Zeolites are crystalline, hydrated aluminosilicates with an open-framework structure. Unique structural features make them very useful ion-changers, adsorbents and catalysts. The catalytic use of zeolites has expanded from traditional use in the petrochemical industry and refineries to use in the catalytic degradation of various environmental pollutants and the synthesis of fine chemicals. Progress on the use of zeolites has been achieved in biomass conversion to fuels and valuable industrial bio-based chemicals.
- clinoptilolite
- natural zeolite
- catalysis
1. Introduction
Today, environmental pollution is one of the most important topics of worldwide discussion. An increase in the population along with rapid industrialization and urbanization have led to damage of the environment and consequently to serious damage to human health. Furthermore, the situation is becoming more complex due to insufficient attention and control of the discharging of many pollutants to the environment, such as heavy metals, pharmaceuticals, pesticides, organic dyes, etc. Many of these pollutants are persistent, toxic and carcinogenic. Also, the generation of large amounts of solid waste and its inappropriate disposal negatively affect the environment.
Consequently, research efforts are focused on developing new materials and technologies that can minimize environmental pollution. According to the principles of sustainable development, they are necessary to be not only effective but also environmentally and economically acceptable. In this regard, catalysis is one of the main fields that can give a significant contribution to the field of green chemistry and environmental protection. About 90% of all industrial processes are catalyzed, so the choice of catalyst is one of the key parameters for the sustainability of applied technologies. A large number of both homogeneous and heterogeneous catalysts are in use. Different kinds of solids, including activated carbon-based materials, mesoporous silica, clays, zeolites and zeolite-like materials have been studied in catalysis [1][2][3][4][5][6][7].
Zeolites are hydrated aluminosilicates with unique structural features and a chemical composition that is applicable in many areas. In the second part of the 20th century, there was an expansion of synthetic zeolites with new structural features, which led to the neglect of natural zeolites in many scientific studies. However, the 21st century, as the century of green chemistry, brought natural zeolites back into the spotlight of scientific interest. Many regions in the world have deposits with high contents of zeolites with high purity. Due to their low price and availability, they have become a good basis for the development of new green adsorbents and catalysts.
Zeolites are hydrated aluminosilicates with unique structural features and a chemical composition that is applicable in many areas. In the second part of the 20th century, there was an expansion of synthetic zeolites with new structural features, which led to the neglect of natural zeolites in many scientific studies. However, the 21st century, as the century of green chemistry, brought natural zeolites back into the spotlight of scientific interest. Many regions in the world have deposits with high contents of zeolites with high purity. Due to their low price and availability, they have become a good basis for the development of new green adsorbents and catalysts.
2. A Brief Description of Zeolite Structures
Zeolites are crystalline, open-framework aluminosilicates. They are composed of three-dimensional frameworks consisting of [SiO4]4– and [AlO4]5– tetrahedral units linked via oxygen atoms (Figure 1). The lattice of zeolites is negatively charged, and its electroneutrality is achieved by extraframework cations - alkali and earth alkaline cations. These cations interact with the lattice via electrostatic interactions and are movable, which gives zeolites an ion-exchange property. Ion-exchange takes place to varying degrees depending on nature of zeolites, nature of cations present in zeolite lattice and solution as well as on experimental conditions such as static or dynamic regime, solid:liquid ratio, contact time, reaction temperature, initial concentration, pH, contact time.
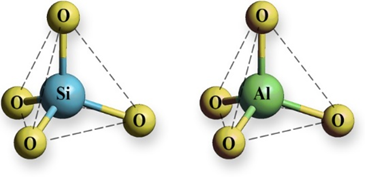
Figure 1. [SiO4]4− and [AlO4]5− tetrahedral units of a zeolite lattice.
Porosity of the lattice provides a large specific area, being several hundred to thousand square meters per gram, which gives zeolites good adsorptive properties. The shape and openings of cavities and channels affect the adsorptive behavior allowing only species of proper dimensions and geometries to enter and diffuse throughout the lattice.
Zeolites show also catalytic properties. Hydrogen ions can be accommodated in the zeolite lattice instead of metal cations. This brings Brönsted acidity and makes zeolites catalytically active. The presence of three-coordinated Al and/or extra-framework Al species in the lattice brings Lewis acid sites (Figure 2). The acidity of zeolites can be significantly enriched through different chemical and/or thermal treatments.
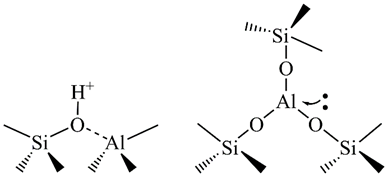
Figure 2. The Brönsted (left) and Lewis (right) acid sites in a zeolite lattice.
