatforms for cancer theranostics. J. Control. Release 2022, 348, 706–722.
- Wang, M.; Xin, Y.; Cao, H.; Li, W.; Hua, Y.; Webster, T.J.; Zhang, C.; Tang, W.; Liu, Z. Recent advances in mesenchymal stem cell membrane-coated nanoparticles for enhanced drug delivery. Biomater. Sci. 2021, 9, 1088–1103.
- Takayama, Y.; Kusamori, K.; Nishikawa, M. Mesenchymal stem/stromal cells as next-generation drug delivery vehicles for cancer therapeutics. Expert Opin. Drug Deliv. 2021, 18, 1627–1642.
- Starnes, C.O. Coley’s toxins. Nature 1992, 360, 23.
- Sartorio, M.G.; Pardue, E.J.; Feldman, M.F.; Haurat, M.F. Bacterial Outer Membrane Vesicles: From Discovery to Applications. Annu. Rev. Microbiol. 2021, 75, 609–630.
- Liu, J.; Liew, S.S.; Wang, J.; Pu, K. Bioinspired and Biomimetic Delivery Platforms for Cancer Vaccines. Adv. Mater. 2022, 34, e2103790.
- Li, M.; Zhou, H.; Yang, C.; Wu, Y.; Zhou, X.; Liu, H.; Wang, Y. Bacterial outer membrane vesicles as a platform for biomedical applications: An update. J. Control. Release 2020, 323, 253–268.
- Holay, M.; Guo, Z.; Pihl, J.; Heo, J.; Park, J.H.; Fang, R.H.; Zhang, L. Bacteria-Inspired Nanomedicine. ACS Appl. Bio Mater. 2021, 4, 3830–3848.
- Krishnan, N.; Kubiatowicz, L.J.; Holay, M.; Zhou, J.; Fang, R.H.; Zhang, L. Bacterial membrane vesicles for vaccine applications. Adv. Drug Deliv. Rev. 2022, 185, 114294.
- Long, Q.; Zheng, P.; Zheng, X.; Li, W.; Hua, L.; Yang, Z.; Huang, W.; Ma, Y. Engineered bacterial membrane vesicles are promising carriers for vaccine design and tumor immunotherapy. Adv. Drug Deliv. Rev. 2022, 186, 114321.
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759.
- Najafi, S.; Majidpoor, J.; Mortezaee, K. Extracellular vesicle-based drug delivery in cancer immunotherapy. Drug Deliv. Transl. Res. 2023, 1–17.
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular vesicles: A bright star of nanomedicine. Biomaterials 2021, 269, 120467.
- Liu, S.; Wu, X.; Chandra, S.; Lyon, C.; Ning, B.; Jiang, L.; Fan, J.; Hu, T.Y. Extracellular vesicles: Emerging tools as therapeutic agent carriers. Acta Pharm. Sin. B 2022, 12, 3822–3842.
- Liu, C.; Wang, Y.; Li, L.; He, D.; Chi, J.; Li, Q.; Wu, Y.; Zhao, Y.; Zhang, S.; Wang, L.; et al. Engineered extracellular vesicles and their mimetics for cancer immunotherapy. J. Control. Release 2022, 349, 679–698.
- Ruan, S.; Greenberg, Z.; Pan, X.; Zhuang, P.; Erwin, N.; He, M. Extracellular Vesicles as an Advanced Delivery Biomaterial for Precision Cancer Immunotherapy. Adv. Healthc. Mater. 2022, 11, e2100650.
- Chen, H.Y.; Deng, J.; Wang, Y.; Wu, C.Q.; Li, X.; Dai, H.W. Hybrid cell membrane-coated nanoparticles: A multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020, 112, 1–13.
- Wang, D.; Liu, C.; You, S.; Zhang, K.; Li, M.; Cao, Y.; Wang, C.; Dong, H.; Zhang, X. Bacterial Vesicle-Cancer Cell Hybrid Membrane-Coated Nanoparticles for Tumor Specific Immune Activation and Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 41138–41147.
- Liu, Y.; Sukumar, U.K.; Kanada, M.; Krishnan, A.; Massoud, T.F.; Paulmurugan, R. Camouflaged Hybrid Cancer Cell-Platelet Fusion Membrane Nanovesicles Deliver Therapeutic MicroRNAs to Presensitize Triple-Negative Breast Cancer to Doxorubicin. Adv. Funct. Mater. 2021, 31, 2103600.
- Zhang, T.; Liu, H.; Li, L.; Guo, Z.; Song, J.; Yang, X.; Wan, G.; Li, R.; Wang, Y. Leukocyte/platelet hybrid membrane-camouflaged dendritic large pore mesoporous silica nanoparticles co-loaded with photo/chemotherapeutic agents for triple negative breast cancer combination treatment. Bioact. Mater. 2021, 6, 3865–3878.
- Liu, W.L.; Zou, M.Z.; Liu, T.; Zeng, J.Y.; Li, X.; Yu, W.Y.; Li, C.X.; Ye, J.J.; Song, W.; Feng, J.; et al. Cytomembrane nanovaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells. Nat. Commun. 2019, 10, 3199.
- Gao, W.; Fang, R.H.; Thamphiwatana, S.; Luk, B.T.; Li, J.; Angsantikul, P.; Zhang, Q.; Hu, C.M.; Zhang, L. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015, 15, 1403–1409.
- Patel, R.B.; Ye, M.; Carlson, P.M.; Jaquish, A.; Zangl, L.; Ma, B.; Wang, Y.; Arthur, I.; Xie, R.; Brown, R.J.; et al. Development of an In Situ Cancer Vaccine via Combinational Radiation and Bacterial-Membrane-Coated Nanoparticles. Adv. Mater. 2019, 31, e1902626.
- Dash, P.; Piras, A.M.; Dash, M. Cell membrane coated nanocarriers—An efficient biomimetic platform for targeted therapy. J. Control. Release 2020, 327, 546–570.
- Le, Q.V.; Lee, J.; Lee, H.; Shim, G.; Oh, Y.K. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm. Sin. B 2021, 11, 2096–2113.
- Luk, B.T.; Hu, C.M.; Fang, R.H.; Dehaini, D.; Carpenter, C.; Gao, W.; Zhang, L. Interfacial interactions between natural RBC membranes and synthetic polymeric nanoparticles. Nanoscale 2014, 6, 2730–2737.
- Xu, C.H.; Ye, P.J.; Zhou, Y.C.; He, D.X.; Wei, H.; Yu, C.Y. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater. 2020, 105, 1–14.
- Rao, L.; Cai, B.; Bu, L.L.; Liao, Q.Q.; Guo, S.S.; Zhao, X.Z.; Dong, W.F.; Liu, W. Microfluidic Electroporation-Facilitated Synthesis of Erythrocyte Membrane-Coated Magnetic Nanoparticles for Enhanced Imaging-Guided Cancer Therapy. ACS Nano 2017, 11, 3496–3505.
- Zhang, J.; Gao, W.; Fang, R.H.; Dong, A.; Zhang, L. Synthesis of Nanogels via Cell Membrane-Templated Polymerization. Small 2015, 11, 4309–4313.
