Biobanks provide a platform for innovative biomedical research. Recent scientific advances in cryopreservation have enabled the prospect of establishing “living biobanks” that store viable, functional tissue or replicable cell types for years to decades.
- patient-derived cancer models
- living biobanks
1. Introduction
Traditional cancer models including cell lines and animal models have limited applications in both basic and clinical cancer research. Genomics-based precision oncology only help 2–20% patients with solid cancer. Functional diagnostics and patient-derived cancer models are needed for precision cancer biology. RIn this researchers view, we will summarize applications of conditional cell reprogramming (CR) in cancer research and next generation living biobanks (NGLB). Together with organoids, CR has been cited in two NCI (National Cancer Institute, USA) programs (PDMR: patient-derived cancer model repository; HCMI: human cancer model initiatives. HCMI will be distributed through ATCC). Briefly, the CR method is a simple co-culture technology with a Rho kinase inhibitor, Y-27632, in combination with fibroblast feeder cells, which allows researchersus to rapidly expand both normal and malignant epithelial cells from diverse anatomic sites and mammalian species and does not require transfection with exogenous viral or cellular genes. Establishment of CR cells from both normal and tumor tissue is highly efficient. The robust nature of the technique is exemplified by the ability to produce 2 × 106 cells in five days from a core biopsy of tumor tissue. Normal CR cell cultures retain a normal karyotype and differentiation potential and CR cells derived from tumors retain their tumorigenic phenotype. CR also allows reusearchers to enrich cancer cells from urine (for bladder cancer), blood (for prostate cancer), and pleural effusion (for non-small cell lung carcinoma). The ability to produce inexhaustible cell populations using CR technology from small biopsies and cryopreserved specimens has the potential to transform biobanking repositories (NGLB: next-generation living biobank) and current pathology practice by enabling genetic, biochemical, metabolomic, proteomic, and biological assays, including chemosensitivity testing as a functional diagnostics tool for precision cancer medicine.
2. Patient-Derived Cancer Models and Cell RCultures are Needed for Precision Oncology
2.1. iPS (Induce Pluripotent Stem) Cells
2.2. Organoid Cultures
2.3. Patient-Derived Xenografts (PDX)
2.4. Conditionally Reprogrammed Cells (CRCs)
3. Next-Generation Living Biobanks (NGLB)
Living Biobanks will have a significant impact across basic biological research, medicine and the biopharma industry; however, the effects of such applications are underexplored. Recent approaches, for example, patient-derived xenograft models, organoids, conditionally reprogrammed cells, induced pluripotent cells, and other cancer precision medicine applications, represent an unexhausted resource of living biobanks. However, these concepts are applied in very many different ways by the academic and private sectors, representing an actively growing field that has yet to reach clinical consensus or maturity. Here reswearchers summarize application of CR technology in living biobanks. 28–29 March 2011, rwesearchers first presented “bring biobank to life” at BRN 2011 Symposium “Advancing Cancer Research Through Biospecimen Science” of NCI when researcherswe started CR technology [26][40][12,84]. Editor in Chief of American Journal of Pathology proposed applications of CR technology in living biobanks [40][84]. Eventually, more and more articles came out for tumor living biobanks as the applications of CRC and organoid cultures [13][19][22][28][36][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][37,43,46,51,59,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102]. However, that will be impossible task in terms of efforts, work load and cost if one would like to establish living biobanks from every patient in the hospital for the immediate use of personalized medicine. In order to balance of distribution of disease types for research and industry, it’s extremely important to cryopreserved tissue specimens and will generate cell cultures using CR and/or organoids technologies. Thus, rwesearchers brought forth a new concept: next generation living biobank (NGLB) (Figure 16). Except for immediate use of personalized diagnostics and treatment, researchwers suggest to collect and cryopreserve specimens including surgical specimens, core biopsies, needle biopsies, brushed cells, or cells from liquid biopsies (blood, urine, etc.). These specimens should be collected and stored together with their corresponding -omics (genomic, transcriptomic, proteomic, metabolomic, etc.) and clinical information. Then, CR technology will be used to generate an unexhausted cell cultures for living cell banks for future demands in drug discovery, disease modeling, regenerative medicine. These cryopreserved specimens, their corresponding CR cells and CRC- derivatives together compose of NGLB (Figure 1). Compared to regular living biobanks, this NGLB strategy would be a highly efficient platform with the minimal time, money, and other support, this may also avoid redundant cell lines from common diseases, for example, lung cancers. Most importantly, this NBLG includes cryopreserved specimens with broader coverage of disease types, which allow reusearchers to generate cell cultures from rare disease using CR or organoids cultures, since there are no available cell models or very few cell lines for many rare diseases, for example, adenoid cystic carcinoma (ACC), neuroendocrine cancer, etc. ResearchersWe also list properties of conventional cell lines, CRC, organoids and PDX in basic and translational cancer research (Table 1).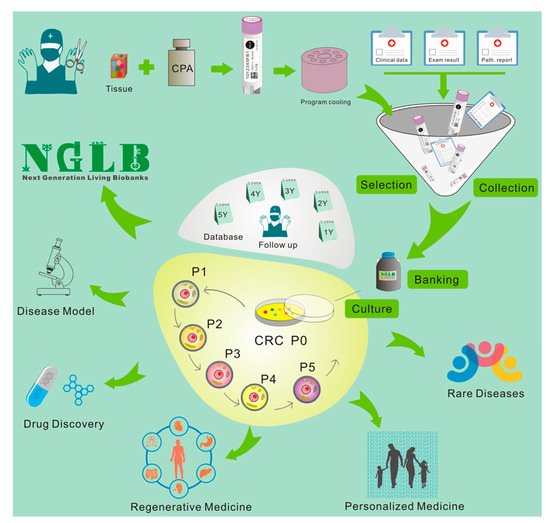
| Conventional Cell Lines | Organoids | PDX | CRC | |
|---|---|---|---|---|
| Sample size | ||||
| FNA | − | +/− | − | +++ |
| Core Biopsy | − | + | − | +++ |
| Surgical Specimens | + | +++ | ++ | +++ |
| Timing | dozen days | 1-5 weeks | 1-5mont | 1-10 days |
| Success rate of initiation | + 0–10% | ++ (5–80%) | ++ (2–30%) | +++50–100% |
| Tumor type specific | ||||
| Rapid Expansion | +++ | ++ | + | +++ |
| Matched Normal con | − | + | - | + |
| Karyotypic stability | − | ++ | N/A | ++ |
| 3D growth | − | + | +++ | − |
| Representation of tumor | + | ++ | ++ | ++ |
| Genetic manipulation | +++ | ++ | − | ++ |
| Maintenance (passage) | +++ | ++ | + | +++ |
| LT drug screens | +++ | – | + | +++ |
| HT drug screens | +++ | ++ | − | +++ |
| Heterogeneity | − | ++ | +++ | ++ |
| Tumor–stroma interaction | − | − | ++ | − |