3. Clinoptilolite
According to the structural database of the International Zeolite Association (IZA), currently about 60 different zeolite structures belong to natural zeolites and 248 for synthetic ones[8]. Among the natural zeolites, clinoptilolite (CLI) is one of the most abundant and the most widely studied natural zeolites. Deposits of CLI-rich tuffs are found in many countries including deposits in China, the United States, Indonesia, New Zealand, Cuba and the Republic of Korea. In Europe, the abundant deposits are found in Turkey, Hungary, Slovenia, Slovakia, Ukraine, Italy, Romania and Serbia[9][10][11]. Depending on the location of deposits, the CLI content varies between 60% and 90%, whereas feldspars, clays and quartz are the most common present satellite phases.
CLI is a member of the heulandite (HEU) group of natural zeolites. CLI and HEU are isostructural and differ only by the Si/Al molar ratio, which influences their thermal stability. The Si/Al ratio of CLI is in the range 4.0–5.3, in contrast to HEU, for which this ratio is lower than 4.0[9][12][13]. A higher Si/Al ratio of CLI makes CLI more thermally stable (up to 800 °C) than HEU (up to 550 °C).
The framework of the CLI consists of three types of channels: A - formed from 10-membered rings, B - formed from 8-membered rings and C - formed from 8-membered rings. The channels A (0.3 x 0.76 nm) and B (0.33 x 0.46 nm) are parallel to the c-axis, while the C channel (0.26 x 0.47 nm) is parallel to the a-axis[14][15].
3.1. Modification of CLI
Due to its structural features and availability, CLI has been recognized as a perspective candidate for the synthesis of different types of materials that have been used in ion-exchange, adsorption and catalysis. In order to expand the range of CLI applications its modification by various chemical and/or thermal treatments has received much attention in many research works.
Numerous studies have shown that the ion-exchange capacity of CLI can be significantly increased through modification. The most commonly used method involves the conversion of parent CLI into a homoionic form usually by treating CLI with a concentrated solution of NaCl [16][17][18][19][20]. This method involves an ion-exchange reaction between cations present in the CLI lattice and cations in solution, as well as diffusion of the outside cations through the lattice. Ion exchange mediated by transition metal cations typically includes the chemisorption or complexation of the cations and/or oxide precipitation onto the CLI surface.
The surface charge and polarity of CLI can be adjusted using suitable methods that involve the adsorption of various anions and/or nonpolar organics. Among the most commonly studied methods is the adsorption of different surfactants, such as quaternary ammonium compounds (tetraethylammonium, hexadecyltrimetylammonium, cetylpyridinium or octadecyltrimethylammonium ion) [21][22]]. One or two surfactant layers cover the surface depending on the surfactant concentration.
In addition, different organic species, such as polymers (polypyrrole, polyaniline, polydomin, chitosan, polyethylenimine, etc.) or amines (n-octadecylamine, n-butylamine, tetrapropylamine, monoethanolamine, 1-dodecylamine, 1-hexadecylamine, etc.) have been applied to provide the presence of multifunctional groups onto the CLI surface and additional binding sites [23][24][25][26][27].
Various studies confirmed that the catalytic performance of CLI can be significantly improved by covering the CLI surface with various metal oxide particles, such as Ni, Ga, Ti, Sn and Zr. The CLI structure fulfills multiple roles: it contributes to the crystallization of nano particles, prevents their agglomeration and efficiently immobilizes them[28][29][30][31].
3.2. Preparation of CLI-Based Catalysts
One of the most commonly used methods includes the conversion of CLI into a Fe(III)-form (Fe2O3-containing CLI, FeCLI). The methods are based on a three-step procedure: 1) conversion of CLI to NaCLI, 2) conversion of NaCLI to FeCLI accompanied by the addition of NaOH and 3) calcination of FeCLI at about 550 °C [32][33]. In the last step, nano Fe2O3 crystallizes onto the CLI surface. FeCLI appears to be a suitable catalyst for Fenton-like reactions offering advantages, such as activity even at neutral pH, the degradation of different organic pollutants (organic dyes, personal care products, phenol, furfural, etc.) through adsorption and oxidation and recyclability with minimal iron leaching [34][35][36][37][38].
The preparation of metal-containing CLI (MOCLI) catalysts very often requires pretreatment of the CLI and its transformation to HCLI. HCLI has a larger specific surface area and possesses acidity[28].
MOCLI can also be obtained via precipitation, impregnation, hydrothermal crystallization, sol-gel and solid-state dispersion [39][40][41][42][43]. The catalytic performance of MOCLI shows dependence on the modification parameters including the metal concentration, reaction temperature, acidity, calcination temperature, etc.[39][42][43].
Since the acidity of a catalyst plays a crucial role in many catalytic reactions, many studies have been focused on HCLI preparation. Two treatments proved to be suitable: a) conversion of CLI to NH4-CLI, in which an ion-exchange reaction mediated by NH4+ ions, followed by the calcination of NH4-CLI at about 500 °C, gives HCLI and b) the direct conversion of CLI to HCLI using an acid treatment. Most commonly, strong mineral acids, such as HCl, HNO3 or H2SO4 are used. To minimize the loss of crystallinity of the CLI lattice, mild treatment conditions are preferable [44]. In the acid treatment, exchangeable cations from the CLI lattice are replaced by hydronium ions, which is accompanied by partial dealumination of the lattice (Figure 3). Al leaching leads to the formation of lattice vacancies and the appearance of extra-framework aluminum species (octahedrally and five-coordinated Al species) present in the pore system [44][45]. These species create strong acid Lewis sites, in contrast to Brönsted acid sites, which are formed by bridging hydroxyl groups (Si–(OH)–Al).
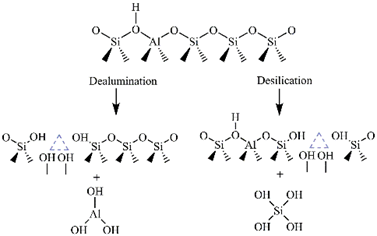
Figure 3. Schematic illustration of the dealumination and desilication of CLI.
The acid treatment of CLI improves both porosity and catalytic performance. Raw CLI has a relatively low specific surface area (up to 40 m2 g–1), as well as micropore volumes. Considerable increases in the specific surface area (even five times), micropore volume and external surface area have been observed during the acid treatment [15][44][46]. This is ascribed to partial dealumination of the aluminosilicate lattice, resulting in opening of the pore system. Also, almost complete replacement of the metal cations by H+ cations occurs and contributes to more free space within the CLI lattice [44][45].
The acidity of CLI can be improved also by desilication. The method includes a partial removal of Si4+ during an alkaline treatment (usually by applying NaOH). Si removal can occur at different extents, from about 0.5 wt.% (using 0.05 mol NaOH dm–3) to 7.6 wt.% (by 0.8 mol NaOH dm–3) without damage to the crystal structure. A higher degree of desilication causes a partial loss of the CLI crystallinity [47][48][49][50]. It is worth noting that desilication does not lead to a significant increase in the CLI specific surface area in comparison to dealumination [47]. Desilication contributes significantly to the acidity increasing both the Brönsted and Lewis acidity [49].
3,3 Catalytic Performance of CLI
The availability and structural features of CLI result in its widespread application in many areas, including various industrial processes, agriculture, veterinary fields, medicine and environmental protection [51][52][53][54][55][56][57].
Moreover, the possibility of the chemical modification of the CLI and its thermal stability make CLI is a good candidate for catalytic applications. CLI shows catalytic activity in the degradation of different environmental pollutants, including organic dyes which are extensively used in textile, paper, food and cosmetic industries. CLI has shown to be good support for the photocatalytically active particles of metal oxide (MO), such as TiO2, ZnO, SnO2, CuO, NiO, etc.[28][42][58][59]. It has been found that the adsorption affinity of the CLI towards organic dyes brings more organic dye molecules near the catalyst surface where the production of hydroxyl radicals occurs. This leads to the creation of many active sites for the adsorption of intermediates and performance of catalytic reactions. The enhanced reactivity of MOCLI is ascribed to a synergistic effect between the MO particles and the CLI lattice. The CLI lattice prevents the aggregation of MO particles by fixing them onto ion-exchange sites and also enables electron-hole recombination, which both contribute to the photocatalytic reaction [28][58][60][61]. Additionally, CLI has been used for the degradation of pharmaceuticals including the most frequently used antibiotics, beta-blockers, diuretics, antihistamines, etc., herbicides and pesticides that are recognized as emerging pollutants that pose a severe threat to the environment and risk to human health due to their low biodegradability, high persistence and bio-accumulation[40][62][63][64][65].
Nowadays, CLI and its modified forms have been also applied in biomass conversion to biofuels, biofuel additives and important industrial chemicals [29][66][67]. Additionally, CLI has also been recognized as a suitable candidate for reduction of nitrogen oxides (NOx; mainly NO and NO2) through the selective catalytic reduction process which is one of the most widely applied technologies for NOx reduction. Even non-modified CLI possesses catalytical activity, which was ascribed to the presence of iron species, which are the main impurity in the zeolitic tuffs [68][69][70]. The catalytic performance can be enhanced through the immobilization of active metal oxide particles at the CLI surface. Iron oxides were mostly studied because of their outstanding thermal stability, environmental compatibility, weak oxidizing properties and high tolerance to the presence of SOx in flue gas [68][69][71][72].
References
- Perez, F.M.; Santori, G.F.; Pompeo, F.; Nichio, N.N. Silica-resin-bentonite nanocomposite and its application in catalysis. Minerals 2022, 12(12), 1486. https://doi.org/10.3390/min12121486
- Valášková, M.; Kočí, K.; Madejová, J.; Matějová, L.; Pavlovský, J.; Barrocas, B.T.; Klemencová, K. α-Fe2O3 nanoparticles/iron-containing vermiculite composites: Structural, textural, optical and photocatalytic properties. Minerals 2022, 12, 607. https://doi.org/10.3390/min12050607
- Li, X.; Peng, K. MoSe2/Montmorillonite composite nanosheets: Hydrothermal synthesis, structural characteristics, and enhanced photocatalytic activity. Minerals 2018, 8(7), 268. https://doi.org/10.3390/min8070268
- Di Lorenzo, F.; Ruiz-Aguido, C.; Ibañez-Velasco, A.; Gil-San Millan, R.; Navarro, J.A.R.; Ruiz-Agudo, E.; Rodriguez-Navarro, C. The carbonation of wollastonite: A model reaction to test natural and biomimetic catalysts for enhanced CO2 sequestration. Minerals 2018, 8(5), 209. https://doi.org/10.3390/min8050209
- Yu, X.; Williams, C.T. Recent advances in the applications of mesoporous silica in heterogeneous catalysis. Catal. Sci. Technol. 2022, 12, 5765–5794. https://doi.org/10.1039/D2CY0000Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. https://doi.org/10.1038/s41578-021-00347-3Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: a review. RSC Adv. 2018, 8, 5197–5211. https://doi.org/10.1039/C7RA12924F
- Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. https://doi.org/10.1038/s41578-021-00347-3Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: a review. RSC Adv. 2018, 8, 5197–5211. https://doi.org/10.1039/C7RA12924F
- Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: a review. RSC Adv. 2018, 8, 5197–5211. https://doi.org/10.1039/C7RA12924F
- https://europe.iza-structure.org/IZA-SC/ftc_table.php (accessed on 13 May 2023)
- Ambrozova, P.; Kynicky, J.; Urubek, T., Nguyen, V.D. Synthesis and modifications of clinoptilolite. Molecules 2017, 22(7), 1107. https://doi.org/10.3390/molecules22071107
- Mormone, A.; Piochi, M. Mineralogy, geochemistry and genesis of zeolites in cenozoic pyroclastic flows from the Asuni Area (Central Sardinia, Italy). Minerals 2020, 10(3), 268. https://doi.org/10.3390/min10030268
- Kowalczyk P.; Sprynskyy, M.; Terzyk, A.P.; Lebedynets, M.; Namieśnik, J.; Buszewski, B. Porous structure of natural and modified clinoptilolites. J. Colloid. Interface Sci. 2006, 297(1), 77–85. https://doi.org/10.1016/j.jcis.2005.10.045
- Mansouri, N.; Rikhtegar, N.; Panahi, S.H.; Atabi, F.; Shahraki, B.K. Porosity, characterization and structural properties of natural zeolite—Clinoptilolite—As a sorbent. Environ. Prot. Eng. 2013, 39, 139–152. DOI: 10.5277/EPE13011
- Godelitsas, A.; Armbruster, T. HEU-type zeolites modified by transition elements and lead. Micropor. Mesopor. Mat. 2003, 61, 3–24. https://doi.org/10.1016/S1387-1811(03)00352-4
- Dziedzicka, A.; Sulikowski, B.; Ruggiero-Mikolajczyk, M. Catalytic and physicochemical properties of modified natural clinoptilolite, Catal. Today 2016, 259, 50–58. https://doi.org/10.1016/j.cattod.2015.04.039
- Rajic, N.; Stojakovic, Dj.; Daneu, N.; Recnik, A. The formation of oxide nanoparticles on the surface of natural clinoptilolite, J. Phys. Chem. Solids 2011, 72, 800–803. https://doi.org/10.1016/j.jpcs.2011.03.018
- Tarasevich, Y.I.; Krysenko, D.A.; Polyakov, V.E.; Aksenenko, E.V. The heats of exchange of transition metal ions on the Na form of clinoptilolite. Russ. J. Phys. Chem. A 2008, 82, 1506–1511. https://doi.org/10.1134/S0036024408090185
- Mozgawa, W.; Krol, M.; Pichor, W. Use of clinoptilolite for the immobilization of heavy metal ions and preparation of autoclaved building composites. J. Hazard. Mater. 2009, 168, 1482–1489. https://doi.org/10.1016/j.jhazmat.2009.03.037
- Rajić, N.; Stojaković, Đ., Jovanović, M.; Zabikovec Logar, N.; Mazaj, M.; Kaučić, V. Removal of nickel(II) ions from aqueous solutions using the natural clinoptilolite and preparation of nano-NiO on the exhausted clinoptilolite. Appl. Surf. Sci. 2010, 57, 1524 –1532. https://doi.org/10.1016/j.apsusc.2010.08.09019. Mihaly-Cozmuta, L.; Mihaly-Cozmuta, A.; Peter, A.; Nicula, C.; Tutu, H.; Silipas, D.; Indrea, E. Adsorption of heavy metal cations by Na-clinoptilolite: Equilibrium and selectivity studies. J. Environ. Manage. 2014, 137, 69–80. https://doi.org/10.1016/j.jenvman.2014.02.00720. Guan, H.; Bestland, E.; Zhu, C.; Zhu, H.; Albertsdottir, D.; Hutson, J.; Simmons, C.T.; Ginic-Markovic, M.; Tao, X.; Ellis, A.V. Variation in performance of surfactant loading and resulting nitrate removal among four selected natural zeolites. J. Hazard. Mater. 2010, 183, 616–621. https://doi.org/10.1016/j.jhazmat.2010.07.069
- Mihaly-Cozmuta, L.; Mihaly-Cozmuta, A.; Peter, A.; Nicula, C.; Tutu, H.; Silipas, D.; Indrea, E. Adsorption of heavy metal cations by Na-clinoptilolite: Equilibrium and selectivity studies. J. Environ. Manage. 2014, 137, 69–80. https://doi.org/10.1016/j.jenvman.2014.02.007
- Guan, H.; Bestland, E.; Zhu, C.; Zhu, H.; Albertsdottir, D.; Hutson, J.; Simmons, C.T.; Ginic-Markovic, M.; Tao, X.; Ellis, A.V. Variation in performance of surfactant loading and resulting nitrate removal among four selected natural zeolites. J. Hazard. Mater. 2010, 183, 616–621. https://doi.org/10.1016/j.jhazmat.2010.07.069
- Schick, J.; Caullet, P.; Paillaud, J.L.; Patarin, J.; Mangold-Callarec, C. Nitrate sorption from water on a Surfactant-Modified Zeolite. Fixed-bed column experiments. Micropor. Mesopor. Mat. 2011, 142, 549–556. https://doi.org/10.1016/j.micromeso.2010.12.03922. Olad, A.; Ahmadi, S.; Rashidzadeh, A. Removal of nickel (II) from aqueous solutions with polypyrrole modified clinoptilolite: Kinetic and isotherm studies. Desalin. Water Treat. 2013, 51, 7172–7180. https://doi.org/10.1080/19443994.2013.771285
- Olad, A.; Ahmadi, S.; Rashidzadeh, A. Removal of nickel (II) from aqueous solutions with polypyrrole modified clinoptilolite: Kinetic and isotherm studies. Desalin. Water Treat. 2013, 51, 7172–7180. https://doi.org/10.1080/19443994.2013.771285
- Zaremotlagh, S.; Hezarkhani, A. Removal of textile dyes from aqueous solution by conducting polymer modified clinoptilolite. Environ. Earth Sci. 2014, 71, 2999–3006. https://doi.org/10.1007/s12665-013-2676-5
- Guzel, P.; Aydın, Y.A.; Deveci Aksoy, N. Removal of chromate from wastewater using amine-based-surfactant-modified clinoptilolite. Int. J. Environ. Sci. Technol. 2016, 13, 1277–1288. https://doi.org/10.1007/s13762-016-0954-y
- Zhao, Y.; Zhao, X.; Deng, J.; He, C. Utilization of chitosan–clinoptilolite composite for the removal of radiocobalt from aqueous solution: Kinetics and thermodynamics. J. Radioanal. Nucl. Chem. 2016, 308, 701–709. https://doi.org/10.1007/s10967-015-4475-9
- Ambrozova, P.; Kynicky, J.; Urubek, T.; Nguyen, V.D. Synthesis and modification of clinoptilolite. Molecules 2017, 22, 1107. https://doi.org/10.3390/molecules22071107
- Mortazavi, N.; Bahadori, M.; Marandi, A.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I. Enhancement of CO2 adsorption on natural zeolite, modified clinoptilolite with cations, amines and ionic liquids. Sustain. Chem. Pharm. 2021, 22, 100495. https://doi.org/10.1016/j.scp.2021.100495
- Šuligoj, A.; Pavlović, J.; Arčon, I.; Rajić, N.; Novak Tušar, N. SnO2-containing clinoptilolite as a composite photocatalyst for dyes removal from wastewater under solar light. Catalysts 2020, 10, 253. https://doi.org/10.3390/catal10020253
- Pavlović, J.; Popova, M.; Mihalyi, R.M.; Mazaj, M.; Mali, G.; Kovač, J.; Lazarova, H.; Rajić, N. Catalytic activity of SnO2- and SO4/SnO2-containing clinoptilolite in the esterification of levulinic acid. Micropor. Mesopor. Mater. 2019, 27, 10–18. https://doi.org/10.1016/j.micromeso.2018.12.009
- Yang, G.; Pidko, E.A.; Hensen, E.J.M. Structure, stability, and Lewis acidity of mono and double Ti, Zr, and Sn framework substitutions in BEA zeolites: A periodic density functional theory study, J. Phys. Chem. 2013, 117, 3976–3986. https://doi.org/10.1021/jp310433r
- Zhang, G.; Feng, P.; Zhang, W.; Liu, H.; Wang, C.; Ma, H.; Wang, D.; Tian, Z. Single isomerization selectivity of glucose in methanol over Sn-BEC zeolite of homogenous Sn distribution, Micropor. Mesopor. Mat. 2017, 247, 158–165. https://doi.org/10.1016/j.micromeso.2017.03.052
- Huo, H.; Lina, H.; Donga, Y.; Cheng, H.; Wang, H.; Cao, L. Ammonia-nitrogen and phosphates sorption from simulated reclaimed waters by modified clinoptilolite. J. Hazard. Mater. 2012, 229-230, 292–297. https://doi.org/10.1016/j.jhazmat.2012.06.001
- Guaya, D.; Valderrama, C.; Farran, A.; Cortina, J.L. Modification of a natural zeolite with Fe(III) for simultaneous phosphate and ammonium removal from aqueous solutions. J. Chem. Technol. Biotechnol. 2016, 91, 1737–1746. https://doi.org/10.1002/jctb.4763
- Tekbaş, M.; Yatmaz, H. C.; Bektaş, N. Heterogeneous photo-Fenton oxidation of reactive azo dye solutions using iron exchanged zeolite as a catalyst. Micropor. Mesopor. Mat. 2008, 115(3), 594–602. https://doi.org/10.1016/j.micromeso.2008.03.001
- Bayat, M.; Sohrabi, M.; Royaee, S. J. Degradation of phenol by heterogeneous Fenton reaction using Fe/clinoptilolite. J. Ind. Eng. Chem. 2012, 18(3), 957–962. https://doi.org/10.1016/j.jiec.2011.09.004
- Gonzalez-Olmos, R.; Martin, M. J.; Georgi, A.; Kopinke, F.D.; Oller, I.; Malato, S. Fe-zeolites as heterogeneous catalysts in solar Fenton-like reactions at neutral pH. Appl. Catal. B: Environ. 2012, 125, 51–58. https://doi.org/10.1016/j.apcatb.2012.05.022
- Mousavi-Mortazavi, S.; Nezamzadeh-Ejhieh, A. Supported iron oxide onto an Iranian clinoptilolite as a heterogeneous catalyst for photodegradation of furfural in a wastewater sample. Desalin. Water Treat. 2015, 57(23), 10802–10814. https://doi.org/10.1080/19443994.2015.1036465
- Anis, M.; Haydar, S. Heterogeneous Fenton oxidation of caffeine using zeolite-supported iron nanoparticles. Arab. J. Sci. Eng. 2018, 44(1), 315–328. https://doi.org/10.1007/s13369-018-3659-3
- Wang, C.; Shi, H.; Li, Y. Synthesis and characterization of natural zeolite supported Cr-doped TiO2 photocatalysts. Appl. Surf. Sci. 2012, 258(10), 4328–4333. https://doi.org/10.1016/j.apsusc.2011.12.108
- Heidari, Z.; Alizade, R.; Ebadi, A.; Oturan, N.; Oturan, M.A. Efficient photocatalytic degradation of furosemide by a novel sonoprecipited ZnO over ion exchanged clinoptilolite nanorods. Sep. Purif. Technol. 2020, 242, 116800. https://doi.org/10.1016/j.seppur.2020.116800
- Shen, Y.; Zhou, P.; Zhao, S.; Li, A.; Chen, Y.; Bai, J.; Han, C.; Wei, D.; Ao, Y. Synthesis of high-efficient TiO2/clinoptilolite photocatalyst for complete degradation of xanthate. Miner. Eng. 2020, 159, 106640. https://doi.org/10.1016/j.mineng.2020.106640
- Ullah, R.; Liu, C.; Panezai, H.; Gul, A.; Sun, J.; Wu, X. Controlled crystal phase and particle size of loaded-TiO2 using clinoptilolite as support via hydrothermal method for degradation of crystal violet dye in aqueous solution. Arab. J. Chem. 2020, 13, 4092–4101. https://doi.org/10.1016/j.arabjc.2019.06.011
- Ullah, R.; Sin, J.; Gul, A.; Munir, T.; Wu, X. Evaluations of physico-chemical properties of TiO2/clinoptilolite synthesized via three methods on photocatalytic degradation of crystal violet. Chin. J. Chem. Eng. 2021, 33, 181–189. https://doi.org/10.1016/j.cjche.2020.09.045
- Garcia-Basabe, Y.; Rodriguez-Iznaga, I.; de Menorval, L.-C.; Llewellyn, P.; Maurin, G.; Lewis, D.W.; Binions, R.; Autie, M.; Ruiz-Salvador, A.R. Step-wise dealumination of natural clinoptilolite: Structural and physicochemical characterization. Micropor. Mesopor. Mater. 2010, 135, 187–196. https://doi.org/10.1016/j.micromeso.2010.07.008
- Farías, T.; Ruiz-Salvador, A.R.; Velazco, L.; de Ménorval, L.C.; Rivera, A. Preparation of natural zeolitic supports for potential biomedical applications. Mater. Chem. Phys. 2009, 118, 322–328. https://doi.org/10.1016/j.matchemphys.2009.07.054
- 46. Wojciechowska, K. The influence of desilication/dealumination processes on the physicochemical properties of clinoptilolite. Clay Miner. 2019, 54, 111–119. https://doi.org/10.1180/clm.2019.17
- Lin, H.; Liu, Q.; Dong, Y.; He, Y.; Wang, L. Physicochemical properties and mechanism study of clinoptilolite modified by NaOH, Micropor. Mesopor. Mat. 2015, 218, 174–179. https://doi.org/10.1016/j.micromeso.2015.07.017
- Ates A. Effect of alkali-treatment on the characteristics of natural zeolites with different compositions, J. Colloid Interf. Sci. 2018, 23, 266–281. https://doi.org/10.1016/j.jcis.2018.03.115
- Souza, V.C.; Villarroel-Rocha, J.; Araújo, M.J.G.; Sapag, K.; Pergher, S.B.C. Basic treatment in natural clinoptilolite for improvement of physicochemical properties, Minerals 2018, 8(12), 595–609. https://doi.org/10.3390/min8120595
- Wang, C.; Leng, S.; Guo, H.; Cao, L.; Huang, J. Acid and alkaline treatments for regulation of hydrophilicity/hydrophobicity of natural zeolite, Appl. Surf. Sci. 2019, 478, 319–326. https://doi.org/10.1016/j.apsusc.2019.01.263
- Gennaro, B.; Catalanotti, L.; Cappelletti, P.; Langella, A.; Mercurio, M.; Serri, C.; Biondi, M.; Mayol, L. Surface modified natural zeolite as a carrier for sustained diclofenac release: A preliminary feasibility study, Colloid. Surface. B 2015, 130, 101–109. https://doi.org/10.1016/j.colsurfb.2015.03.052
- Pavlović, J.; Krogstad, T.; Rajić, N. Applicability of zeolites in potassium and nitrate retention in different soil types, J. Serb. Chem. Soc. 2017, 82, 1303–1314. https://doi.org/10.2298/JSC170704106P
- Hailu, Y.; Tilahum, E.; Brhane, A.; Resky, H.; Sahu, O. Ion exchanges process for calcium, magnesium and total hardness from ground water with natural zeolite, Groundw. Sustain. Dev. 2019, 8, 457–469. https://doi.org/10.1016/j.gsd.2019.01.009
- Vollprecht, D.; Frühauf, S.; Stocker, K.; Ellersdorfer, M. Ammonium sorption from landfill leachates using natural and modified zeolites: Pre-tests for a novel application of the ion exchanger loop stripping process. Minerals 2019, 9(8), 471. https://doi.org/10.3390/min9080471
- Yi, N.; Wu, Y.; Fan, L.; Hu, S. Remediating Cd-contaminated soils using natural and chitosan-introduced zeolite, bentonite, and activated carbon, Pol. J. Environ. Stud. 2019, 28, 1461–1468. https://doi.org/10.15244/pjoes/89577
- Pavlović, J.; Šuligoj, A.; Oprešnik, M.; Novak Tušar, N.; Zabukovec Logar, N.; Rajić, N. Studies of clinoptilolite-rich zeolitic tuffs from different regions and their activity in photodegradation of methylene blue. Catalysts 2022, 12(2), 224. https://doi.org/10.3390/catal12020224
- Haemmerle, M.; Tschegg, C. Sorption of natural siderophores onto clinoptilolite-tuff and its controlled-release characteristics. Minerals 2023, 13(5), 611. https://doi.org/10.3390/min13050611
- Dzinum, H.; Othman, M.H.; Ismail, A.F. Photocatalytic performance of TiO2/Clinoptilolite: Comparison study in suspension and hybrid photocatalytic membrane reactor. Chemosphere 2019, 228, 241–248. https://doi.org/10.1016/j.chemosphere.2019.04.118
- Tetteh, E.K.; Rathilal, S. Adsorption and photocatalytic mineralization of bromophenol blue dye with TiO2 modified with clinoptilolite/activated carbon. Catalysts 2021, 11(1), 7. https://doi.org/10.3390/catal11010007
- Nezamzadeh-Ejhieh, A.; Zabihi-Mobarakeh, H. Heterogeneous photodecolorization of mixture of methylene blue and bromophenol blue using CuO-nano-clinoptilolite. J. Ind. Eng. Chem. 2014, 20, 1421–1431. https://doi.org/10.1016/j.jiec.2013.07.027
- Bahrami, M.; Nezamzadeh-Ejhieh, A. Effect of the supported ZnO on clinoptilolite nano-particles in the photodecolorization of semi-real sample bromothymol blue aqueous solution. Mater. Sci. Semicon. Proc. 2015, 30, 275–284. https://doi.org/10.1016/j.mssp.2014.10.006
- Ajoudanian, N.; Nezamzadeh-Ejhieh, A. Enhanced photocatalytic activity of nickel oxide supported on clinoptilolite nanoparticles for the photodegradation of aqueous cephalexin. Mater. Sci. Semicond. Process 2015, 36, 162–169. https://doi.org/10.1016/j.mssp.2015.03.042
- Arabpour, N.; Nezamzadeh-Ejhieh, A. Photodegradation of cotrimaxazole by clinoptilolite-supported nickel oxide. Process Saf. Environ. Pro. 2016, 102, 431–440. https://doi.org/10.1016/j.psep.2016.04.025
- Davari, N.; Farhadian, M.; Solaimany Nazar, A.R.; Homayoonfal, M. Degradation of diphenhydramine by the photocatalysts of ZnO/Fe2O3 and TiO2/Fe2O3 based on clinoptilolite: Structural and operational comparison. J. Environ. Chem. Eng. 2017, 5, 5707–5720. https://doi.org/10.1016/j.jece.2017.10.052
- Mehrabadi, Z.; Faghihian, H. Clinoptilolite modified with TiO2 for simultaneous elimination of two herbicides; 2,4-D and MCPA by UV and sunlight-assisted photocatalytic degradation. Mater. Res. Bull. 2019, 119, 110569. https://doi.org/10.1016/j.materresbull.2019.110569
- Helmi, M.; Ghadiri, M.; Tahvildari, K.; Hemmati, A. Biodiesel synthesis using clinoptilolite-Fe3O4-based phosphomolybdic acid as a novel magnetic green catalyst from salvia mirzayanii oil via electrolysis method: Optimization study by Taguchi method. J. Environ. Chem. Eng. 2021, 9, 105988. https://doi.org/10.1016/j.jece.2021.105988
- Aghel, B.; Gouran, A.; Nasirmanesh, F. Transesterification of waste cooking oil using clinoptilolite/industrial phosphoric waste as green and environmental catalysts. Energy 2022, 244, 123138. https://doi.org/10.1016/j.energy.2022.123138
- Saramok, M.; Szymaszek, A.; Inger, M.; Antoniak-Jurak, K.; Samojeden, B.; Motak, M. Modified zeolite catalyst for a NOx selective catalytic reduction process in nitric acid plants. Catalysts 2021, 11(4), 450. https://doi.org/10.3390/catal11040450
- Saramok, M.; Inger, M.; Antoniak-Jurak, K.; Szymaszek-Wawryca, A.; Samojeden, B.; Motak, M. Physicochemical features and NH3-SCR catalytic performance of natural zeolite modified with iron–The effect of Fe Loading. Catalysts 2022, 12, 731. https://doi.org/10.3390/catal12070731
- Gelves, J.R.; Dorkis, L.; Márquez, M.A.; Álvarez, A.C.; González, L.M.; Villa, A. L. Activity of an iron Colombian natural zeolite as potential geo-catalyst for NH3-SCR of NOx. Catal. Today 2019, 320, 112–122. https://doi.org/10.1016/j.cattod.2018.01.025
- Long, R.Q.; Yang, R.T. Selective catalytic reduction of NO with ammonia over Fe3+–exchanged mordenite (Fe–MOR): Catalytic performance, characterization, and mechanistic study. J. Catal. 2002, 207, 274–285. https://doi.org/10.1006/jcat.2002.3521
- Szymaszek, A.; Samojeden, B.; Motak, M. Selective catalytic reduction of NOx with ammonia (NH3-SCR) over transition metal-based catalysts-Influence of the catalysts support. Physicochem. Probl. Miner. Process. 2019, 55, 1429–1441. https://doi.org/10.5277/ppmp19066
